The prescribing of Lithium is common in psychiatric clinical practice. The aim of this study was to identify the most common endocrine side effects associated with this drug and to clarify the pathophysiological basis. A systematic review was conducted in Psycinfo, Embase, PubMed, and Scopus. A computerised search for information was performed using a PICO (patient, intervention, comparative, outcomes) strategy.
The main neuroendocrine alterations were reported in kidneys, thyroid and parathyroid glands, pancreas, and the communication pathways between the pituitary and adrenal glands. The pathophysiological mechanisms are diverse, and include the inhibition of the thyroid adenylate cyclase sensitive to the thyroid stimulant hormone (TSH) sensitive adenylate cyclase, which causes hypothyroidism. It also reduces the expression of aquaporin type 2, which is associated with nephrogenic diabetes insipidus, and the loss of the ionic balance of calcium that induces hyperparathyroidism and hypercalcaemia. Other considerations are related to alterations in the hypothalamic-pituitary-adrenal axis and a decrease in the production of catecholamines. Finally, another side-effect is the glycaemic dysregulation caused by the insulin resistance. Periodical clinical and para-clinical evaluations are necessary. The author proposes an evaluation scheme.
La prescripción de carbonato de litio es común en la actividad psiquiátrica cotidiana. El objetivo es identificar las alteraciones endocrinas secundarias y sus bases fisiopatológicas. La revisión de la literatura se realizó en Psycinfo, EMBASE, PubMed y Scopus. Se efectuó una búsqueda computarizada de información utilizando la estrategia PICO. Las alteraciones más comunes están en riñones, tiroides, paratiroides, páncreas y vías neuroendocrinas. Los mecanismos fisiopatológicos subyacentes son diversos, y destacan la inhibición de la adenilato ciclasa tiroidea sensible a tirotropina como causa de hipotiroidismo, la expresión reducida de acuaporina 2 como causa de diabetes insípida nefrogénica, la pérdida del equilibrio iónico del calcio y la presencia de hiperparatiroidismo e hipercalcemia. En el eje hipotálamo-hipófiso-adrenal, se documenta una disminución en la producción de catecolaminas. Finalmente, se documenta la desregulación en el control de la glucemia al aumentar la resistencia a la insulina. Es necesario conocer estas eventualidades e identificarlas tempranamente a través de evaluaciones periódicas. Se propone un esquema de evaluación integral, sin que implique un algoritmo de tratamiento.
Lithium carbonate remains a drug of choice for bipolar disorder. Research has shown its usefulness in the acute and maintenance treatment of manic episodes, and as an augmentation agent for depressive symptoms,1 irrespective of its use in other types of psychiatric disorders.2 Derived from Greek and meaning “stone”, lithium was discovered in a mineral, is assigned atomic number 3 and has a density lower than water. It is a chemical element with the symbol Li, belonging to the group of alkali metals. It is monovalent and highly reactive and is categorised in Group Ia of the periodic table of elements. In powder form, it shares chemical characteristics with sodium and potassium.3
Its antimanic effect was first reported in 1949, but it was not approved by the United States Food and Drug Administration until 1970; four years later, the use of lithium was authorised for the prevention of relapses in said disorder.4–6 According to some studies, approximately 30% and 60% of patients, respectively, will present a complete or partial response to lithium.7–9
In a meta-analysis published in 2005,10 it was documented that the annual incidence of lithium prescriptions to patients with mood disorders was around 0.1/1000, which is similar to the findings of Timmer in 199911 and Manji in 2001.12 However, a 2008 publication13 establishes the annual prescription incidence in the Netherlands at 0.2/1000 psychiatric patients, although the authors do not specify the reasons for administration. Around 90% of patients will suffer side effects from treatment.14 Considering the significance of this matter in daily psychiatric practice, we felt it was important to review the topic.
The primary objective of this work is to document the endocrine disorders most frequently associated with prescription and their physiopathological bases. To meet this objective, the work was divided into three sections. The first addresses very general aspects referring to the pharmacokinetics and pharmacodynamics of the product, an exercise which is by no means redundant, since these aspects have allowed us to understand some of the favourable and adverse effects of the product.
