Anterior temporal lobectomy (LTA) is a surgical procedure commonly used for the treatment of temporal lobe epilepsy refractory to medical management, with high success rates in the control of seizures. However, an important association with psychiatric illnesses has been described that can alter the postoperative outcome in these patients.
MethodsA series of 2 cases of patients who, despite successful crisis control, developed psychiatric complications in the postoperative period of anterior temporal lobectomy.
ResultsThe cases included a male patient with no history of previous mental illness, who developed a major depressive episode in the postoperative period, and a female patient with previous psychosis who presented as a surgical complication exacerbation of psychosis, diagnosed with paranoid schizophrenia.
ConclusionsPsychiatric disorders can occur in postoperative temporal lobe epilepsy patients with or without a history of mental illness. The most frequent alterations reported are depression, anxiety, psychosis and personality disorders. The inclusion of psychiatric evaluations in the pre- and post-surgical protocols can lead to an improvement in the prognosis of the neurological and mental outcomes of the patients undergoing the intervention.
La lobectomía temporal anterior (LTA) es un procedimiento quirúrgico comúnmente utilizado para el tratamiento de la epilepsia del lóbulo temporal refractario al tratamiento médico, con altas tasas de éxito en el control de las crisis. Sin embargo, se ha descrito una asociación importante con enfermedades psiquiátricas que puede afectar al resultado posquirúrgico en estos pacientes.
MétodosSe exponen 2 casos representativos de pacientes que sufrieron complicaciones psiquiátricas en el posoperatorio de lobectomía temporal anterior, a pesar del control exitoso de las crisis.
ResultadosUn varón sin antecedentes de enfermedad mental que sufre un episodio depresivo mayor en el periodo posoperatorio mediato, y una mujer con psicosis previa que evidencia exacerbación de su afección como complicación quirúrgica.
ConclusionesLa enfermedad psiquiátrica se puede presentar en pacientes posoperatorios de epilepsia de lóbulo temporal tanto con antecedentes de enfermedad mental como sin ellos. Las alteraciones más frecuentes reportadas son depresión, ansiedad, psicosis y trastornos de la personalidad. La inclusión de evaluaciones psiquiátricas en los protocolos prequirúrgicos y posquirúrgicos pueden llevar a una mejora en el pronóstico de los resultados neurológicos y mentales de los pacientes sometidos a la intervención.
Epilepsy is a chronic, progressive condition that requires medical treatment. Patients with this condition often respond well to anticonvulsant medications. However, around 35% persist with seizures despite taking appropriate medications at optimal doses.1–4 For this subgroup of patients, epilepsy surgery is a treatment option.
Anterior temporal lobectomy (ATL) is the most frequently performed intervention, due to its high success rates in seizure control.5–8 An important association with psychiatric pathology has been described in patients with temporal lobe epilepsy (TLE) who underwent surgery.9,10 The disorders frequently referred to are those of mood, thinking and personality.11,12 The appearance of these disorders has not been fully explained, but it is believed that they are related to involvement of the anterior temporal cortex and the limbic system.10
At the Fundación Centro Colombiano De Epilepsia y Enfermedades Neurológicas Jaime Fandiño Franky-FIRE [Colombian Foundation and Centre for Epilepsy and Neurological Diseases], 321 temporal lobectomies have been performed from 1989 to date. The aim of this case report is to describe the association of psychiatric illness in patients who have undergone temporal lobectomy, highlighting the importance of psychiatric evaluation.
Case 1MC is a male patient with no previous medical history or psychiatric disorders. At age 32, he started to have focal dyscognitive seizures, associated with oral and search automatisms. During the postictal period, he presented psychomotor agitation. He required treatment with multiple anticonvulsant drugs that did not achieve adequate control of the seizures.
