Alcohol use disorder is one of the main causes of morbidity and mortality in the world. Alcoholic liver disease is a common complication of this disorder, and hepatic encephalopathy is a serious complication of alcoholic cirrhosis. Precipitating factors may be related to infection, gastrointestinal bleeding, dehydration or the effects of psychotropic drugs (e.g. benzodiazepines and non-benzodiazepine hypnotics). We present a case of the hospital management of a patient with a severe alcohol use disorder, cirrhosis and hepatic encephalopathy who developed alcohol withdrawal symptoms while in hospital, and discuss the complexity of the antagonistic management of a GABAergic delirium characteristic of hepatic encephalopathy in the context of a glutamatergic-noradrenergic delirium due to alcohol withdrawal.
El trastorno por consumo de alcohol es una de las principales causas de morbimortalidad en el mundo. La enfermedad hepática alcohólica es una complicación común de este trastorno y la encefalopatía hepática es una seria comorbilidad de la cirrosis alcohólica. Los factores precipitantes pueden relacionarse con infección, sangrado gastrointestinal, deshidratación o efectos de psicofármacos (p. ej., benzodiacepinas e hipnóticos no benzodiacepínicos). Se expone un caso del manejo hospitalario de un paciente con un trastorno severo por consumo de alcohol, cirrosis y encefalopatía hepática, quien desarrolla síntomas de abstinencia alcohólica durante su hospitalización y la complejidad del manejo antagónico de un delirium gabaérgico propio de la encefalopatía hepática en el contexto de un delirium glutamatérgico-noradrenérgico por abstinencia alcohólica.
Alcohol use disorder is a significant cause of morbidity and mortality. Hepatic encephalopathy (HE) is a relatively common condition in this clinical scenario, and is a neuropsychiatric syndrome that primarily affects patients with advanced chronic liver disease and those with significant portosystemic shunts. It occurs in 20-80% of patients with liver cirrhosis and can be diagnosed once any other neurological disease that might present with an encephalopathic clinical picture has been ruled out.
Among the factors most closely related to HE, alcoholism appears to be the leading cause. Colombia is no stranger to this reality, with alcohol misuse seen in one in 15 inhabitants, beginning at 10 and 11 years of age on average. Severe alcohol use disorder occurs in up to 6% of the population over 45 years of age, as in the case of the patient discussed here.1 In those who go on to develop decompensated cirrhosis, mortality rates are very high, at 71%, 84% and 90% after 5, 10 and 15 years of follow-up, respectively.2 This high mortality rate does not come down until 1.5 years after stopping alcohol use.2 Chronic liver cirrhosis can present with other comorbidities (hepatitis B and C virus, primary biliary cirrhosis, alcoholic liver disease). Of these, alcoholism has the highest short-term mortality rates.3
This entity is characterised by a series of clinical manifestations associated with behavioural changes, delirium, motor dysfunction, altered brain stem reflexes and respiratory pattern. Clinical diagnosis required the following elements: interview, physical/mental examination findings (changes in consciousness, extrapyramidal signs, the "flapping" sign, asterixis or flapping tremor), neurological evaluations, laboratory tests and electroencephalography (EEG).4,5
Drugs such as benzodiazepines are a cause of acute encephalopathy when used in sedation, alcohol abstinence and convulsion protocols in a hospital setting. Of the various hypotheses formulated, the most widely accepted is the ammonia (NH3) hypothesis. The NH3 primarily originates from bacterial metabolism of proteins and glutamine in the lower digestive tract, and to a lesser extent, from the metabolism of the kidney and skeletal muscle. Under normal conditions, circulating NH3 travels to the liver, where it is transformed into urea, and excreted in urine and faeces. In patients with HE, circulating NH3 is not adequately eliminated, leading to an increase in plasma concentrations. It then crosses the blood-brain barrier and triggers a series of changes that explain the syndrome's clinical picture. The gamma-aminobutyric acid (GABA) hypothesis relates to hyperactivity of the GABAergic system associated with the formation of endogenous benzodiazepines linked to the previous cycle, and has been demonstrated in experimental models. It is associated with a favourable response of HE-related delirium to benzodiazepine receptor antagonists such as flumazenil.6Table 1 summarises the classification based on the West Haven clinical criteria.