The second section deals with adverse endocrine effects—the focal point of this paper—placing emphasis on the most documented diseases in the literature and addressing any underlying physiopathological aspects.
With a view to providing more than just a description and, on the basis of scientific evidence, the third part proposes the investigations and laboratory tests that should ideally be requested through a timeline, for the purpose of identifying any prescription-derived endocrine disorder in a timely manner.
This same section also gives brief details of some expert pharmacological recommendations that could be documented, in light of evident secondary endocrine disorders. The authors of this project feel they should state that the third part of the primary objective is not intended as a treatment algorithm. Nor was the project's objective to address topics such as clinical uses, other adverse reactions or contraindications.
Search methodologyIn accordance with evidence-based practice, a computerised information search was performed using the PICO (patient, intervention, comparison, outcome) strategy. All of the components related to the problem identified were described and the research question was formed: What are the most notable endocrine disorders caused by lithium use and what are the physiopathological bases proposed to date, taking into account the target organs involved?
The digital databases used were Science Citation Index, PubMed and EMBASE, although PsycINFO and Scopus were also employed; Boolean operators AND, NOT and OR were used to make combinations of words with terms according to the Medical Subject Headings. The keywords used were lithium, lithium carbonate, adverse effects, endocrine system diseases, physiopathology, pharmacokinetics, pharmacodynamics and prescription. The qualitative criteria used to select the material were: (a) editorials, original articles, reviews, systematic reviews and meta-analyses published from 1980 to 2014 or earlier; (b) English or Spanish language, and (c) works with a scientific methodology and editorials with specific contributions. Works that repeated information or which had evident methodological biases were excluded. Eventually, 102 references were selected according to their relevance and theoretical or practical contribution. Articles from before the 1980s were only considered if they were contributions that continued to be cited, and which also met the established criteria.
Pharmacokinetics and pharmacodynamicsLithium tends to reach homogeneous concentrations in the different distribution spaces, except for cerebrospinal fluid. It does not bind to plasma proteins, has a half-life of 20h in adults, achieves equilibrium after 5–7 days of regular consumption, and, when administration ceases, it is quickly eliminated and plasma levels fall within 12–24h of the final dose of the medicine.15
It is primarily eliminated by the kidneys, although it is filtered in the glomerulus, with 80% reabsorption in the proximal convoluted tubules; lithium excretion depends on the glomerular filtration rate and around 95% of the dose is excreted in urine without undergoing biotransformation.15,16 There is no evidence of active metabolites.
In cells, lithium is known to alter sodium transportation in the neuronal membrane and myocyte, to reduce the concentration, storage and release of endogenous catecholamines and to cause increased serotonin synthesis.17 Despite this empirical evidence, the exact mechanism through which lithium functions as a mood stabiliser is unknown.3,17
For several decades it has been proposed that lithium inhibits the enzyme adenylyl cyclase. However, it is currently postulated that the blockage of inositol phosphate recycling is the probable mechanism of action. This blockage has been shown to disable the neurons’ capacity to generate second postsynaptic messengers.18
Endocrine disordersFirst to note is nephrogenic diabetes insipidus, which is estimated in 25–50% of cases treated with this drug19,20; the onset of hypothyroidism is also discussed, affecting 20–30% of patients, as well as hyperparathyroidism, in 5–10% of cases.21–24 Disorders of the hypothalamic-pituitary-adrenal axis25–27 and the pancreas are also common.28–33
Nephrogenic diabetes insipidusThe kidney's function is to eliminate metabolic waste and regulate the volume and composition of bodily fluids.34–36 Lithium causes a reduction in vasopressin by inhibiting the response of renal adenylyl cyclase which is sensitive to said hormone. In turn, this inhibition induces the translocation of aquaporin 2 (AQP2), present in the collecting tubule apical membrane,37 resulting in the onset of diabetes insipidus.