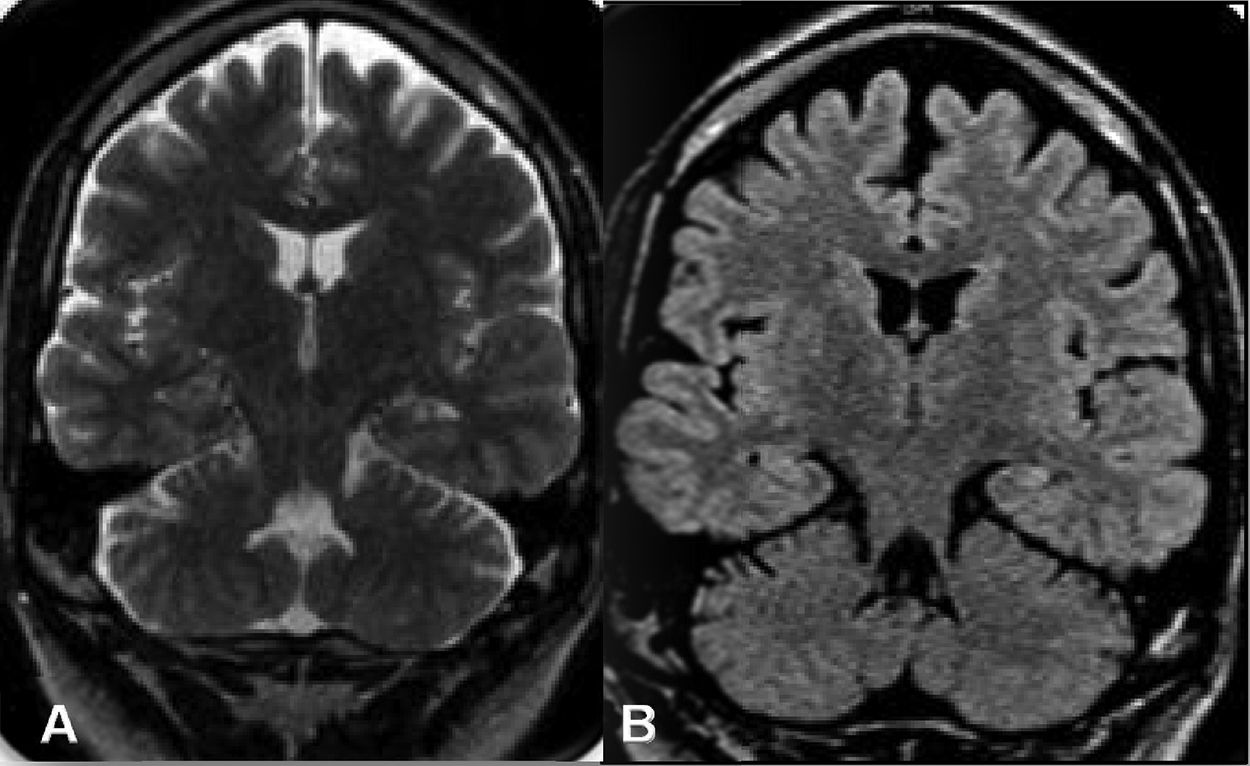
The institutional protocol for epilepsy surgery was carried out, and a brain MRI scan revealed left hippocampal sclerosis (Fig. 1A and B). Videotelemetry showed dyscognitive epileptic seizures with exclusively left temporal electrical activity. Speech and language testing found normal language processing, and neuropsychology testing showed an adequate neurocognitive pattern and mood stability. Therefore, the patient was considered a candidate for a left anterior temporal lobectomy, which was performed without surgical complications.
In the immediate postoperative period, he presented a tendency to cry easily, feelings of hopelessness, disability, isolation, anhedonia and apathy. He was assessed in the psychiatry unit, where he was diagnosed with a severe depressive episode and started on treatment with antidepressants. Despite appropriate treatment, the symptoms have not improved. He is currently dependent and has not resumed his employment. He is free of epileptic seizures after one year of follow-up.
Case 2A woman whose seizures began in her first year of life. Her epilepsy was initially controlled with carbamazepine, but after four years the symptoms changed. Signs of pathological attachment to certain belongings, repetitive behaviours, opposition to authority figures and solitary play appeared.
During adolescence, she developed poor seizure control and her mental symptoms worsened. Family members described persecutory delusional ideation and complex auditory and visual hallucinations which, because they interfered with her behaviour, led to her first psychiatric consultation. In the psychiatric evaluation, she was diagnosed with chronic interictal psychosis, for which medical treatment was initiated. A brain MRI was performed, which revealed a right temporal tumour (Fig. 2A and B). According to the institutional protocol for epilepsy surgery, the medical board decided that the area affected by the tumour was the cause of the seizures, so it was decided to perform LTA despite the risk of increased psychotic symptoms. Histopathology revealed ganglioglioma.
In the immediate postoperative period, the patient suffered an increase in psychotic symptoms. Paranoid schizophrenia was diagnosed. She is currently being treated with antipsychotics, with poor control of delusional ideation, and at 15 years after the intervention, she remains seizure-free.
DiscussionTemporal lobe epilepsy (TLE) is the most common form of drug-resistant epilepsy.5 It is well described that patients with TLE have a greater predisposition to psychiatric disorders than with other types of epilepsy.11–18 The prevalence of psychiatric comorbidity in this group is estimated at 20%–40%, and up to 70% for patients with refractory temporal lobe epilepsy (RTLE).16,19 Among the most common psychiatric disorders are mood disorders, anxiety, psychosis and personality disorders (Table 1).