Clinical Classification of Hepatic Encephalopathy according to the West Haven Criteria
| Grade | Characteristics |
|---|---|
| 0 | No behavioural changes, no asterixis |
| I | Difficulty maintaining and directing attention |
| Disordered sleep and affective behavioural changes. | |
| Asterixis may be present | |
| II | Lethargy, temporal disorientation, amnesia of recent events, productive language difficulties. Asterixis present |
| III | Somnolence, severe confusion, clonus and nystagmus, usually no asterixis |
| IV | Coma |
Alcohol has a range of effects on the central nervous system depending on the volume ingested and the chronicity of use. It acts on various molecular targets, primarily GABA A receptors, NMDA glutamate receptors and voltage-gated calcium channel receptors. In contrast, alcohol withdrawal syndrome is a series of signs and symptoms that typically present within 24 to 48 hours of the last intake in alcohol-dependent individuals. It is a progressive neuropsychiatric syndrome secondary to the central nervous system effects of chronic alcohol use and is exacerbated by some comorbidities of alcoholism. Generally speaking, it involves enhanced transmission in NMDA receptors, reduced GABAergic transmission, and secondary dysregulation of the dopaminergic and noradrenergic system with excessive noradrenergic activity due to desensitisation of the central alpha-2 receptors and conversion from dopamine.7
The rate of associated medical and surgical complications in moderate to severe alcohol withdrawal is high. The incidence of severe alcohol withdrawal in patients admitted to medical or intensive care units (ICU) varies between 5% and 20%. These cases are associated with additional severe complications from a clinical viewpoint, such as increased ventilation requirements, increase length of ICU and hospital stay, increased cognitive sequelae and increased mortality. There is a positive correlation between severity and duration of severe alcohol withdrawal symptoms and the occurrence of pneumonia, heart disease, severe liver disease and anaemia.
To date, alcohol withdrawal mortality remains high. According to Monte et al., it can reach 15% in patients not treated in medical units, versus 2% when the patient receives the respective hospital care.8
Case reportD is a 72-year-old male patient who lives with his wife. He worked as an industrial engineer for over 40 years and has been retired since 56 years of age.
He was initially diagnosed with liver cirrhosis at 67 years of age, Child-Pugh class C prior to admission, recurrent HE, oesophageal varices and small fundal varices, grade i diastolic dysfunction observed as mild mitral valve regurgitation. Treatment on admission with spironolactone 25 mg every 12 hours, lactulose 1 sachet every 8 hours and thiamine 600 mg per day. He has used alcohol habitually since 12 years of age and for the last year has consumed one bottle of whiskey and a quarter litre of aguardiente (Colombian aniseed liquor) per day, generally accompanied by a small drink of brandy with milk every night. During periods in which he claimed to have abstained, he stopped drinking distilled liquors, instead drinking beer with carbonated beverages (Cola y Pola®), consuming 3-5 cans per day. At the time of admission, he had a score of 28 on the Alcohol Use Disorders Identification Test (AUDIT) and had smoked two packs per day for 35 years (70 pack-years).
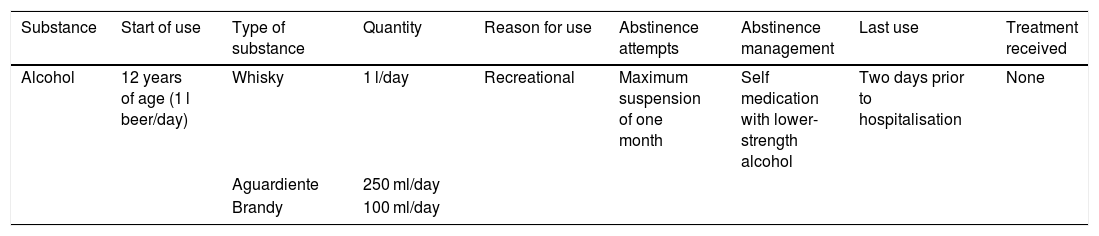
His alcohol use pattern is summarised in Table 2. On admission to hospital, he was dependent for all basic and instrumental activities of daily living. Asterixis, elevated ammonia and severe prostration were documented on admission. His companion described poor adherence to treatment and persistent alcohol use in spite of the recommendations of medical staff treating him. Admitted with a diagnosis of grade iii HE, with a history of continuous alcohol use; it was expected that he might develop symptoms of alcohol withdrawal.