This disease is characterised by the production of abnormally high volumes of dilute urine, with a volume of up to 50ml/kg in 24h and osmolarity <300mOsm/l. Clinically, polyuria or pollakiuria and nocturia are present.35,36 Decreased aquaporin expression affects the renal filtration and reabsorption of liquids. The presence of nephrogenic diabetes insipidus in patients treated with lithium can generally be reversed by discontinuing treatment, although there is evidence that some patients may suffer chronic interstitial nephropathy and irreversible kidney damage after prolonged use.38,39
Proposed mechanism of developing the disorderFor some authors, these mechanisms, although identified, are still not fully understood and probably fail to explain the entirety of the problem.40 In 1994, Unwin et al.41 described decreased aquaporin 2 expression in rat kidney tissue due to lithium treatment. A recent study by Kortenoeven et al.40 showed that lithium decreases the messenger RNA involved in the synthesis of aquaporin 2, whilst reducing the expression of the enzyme glycogen synthase kinase-3 beta (GSK3β). Based on their research, Rao et al.42 propose that decreased GSK3β expression leads to reductions in cyclic adenosine monophosphate (cAMP) in response to arginine vasopressin, which causes a total or partial decrease in aquaporin 2. For the time being, we only have evidence that the deregulation of this transmembrane protein is the demonstrable and key effect of lithium use.43
HypothyroidismThe thyroid gland produces thyroid hormones which are vital for cell differentiation and thus the development and growth of children and adolescents. They are also involved in thermogenic and metabolic homeostasis in adults. In 1968, Schou et al.6 reported a goitre prevalence of 3.6% and an annual incidence of 4% among patients on continuous lithium treatment, compared to 1% of the general population at the time, who were treatment-free. It is currently noted that hypothyroidism and goitre due to this cause generally present within the first two years of treatment, with incidences of 20–30% and 50%, respectively.16 There are reports that decreased thyroid hormone release is more common in patients undergoing prolonged treatment, and that the risk even increases in people living in iodine-deficient areas,16,21 in individuals with a high risk of autoimmune thyroid disease or in those with a family history of thyroid disease.44
In a retrospective study with 718 patients, Johnston et al.45 found a clinical hypothyroidism prevalence of 10.4% in women undergoing prolonged lithium treatment. Kirov et al.46 published that the risk of hypothyroidism increased in women over 50 years of age. It has also been established that 30% of patients treated chronically with lithium suffer from elevated thyroid-stimulating hormone (TSH) which leads to subclinical hypothyroidism and eventually progresses to hypothyroidism with or without goitre.47,48
Proposed mechanisms of thyroid diseaseThe modification of TSH-sensitive adenylyl cyclase activity is particularly noteworthy. There is also evidence that lithium causes a functional modification of G-proteins, prolonging their state of inactivity.49 By preventing molecule activation, lithium promotes a decrease in the production of thyroid hormones, which in turn increases pituitary TSH release. This effect has been deemed critical in the development of hypothyroidism and goitre.49,50
Through experimentation, it has been proven that, with high concentrations of lithium, the iodine organification process is reduced.51 Some authors have highlighted lithium accumulation in thyroid tissue through its intracellular incorporation via active transport as a potential toxic mechanism thereof, as well as reduced iodine absorption by the gland.52,53 Studies performed in animals and humans have shown that lithium can lead to an increased TSH response in the thyrotropin-releasing hormone (TRH) test.54 For Bocchetta et al.,49,50,55 the most noteworthy aspect is that lithium slows down the thyroxine (T4) degradation rate, although for these authors extrathyroidal triiodothyronine (T3) production is not reduced. Current evidence indicates that the peripheral conversion of T4 to T3 is indeed affected.56