The neurophysiological bases of this association are not clearly defined. It is known that the development of psychopathology in patients with TLE may be related to alterations in neuroanatomical and neurochemical networks of the amygdala, hippocampus and possibly other structures of the temporal lobe.10,18,20,21 Kandratavicius et al.18 point out that abnormalities related to neuroinflammation associated with epilepsy, such as glutamate dysregulation and blood-brain barrier dysfunction, could be part of the neurochemical alteration found. There are also morphological variations of the brain associated with the concomitance of these alterations.20 Kondziella et al.22 report that there is a reduction in the volume of the hippocampus, entorhinal cortex, neocortex and amygdala in patients with TLE who suffer from depression and anxiety.
Other authors indicate that alterations in structures outside the limbic system could play an additional role.23,24 Salzberg et al.24 conducted a study in which they demonstrated the existence of hypometabolism in the areas of the orbitofrontal cortex (OFC), both in patients with a history of depression and in those in whom it appeared postoperatively.
Temporal lobe surgery and psychiatric disordersEpilepsy surgery has become a fundamental treatment option for patients with RTLE.16 Various series report 70% absence of seizures at two years of follow-up.10,16,25 The most widely used techniques are anterior temporal lobectomy (ATL) and selective amygdalohippocampectomy.5
Psychiatric complications can affect the surgical outcome in patients after ATL.8,12–14,17,26 Some articles show that psychiatric disorders can appear after the intervention.27–29 Others show that patients with previous mental conditions can improve after surgery, while a small group describes how these symptoms can worsen.14,30
Mood disordersThe most common psychiatric disorder in patients undergoing temporal lobectomy is depression. It is often transitory, occurs within 3–12 months after surgery and persists from 1 to 11 months in most cases.20,25,31,32
Some studies show improvement of this disorder with surgical intervention, especially in those who control their postoperative seizures. Devinsky et al.33 conducted a prospective multicentre study in 358 patients, 90% of whom underwent ATL. They were followed up for two years, using self-assessment scales and a structured interview schedule. They reported that the presurgical depression rate was 22.1%. At the end of follow-up, the rate was halved among those who continued to have seizures and dropped to 8.2% among those who were seizure-free.
The prevalence of de novo depression varies between 4% and 18% among patients after ATL.20 Foong et al.17 described similar rates in patients who had selective amygdalohippocampectomy. In the study conducted by Pope et al.,34 a series of 30 patients who underwent ATL, it was found that five cases suffered de novo depressive episodes in the four years after surgery. The MRI results prior to surgery showed bilateral atrophy of the orbitofrontal cortex, the left cingulate gyrus and the left thalamus in patients who developed depression, indicating that frontal dysfunction may play an important role.
The factors related to the appearance of depression after temporal lobectomy are variable. There is little evidence that postoperative depression is associated with the laterality of the resection. Only one study35 found that patients who have had resection of the right temporal lobe are at higher risk, but this has not been confirmed by others.33,36–39 For most studies, the preoperative presence of depression is the main predictor.13,17,20,32 Other authors indicate that the presence of fear as an aura, focal seizures with secondary generalisation,25,29,37 older age at the time of surgery, little psychosocial support, family history of mood disorders40 and type of temporal surgery (mesial resections)36,41 are factors that may influence the onset of depression.
In a study by Wrench et al.,36 patients who underwent mesial temporal lobe resection were evaluated against those with different resections of the mesial temporal lobe. They found that the prevalence of preoperative chronic depression did not differ between the surgical groups. In the postoperative phase, the mesial temporal resection group experienced a significantly higher rate of recurrent and de novo depression. This is relevant because alteration of the mesial temporal lobe is the most common reason for refractory epilepsy being operated on, and it is believed that dysfunction of these structures leads to an increased risk of this complication, as already mentioned.
On the other hand, it has been described that pharmacological treatment of depression prior to surgery is a good prognostic factor. The study carried out by Blumer et al.,39 found that depressive symptoms subsided or remained stable in half of the patients with previous antidepressant treatment and that in those who interrupted treatment the symptoms worsened.
