Silicone breast implants are considered highly immunogenic adjuvant devices in the human body, however, the medical evidence linking the development of autoimmune diseases and Adjuvant-induced Inflammatory Autoimmunity Syndrome (ASIA) in patients using breast implants is not conclusive. In the following manuscript, an exploratory systematic review was carried out using the Scopus and PubMed databases, including analytical and descriptive observational studies with no time limit. Twenty-one articles were included, which showed that the main autoimmune diseases associated with this group of patients were undifferentiated diseases of connective tissue; most of the patients presented symptoms such as chronic fatigue, arthralgia, fever, and myalgia. The objective of this study is to synthesize and analyse the current medical literature on the frequency of rheumatological diseases concomitant with ASIA in users of silicone breast implants.
Los implantes mamarios de silicona son considerados dispositivos adyuvantes altamente inmunogénicos en el cuerpo humano, sin embargo, la evidencia médica que relaciona el desarrollo de enfermedades autoinmunes y el síndrome de autoinmunidad inflamatoria inducido por adyuvantes (ASIA) en pacientes usuarios de implantes mamarios no es concluyente. En el siguiente manuscrito se presenta una revisión sistemática exploratoria que se realizó empleando las bases de datos de Scopus y PubMed, incluyendo estudios observacionales analíticos y descriptivos sin límite de tiempo. Los 21 artículos incluidos pusieron en evidencia que las principales enfermedades autoinmunes asociadas a este grupo de pacientes fueron las enfermedades no diferenciadas del tejido conectivo, en tanto que la mayoría de los pacientes presentó sintomatología como fatiga crónica, artralgias, fiebre y mialgias. El objetivo del estudio es sintetizar y analizar la literatura médica actual sobre la frecuencia de las enfermedades reumatológicas concomitantes con ASIA en usuarios de implantes mamarios de silicona.
Silicone breast implants (SBI) were first introduced to the world of cosmetic surgical procedures in 1960. In 2012, more than 330,000 augmentation mammoplasties in the United States used SBI.1 Unfortunately, the silicone used in these products is highly reactive for the immune system, which generates antigen-antibody responses with systemic hemolymphatic dissemination. Thus, the use of SBI has been associated with systemic adverse reactions2–4 and even with the development of rheumatological diseases.5
Shoenfeld and Agmon-Levin6 first described the Autoimmune/inflammatory Syndrome Induced by Adjuvants (ASIA), initially in four disorders: silicosis, Gulf War syndrome, macrophagic myofascitis syndrome, and the post-vaccination phenomenon. The most common symptoms associated with this syndrome are chronic fatigue, arthralgia, arthritis, myalgia, fever without an apparent source, memory loss, sleep disorders, depression, xerostomia, and xerophthalmia.2,3,5
It is currently recognized that SBI users can develop ASIA syndrome and autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and adult-onset Still disease (AOSD).1,7,8 Despite this, it is a little-known phenomenon among health professionals. On the other hand, it is necessary to collect scientific information on possible systemic complications, along with genetic and environmental risk factors that predispose to the development of rheumatological diseases. For this reason, the current exploratory systematic review was carried out, which collects updated studies on the diagnosis and therapeutic management of patients with SBI who developed ASIA syndrome, as well as its relationship with other autoimmune diseases.
MethodsAn exploratory systematic review was conducted following the steps proposed by Arksey and O'Malley9 and reviewed by Levac et al.10: 1) identification of the research question; 2) identification of relevant studies; 3) selection of studies; 4) data extraction, and 5) synthesis and reporting of results. This review adhered to the elements of the PRISMA extension for reporting scoping systematic reviews (PRISMA-ScR)11 (Supplementary File 1). The research question was: What is the nature of the existing medical literature describing the relationship between SBI use and ASIA syndrome or other autoimmune diseases?
Analytical observational and descriptive studies were included (cohort studies, cases and controls, case series, and case reports) in English and Spanish, without time limit, with data on patients using SBI and its association with autoimmune diseases and ASIA syndrome. Systematic or narrative reviews, letters to the editor, editorials, opinion articles, clinical management guidelines, protocols, and documents without access to the abstract or full text were excluded.
The PubMed and Scopus databases were included, using key terms as well as Boolean operators (Supplementary file 2). References cited in the included documents, if they met the eligibility criteria and had not been previously identified, were considered for possible final inclusion. The review of the titles and abstracts of the publications found in the databases was carried out by three independent authors (EB, LV, and CA), based on the eligibility criteria. In publications in which there was some doubt about their inclusion, the remaining authors met to decide their inclusion or exclusion.