Other findingsIt should be noted that inhibition of thyroid hormone release predominantly affects females and patients in whom antithyroid antibodies can be detected at the onset of lithium treatment.16
Another significant fact is that the highest annual hypothyroidism rates are found in subjects with positive antibodies, followed by those with negative antibodies or in females.49 Moreover, an additional interesting finding is the presence of autoimmune thyroiditis, which appears to predispose patients to hypothyroidism and goitre during lithium treatment.55 However, the exact role of autoimmunity on drug-induced thyroid deficiency remains unknown.57
Lithium affects many aspects of cellular and humoral immunity, as seen in in vitro and in vivo studies, although the bone of contention is whether lithium alone can induce thyroid autoimmunity.49 As regards the presence of antithyroid antibodies and the role of lithium in the development of the disease, studies performed in the 1980s58–60 did not detect differences in the antibodies present before or after treatment. More recent findings have shown the development of antithyroid antibodies in young patients of both genders only a few years after lithium exposure.50
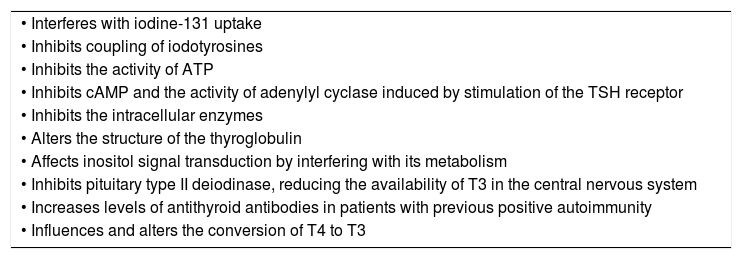
At present, there are case reports on the onset of lithium-induced Hashimoto's encephalopathy, with elevated antithyroid microsomal antibody titres in cerebrospinal fluid.61 Finally, it is important to highlight that autoimmunity in the thyroid gland has been associated with affective disorders irrespective of treatment.62–64 The possible underlying mechanisms are summarised in Table 1.23,50,52
Effects of lithium on the thyroid gland.23,50,52
| • Interferes with iodine-131 uptake |
| • Inhibits coupling of iodotyrosines |
| • Inhibits the activity of ATP |
| • Inhibits cAMP and the activity of adenylyl cyclase induced by stimulation of the TSH receptor |
| • Inhibits the intracellular enzymes |
| • Alters the structure of the thyroglobulin |
| • Affects inositol signal transduction by interfering with its metabolism |
| • Inhibits pituitary type II deiodinase, reducing the availability of T3 in the central nervous system |
| • Increases levels of antithyroid antibodies in patients with previous positive autoimmunity |
| • Influences and alters the conversion of T4 to T3 |
ATP: adenosine triphosphate; cAMP: cyclic adenosine monophosphate; TSH: thyroid-stimulating hormone.
The parathyroid glands are responsible for releasing parathyroid hormone (PTH),24 which is the main regulator of calcium physiology.34 Hyperparathyroidism is characterised by excess PTH production, which often causes hypercalcaemia.35,36 In 1989,65 the causal relationship between chronic lithium treatment and this disorder was proven, having been subsequently reproduced in various later works.44,66,67
Lithium is associated with mild hypercalcaemia, but in some cases it causes hyperparathyroidism due to mechanisms which are not at all clear.68 It has been proposed that these phenomena could also be aetiologically related to reduced kidney function in subjects on chronic lithium treatment.67 Experts highlight that it is not always possible to rule out the possibility of previous hyperparathyroidism, due to the fact that in most cases no baseline assessment is available.16