Patient's alcohol use pattern
| Substance | Start of use | Type of substance | Quantity | Reason for use | Abstinence attempts | Abstinence management | Last use | Treatment received |
|---|---|---|---|---|---|---|---|---|
| Alcohol | 12 years of age (1 l beer/day) | Whisky | 1 l/day | Recreational | Maximum suspension of one month | Self medication with lower-strength alcohol | Two days prior to hospitalisation | None |
| Aguardiente | 250 ml/day | |||||||
| Brandy | 100 ml/day |
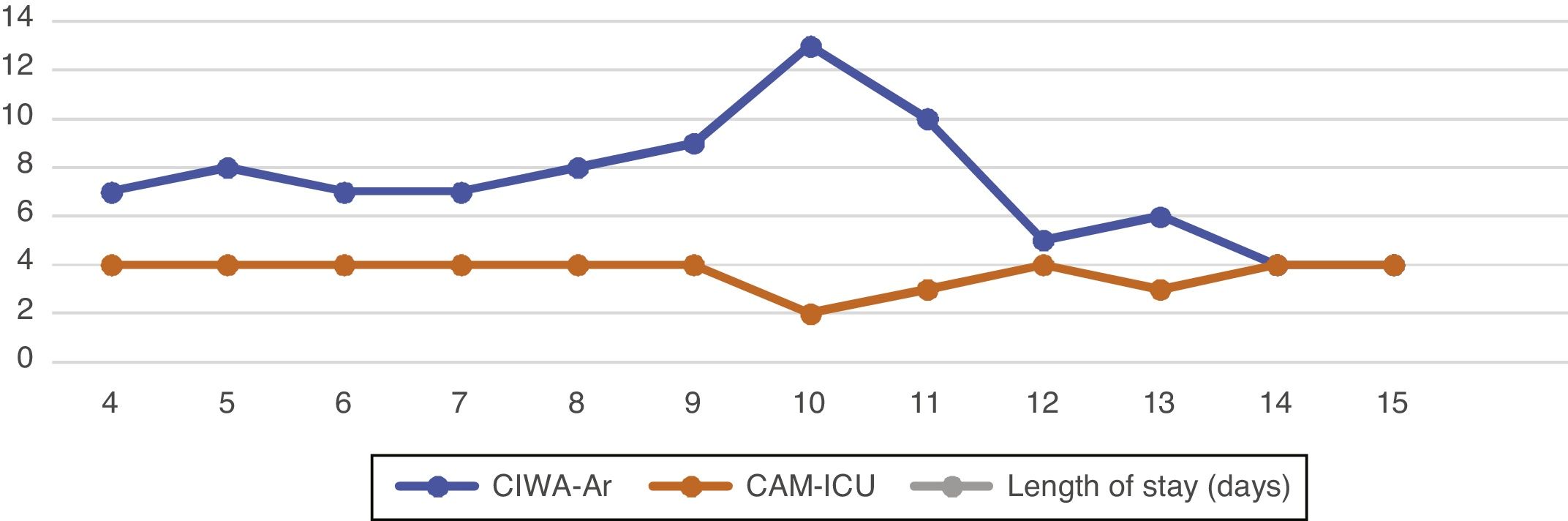
Regarding encephalopathy symptoms, he has a score of 14 (E4 V4 M6) on the Glasgow coma scale, and 4/4 points on the Confusion Assessment Method for the ICU (CAM-ICU) delirium scale. Given his history and ammonia levels, the cause was considered to be of hepatic origin. He was hyponatraemic, which may have increased the degree of somnolence. In view of the above, lactulose 1 sachet every 8 hours was continued, along with rifaximin 400 mg every 8 hours. He was assessed by our department using the Prediction of Alcohol Withdrawal Severity (PAWS) Scale, with a score of 5. In view of this it was decided to start assessment with the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) to monitor and make decisions on the management of possible concurrent alcohol withdrawal, and pharmacological management was started with oral lorazepam 1 mg every 12 hours (the data obtained are shown in Fig. 1).
Patient during clinical evolution with the CAM-ICU and CIWA-Ar scales documenting diagnostic doubts in the evolution based on the suspected aetiopathogenesis.
CAM-ICU: Confusion Assessment Method - Intensive Care Unit; CIWA-Ar: Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised.
Source: A. López, R Chavarría, G. Oviedo.
On day 4 of his stay, he remained inattentive, with marked fluctuations in his level of consciousness, multi-sensory hallucinations, inverted sleep-wake pattern and associated neurovegetative instability. He was diagnosed with delirium of multiple aetiologies. Serial assessments with both the CIWA-Ar and CAM-ICU scales were therefore continued.