Anxiety disorderMost authors have reported a transitory increase in anxiety after ALT, with a frequency between 17% and 54% one month after the intervention, and a decrease in anxiety at three months.41–43 Long-term follow-up studies have confirmed that the highest rates of anxiety were in the first three postoperative months, with a significant reduction at 12–24 months.33,34
The effect of seizure control on the appearance of anxiety disorders after ALT is currently unclear. In their multicentre study, Devinsky et al.33 found a tendency to reduce anxiety symptoms in patients without seizures. In contrast, other authors describe that there is no improvement in anxiety disorders after temporal lobectomy, even with seizure control.42,53
The main factor associated with the appearance of anxiety in the postoperative period is a history of affective disorders.43 Kohler et al.37 reported that patients with TLE who present fear as a typical aura pre-surgically have a 50% higher risk of anxiety and panic attacks after surgery. There is no clarity regarding the effect of surgical lateralisation as an associated factor in the appearance of anxiety disorder. Some authors, such as Bladin et al.42 and Ring et al.,44 have reported an association between resection of the left temporal lobe and persistent anxiety, but this has not been confirmed by other studies.33
Psychotic disordersPost-surgical prevalence rates for de novo psychosis are low. In the study by Christodoulou et al.,45 three of 282 patients suffered postictal psychosis. However, in a recent series published by Cleary et al.,46 higher rates of psychosis were reported, in 4%–8% of cases. It is estimated that this complication has its highest incidence in the first postoperative year.47
There is no clear relationship between the development of psychotic symptoms and seizure control.48 Some authors indicate that after surgery, patients with adequate seizure control also show improvement in psychosis.40 However, others suggest that patients with chronic psychosis often show little improvement after surgery despite being seizure-free,46,49 as happened in our case.
The pathophysiological mechanisms for the development of de novo psychosis are unknown. Studies describe several associated factors, including bilateral epileptiform activity, a history of encephalitis and structural abnormalities, particularly of the amygdala. Focal brain lesions such as gangliogliomas and dysembryoplastic neuroepithelial tumours have been reported more frequently as the aetiology of psychosis in patients with RTLE.9,46,47
There is currently no clear evidence that laterality of surgery is associated with postoperative psychosis. Kohler et al.48 reported a predominance in right temporal lobectomy, but this has not been evidenced in other studies.33,40,47
Personality disordersPatients with a history of personality disorders are at increased risk for postoperative psychiatric complications. In the study by Koch-Stoecker et al.,50 a two-year follow-up of 100 patients who underwent ALT was carried out. It was seen that those with personality disorder in the preoperative period had a higher risk of requiring hospitalisation in a mental unit than patients without this history.
On the other hand, it has been described that right ALT in patients with a history of personality disorder has a high rate of psychiatric complications.50 Some authors report that it may be related to right hemisphere dysfunction, which results in a misperception of emotional signals and impaired coping mechanisms.50,51
Presurgical and postsurgical psychiatric evaluationsVarious studies report that psychiatric symptoms can be exacerbated or can develop after ALT. The inclusion of psychiatric evaluations in the preoperative and postoperative protocols can lead to an improvement in the prognosis of the neurological and mental outcomes of the patients undergoing the intervention. Although these evaluations are not yet fully defined or standardised, it has been proposed to use some scales such as the Hamilton Anxiety Rating Scale (HARS), the Hamilton Depression Rating Scale (HDRS) or the Brief Psychiatric Rating Scale (BPRS) to compare and/or define the appearance of symptoms after surgery.29
Certain preoperative psychiatric conditions must be carefully considered. For example, in patients with chronic interictal psychosis, high percentages of therapeutic non-compliance and little psychosocial support have been evidenced. Some authors consider that these types of patients are not good candidates for surgery. Cases like the previous one have led to the debate on the existence of absolute contraindications to ALT, but currently there are no mental conditions that contraindicate the intervention.17
Psychiatric evaluations are crucial in the postoperative period.52 It is known that the highest percentage of psychiatric disorders are of mood and anxiety.53 Both conditions are characterised by appearing transiently in the early postoperative period, for which reason several studies recommend periodic evaluations during the first year after surgery.16,17,46
ConclusionsTLE is the most common form of drug-resistant epilepsy. People with RTLE have a high predisposition to psychiatric disorders, mainly of mood, and in particular depression, anxiety, psychosis and personality disorders. Most of the studies are small series showing both patients suffering from psychopathology after ALT and those who improve or worsen their pre-existing mental disorders. Despite this, surgery continues to be a valuable tool for patients with RTLE since, in general terms, it improves their quality of life.
Long-term follow-up studies, including preoperative and postoperative psychiatric evaluations, are required to determine the association between the intervention and the psychiatric illness evidenced in some patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Camargo Camargo L, Tejada Angarita KS, Suarez Marín MM, Fandiño Franky J. Alteraciones psiquiátricas tras lobectomía temporal anterior: reporte de casos. Rev Colomb Psiquiat. 2021;50:301–307.