Proposed mechanism of parathyroid diseaseIn vivo experiments reveal that lithium augments the threshold of the calcium-sensing receptor,69–71 while in vitro experimental studies in bovine parathyroid cells indicate that it increases the quantity thereof, thus inhibiting PTH release.72,73 Lithium also promotes reduced urinary calcium excretion, due to increased renal reabsorption secondary to elevated PTH.74 The inhibition of the PTH-sensitive adenylyl cyclase enzyme has been proposed as a possible mechanism of action.75
Interference with cAMP generation in different tissues forms part of the molecular bases to explain the effects of the medicine.9 Clinical trials76 have shown that, after administering 600mg of lithium per day to healthy volunteers, calcaemia is increased but does not affect PTH levels, thus demonstrating that the control or regulation of parathyroid hormone dynamics is acutely altered, with lithaemia within the therapeutic range, but the real damage occurs in the long term.9
It has been published that patients present unique metabolic characteristics, such as reduced urinary calcium excretion, absence of kidney stones, normal urinary cAMP excretion and normal levels of inorganic phosphate in the blood. There have been very similar findings in familial hypocalciuric hypercalcaemia.9 It is interesting to note that hypercalcaemia alone seems to hinder the control of affective symptoms.77
Hypothalamic-pituitary-adrenal axisProposed mechanism of disease of this systemThere is evidence that patients treated with lithium present hypothalamic-pituitary-adrenal axis activation. Studies performed in Europe document increased paraventricular nucleus activity as a possible mechanism, due to the increased expression of c-Fos (protein coded by the fos gene in humans).25,78 c-Fos expression acts as a marker of neuronal activity during an action potential.79 If the c-Fos mRNA is activated in a neuron, this indicates there has been recent activity.80
In vitro studies show that lithium can inhibit or reduce the production of catecholamine hormones in tumour tissue chromaffin cells (pheochromocytoma) by inhibiting glycogen synthase kinase-3 (GSK-3) in the PC12 cell line, which provides evidence of the effect on the catecholaminergic system in response to the administration of lithium salts.26
The reduced concentration of catecholamines could explain the findings of Cappeliez et al.27 in 1981, in relation to how a deficiency in these hormones influences locomotor activity. Some works clearly show that lithium's effects are substantially reduced following adrenalectomies.26
PancreasThe pancreas is an exocrine and endocrine organ, producing hormones such as insulin, glucagon and somatostatin, to name but a few. Under normal physiological conditions, glucose is the most important energy source used by the brain. Lithium, by altering insulin release, leads to impaired glucose tolerance.28,29
Proposed mechanism of gland diseaseThe effect on glucose regulation seems to be mediated by the stimulation of adrenoreceptors. Studies in rats have shown that the intravenous infusion of lithium leads to hyperglycaemia, as well as increased glucagon and a decreased response to the action of insulin.29 Lithium's direct effect on pancreatic alpha-2 cells and the beta-adrenergic receptors leads to reduced insulin secretion and raised glucagon.30
The study performed by Fontela et al.31 confirms that lithium in vitro has an inhibitory effect on the second phase of insulin release. Peripheral lithium has been associated with a similar effect to insulin in glucose metabolism in the skeletal muscle and adipocytes.32 Studies in animal models have shown that lithium promotes a greater glucose uptake by the myocytes, as well as greater glycogen synthesis, thereby implying an increase in adenosine triphosphate (ATP) phosphorylation, the inhibition of the GSK-3 enzyme and an increase in protein 38 mitogen-activated protein kinase (P38 MAPK).33
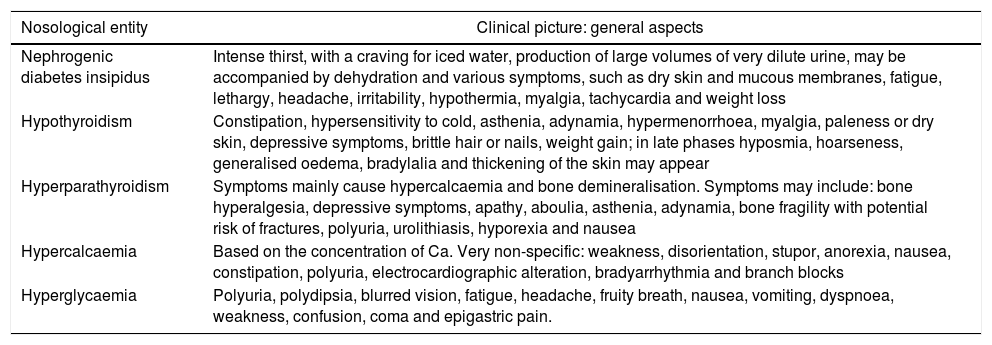
Clinical manifestationsGenerally speaking, Table 234–36 shows the most notable clinical manifestations in each of these disorders.