On the sixth day of his stay he was diagnosed with healthcare-associated pneumonia, managed with piperacillin/tazobactam; given the somnolence now associated with the infectious process, it was decided to reduce the lorazepam dose to 0.5 mg every 12 hours and add intramuscular thiamine 300 mg every 8 hours for five days. Six days later, he presented marked somnolence and was difficult to rouse. In the departmental medical review, suspecting grade iii HE and delirium with a mixed motor subtype, the decision was made to suspend lorazepam and add haloperidol 1 mg at night. Supporting paraclinical tests were requested, with images from nuclear magnetic resonance of the brain indicative of acute ischaemic stroke. Non-specific supratentorial leukoencephalopathy, possibly secondary to chronic small vessel disease (Fazekas II-III). Report: EEG records bursts of slow waves over 2-3 cycles located in broad frontal regions in groups. No triphasic waves, signs of focalisation or epileptic-type paroxysms observed. Abnormal wake-sleep trace of encephalopathic appearance due to increase slow-wave activity in frontal regions; no triphasic waves observed (Fig. 2).
On the 14τη day of his stay, CAM-ICU: 4/4; Richmond Agitation-Sedation Scale (RASS): -1 point; level of consciousness: soporous; the patient passed away three days later.
DiscussionD was classified as a high risk alcoholic (alcohol use disorder) based on his use pattern, which exceeded 80 g of ethanol per day.9 Although the patient was over 68 years of age before he consulted any medical service, a score of 28 on the AUDIT scale documented this impression.10 Abrupt cessation of alcohol consumption, accompanied by a clinical picture of multi-sensory hallucinations and psychomotor agitation, led to a presumptive initial diagnosis of delirium due to alcohol withdrawal.7 The patient had scores between 8 and 14 points on the CIWA-Ar scale. In accordance with institutional guidelines for the management of alcohol withdrawal,11 treatment with lorazepam was started.10
After six days of treatment with benzodiazepines, the patient's clinical picture had evolved on one of predominantly psychomotor inhibition and lethargy. The physical examination revealed signs such as palmar "flapping",4 as well as worsening liver function tests. Suspecting HE, an electroencephalogram was requested, and showed a slow-wave pattern with a predominant beta rhythm and few delta and theta waves. Although this of course is not a clear encephalopathy rhythm, the diffuse slowing is suggestive of an early-stage encephalopathic process.12 The delta waves can be interpreted as a pan-inhibitory state of the central nervous system. It is known that in cases of severe alcohol withdrawal, the encephalographic pattern is generalised low voltage with high frequency, an element that could not be documented at the start of the hospitalisation. Another confirmatory element was the serum ammonium measurement, which was recorded as 147 g/dl, a value six times higher than the baseline. It should be noted that, although ammonia is not currently measured for therapeutic or prognostic ends, elevated levels do corroborate the presence of the disease.13
The above elements guided the decisions to alter the pharmacological management. It was decided to replace lorazepam with haloperidol, which led to an improvement in delirium symptoms. Ultimately, in spite of the gradual improvement of his mental state, the complications that emerged following the diagnosis of healthcare-associated pneumonia, as well as liver failure, resulted in the patient's death.
Closing remarksHE has manifestations associated with a reduction in cerebral metabolism and cerebral oedema. Hyperammonaemia is a fundamental factor in the associated scenario, but other compounds can also contribute, such as neoformation of endogenous benzodiazepines, and it is in this context that delirium with GABAergic characteristics presents.14 The concurrent presentation of alcohol withdrawal symptoms with HE is a clinical and therapeutic challenge. Any exhaustive clinical assessment and diagnostic supports such as encephalography can aid in making an accurate diagnosis and optimising interventions. The presentation of severe alcohol withdrawal varies from person to person. The lack of direct biomarkers and possible genetic variability only add to this difficulty. We consider the adaptation, validation and use of instruments such as the PAWS (Maldonado et al.15) useful in predicting the evolution and severity of alcohol withdrawal syndrome.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: López A, Chavarría R, Oviedo G. Dilema terapéutico: síndrome de abstinencia alcohólica y encefalopatía hepática concurrentes. A propósito de un caso. Rev Colomb Psiquiat. 2021;50:52–56.