Clinical manifestations.34–36
| Nosological entity | Clinical picture: general aspects |
|---|---|
| Nephrogenic diabetes insipidus | Intense thirst, with a craving for iced water, production of large volumes of very dilute urine, may be accompanied by dehydration and various symptoms, such as dry skin and mucous membranes, fatigue, lethargy, headache, irritability, hypothermia, myalgia, tachycardia and weight loss |
| Hypothyroidism | Constipation, hypersensitivity to cold, asthenia, adynamia, hypermenorrhoea, myalgia, paleness or dry skin, depressive symptoms, brittle hair or nails, weight gain; in late phases hyposmia, hoarseness, generalised oedema, bradylalia and thickening of the skin may appear |
| Hyperparathyroidism | Symptoms mainly cause hypercalcaemia and bone demineralisation. Symptoms may include: bone hyperalgesia, depressive symptoms, apathy, aboulia, asthenia, adynamia, bone fragility with potential risk of fractures, polyuria, urolithiasis, hyporexia and nausea |
| Hypercalcaemia | Based on the concentration of Ca. Very non-specific: weakness, disorientation, stupor, anorexia, nausea, constipation, polyuria, electrocardiographic alteration, bradyarrhythmia and branch blocks |
| Hyperglycaemia | Polyuria, polydipsia, blurred vision, fatigue, headache, fruity breath, nausea, vomiting, dyspnoea, weakness, confusion, coma and epigastric pain. |
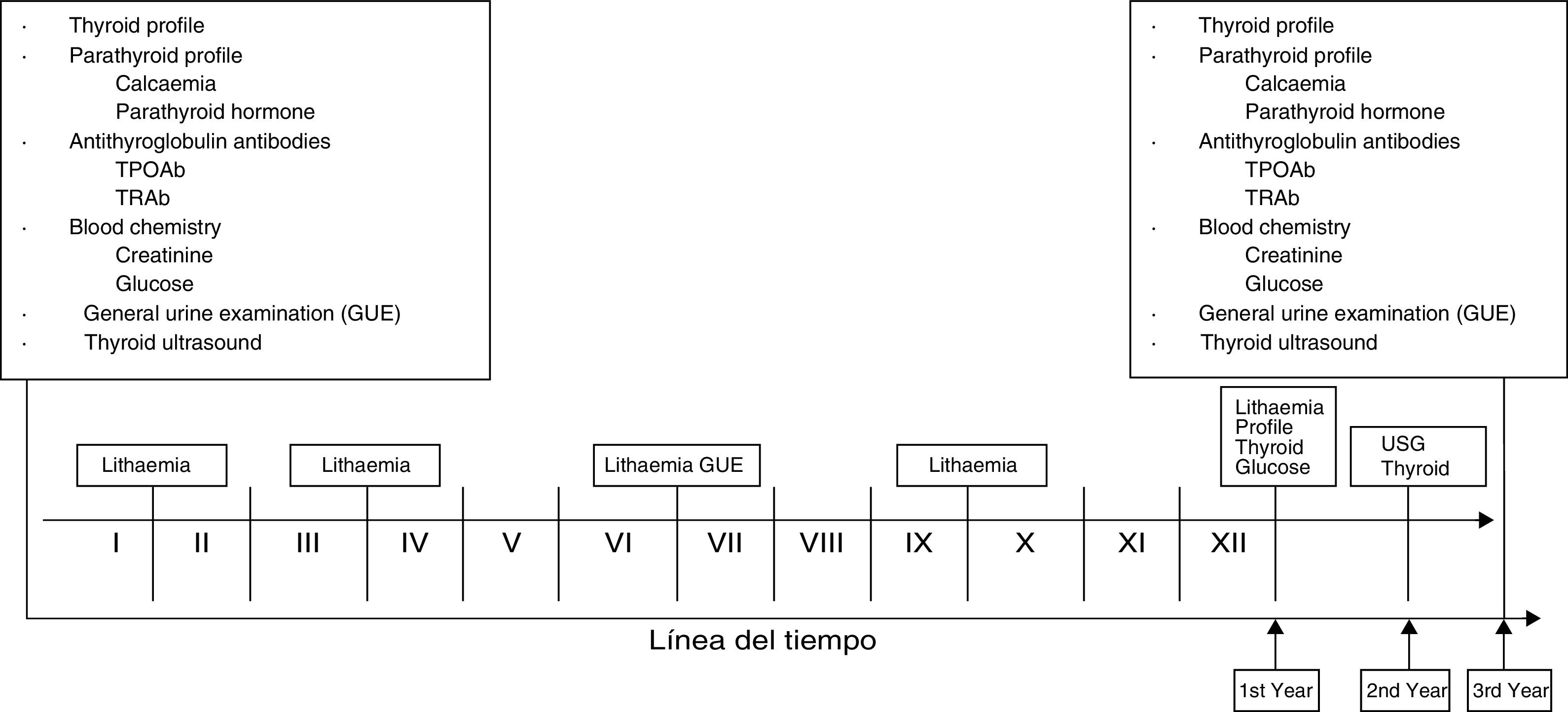
Fig. 149,50,79,80,81 shows the investigations and/or laboratory assessments and tests that should ideally be carried out at baseline and periodically in patients treated with lithium carbonate. This proposal was created in consensus between the authors of this work and is supported by scientific evidence. We decided to include lithaemia which, as we know, is vital. It is important to highlight that the presence of clinical or paraclinical abnormalities at any time during follow-up warrants assessment by the endocrinologist.
Timeline for investigations and laboratory tests.49,50,79–81 Lithaemia: 1st after one month and then every three months. As of the 1st year, every six months. Investigations and laboratory tests: full protocols in senior management. 1st year: thyroid profile and glucose. 2nd year: thyroid ultrasound. 3rd year: protocol similar to baseline. At any time, perform any studies required in correlation with clinical findings. *Roman numerals correspond to the months of the year.
The use of amiloride has been proposed as the medicine of choice at doses of 2–5mg/day.82–85 Other options include hydrochlorothiazide (HCTZ) at doses of 50–100mg/day.86,87
Hyperparathyroidism and hypercalcaemiaThe alternatives may be clinical or surgical.88,89 The use of a product named cinacalcet has also recently been proposed, which has a calcimimetic effect.89–92 The required doses, according to Gregoor,91 showed that on average 30–120mg/day proves effective.
HypothyroidismThe New Zealand clinical practice guidelines93 are pioneering in the treatment of hypothyroidism among lithium-treated bipolar patients.
The guidelines, which were last updated in 2004, state that hypothyroidism responds well to treatment with levothyroxine. A review published in 2006 by Bocchetta et al.49 concluded that 2.1% of females and 0.3% of males required treatment with levothyroxine without discontinuing lithium. Patients under 60 years of age with no clinical data on heart disease may start treatment with 50–100μg/day. Doses are adjusted in increments or decrements of 12.5–25μg/day based on clinical response and TSH concentration.
ConclusionsLithium carbonate has been vital in the treatment of mood disorders, with an indisputable therapeutic effect. Unfortunately, it has also demonstrated a range of adverse effects, placing clinicians in a quandary about whether to prescribe it or not, in spite of the foregoing and the advent of other mood modulators. This decision is without doubt a difficult one, since it is not uncommon for some patients to only benefit from this drug.86
Despite the breadth of information in the literature on adverse events resulting from the use of lithium carbonate, specifically those referring to endocrine disorders, questions continue to be asked on which mechanisms underlie these clinical eventualities.94 These conditions go hand in hand with another reality: the mechanism of action of this product remains uncertain.39,95–98
Prescribing a drug implies challenges, since it can often pose more risks than the disease itself. Endocrine disorders are a clear example of these risks, since, although the literature highlights that they diminish upon the discontinuation of the medicine, some may cause irreversible damage or conditions.99,100 To date, there are no guidelines to steer us in the right direction as regards how to tackle these adverse conditions. Moreover, while it is certain that the first consideration or impulse, so to speak, is to withdraw the prescription, this is not always possible. The recommendation is therefore to fully assess the patients and promote multidisciplinary care for decision-making.
Our proposal to monitor the administration of lithium carbonate, on this occasion, is an approach to what should ideally be done based on the evidence available, considering the angle of endocrine disorders.49,50,79–81 Those of us who work in the clinical field know that ideals are not always feasible or conducive. In this regard, the alternative may therefore be to request investigations which are strictly necessary for the benefit of the patient.
Conflicts of interestThe authors have no conflicts of interest to declare.
To Dr Ricardo Salas Flores, Endocrinologist at the Hospital Regional No. 6 of the Mexican Social Security Institute in Ciudad Madero, Tamaulipas, Mexico, for his suggestions and opinions in this work.
Please cite this article as: García-Maldonado G, Castro-García RdJ. Alteraciones endocrinas vinculadas a la prescripción médica de carbonato de litio. Una revisión narrativa. Rev Colomb Psiquiat. 2019;48:35–43.