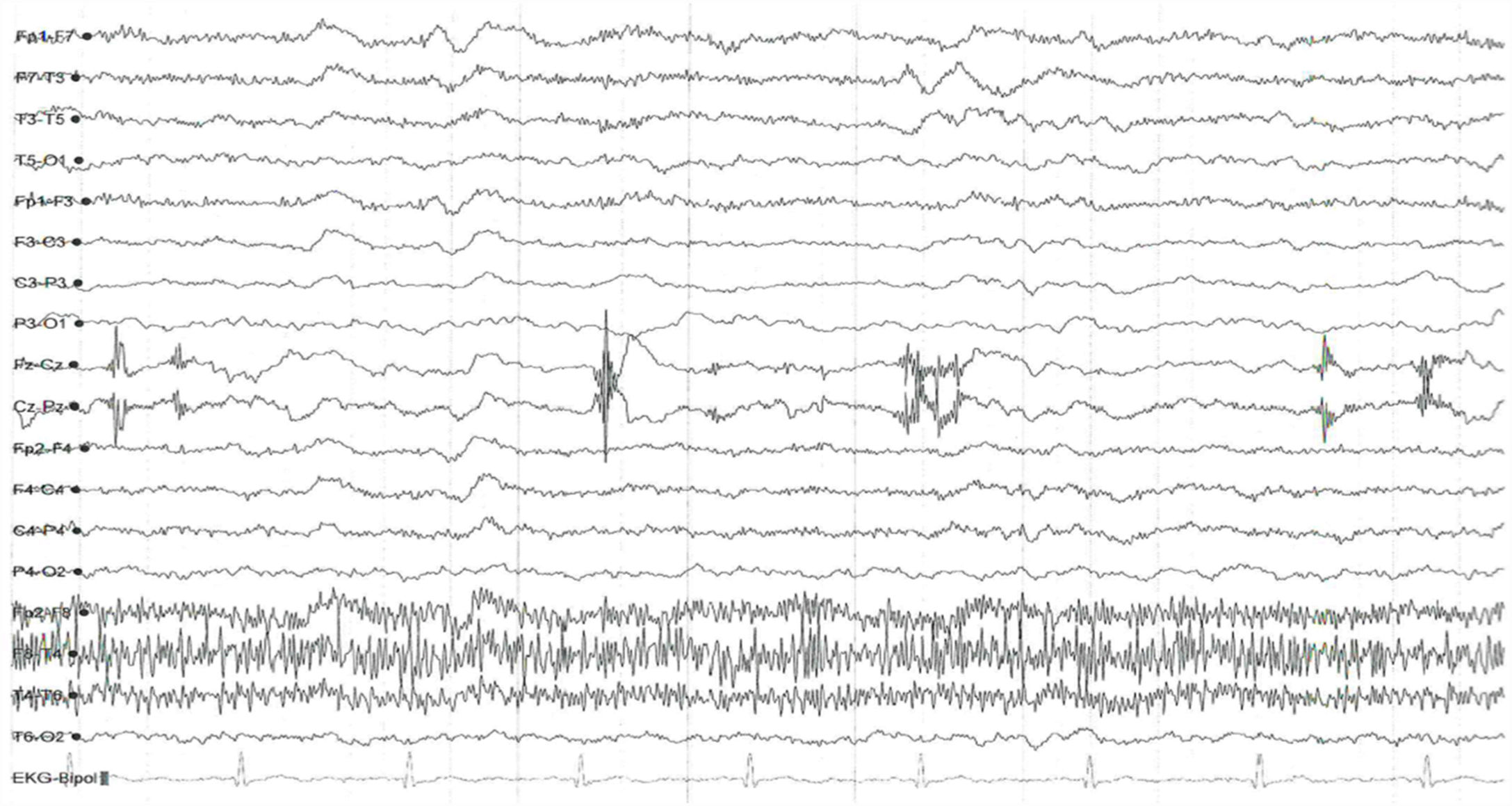

![Electroencephalography trace. As noted in the text, a pattern suggestive of diffuse slowing (Hospital Universitario San Ignacio [San Ignacio University Hospital], 2017). Source: A. López, R Chavarría, G. Oviedo. Electroencephalography trace. As noted in the text, a pattern suggestive of diffuse slowing (Hospital Universitario San Ignacio [San Ignacio University Hospital], 2017). Source: A. López, R Chavarría, G. Oviedo.](https://static.elsevier.es/multimedia/25303120/0000005000000001/v1_202103030824/S253031202100014X/v1_202103030824/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