The included articles were reviewed in full text by all authors. The variables that guided data extraction were authors, year of publication, country, type of document or study, sample size, characteristics of the population under study, objective, main findings, and journal. Finally, a narrative synthesis was conducted of the publications included in the current review.
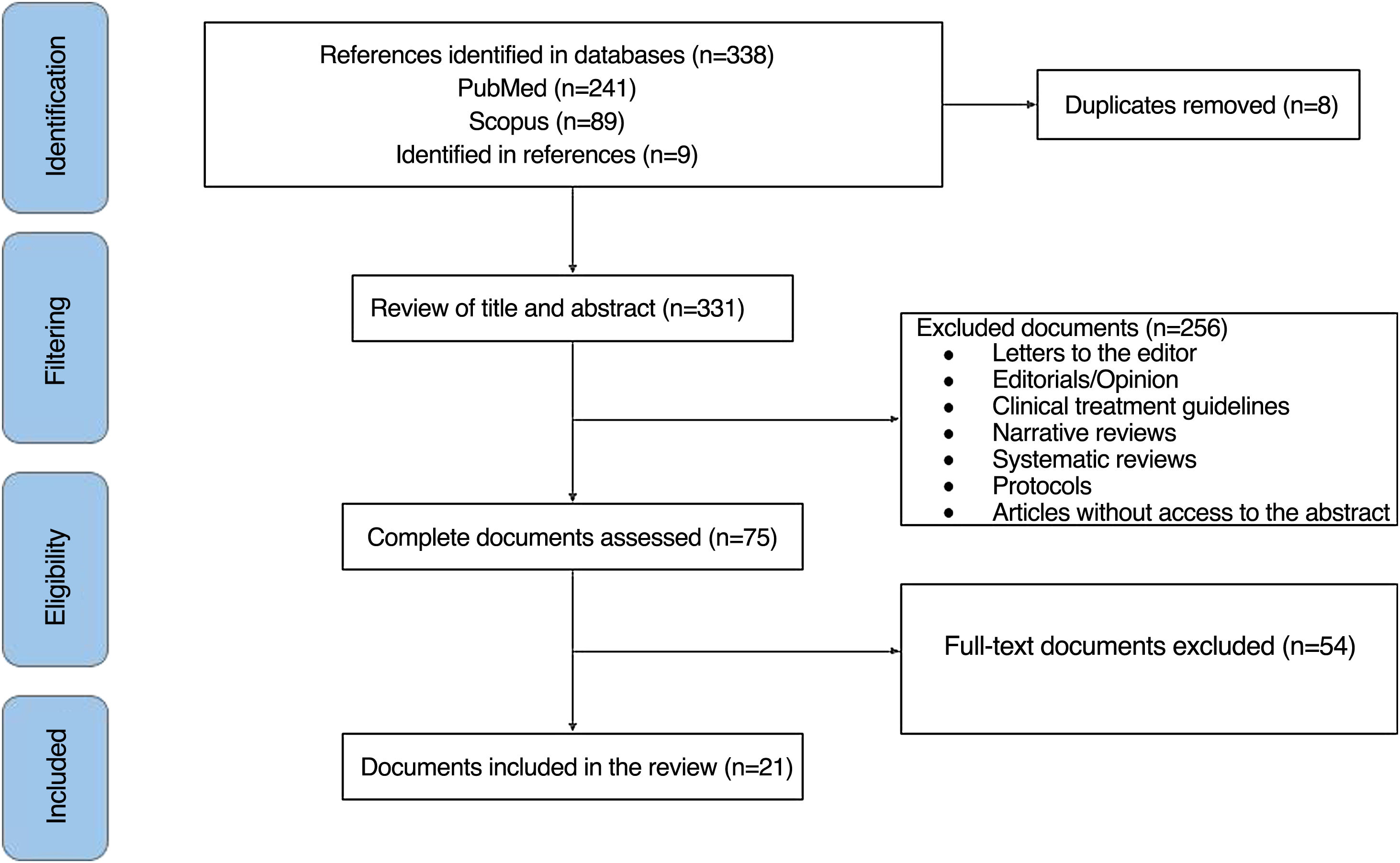
ResultsOf 338 documents identified by the search, 21 articles were finally included (Fig. 1). Case reports (n = 11), case series (n = 5), retrospective cohort studies (n = 3), cross-sectional studies (n = 1), and cases and controls studies (n = 1) were identified, with a total sample of 25,307 subjects. The most common country of origin of the authors was Israel (n = 6), followed by the Netherlands (n = 3), Italy (n = 3), Mexico (n = 2), Poland (n = 2), United States (n = 1), England (n = 1), Spain (n = 1), Serbia (n = 1), and Germany (n = 1). The general features of the documents are found in Table 1. References of the publications included in this review are available in Supplementary File 3.
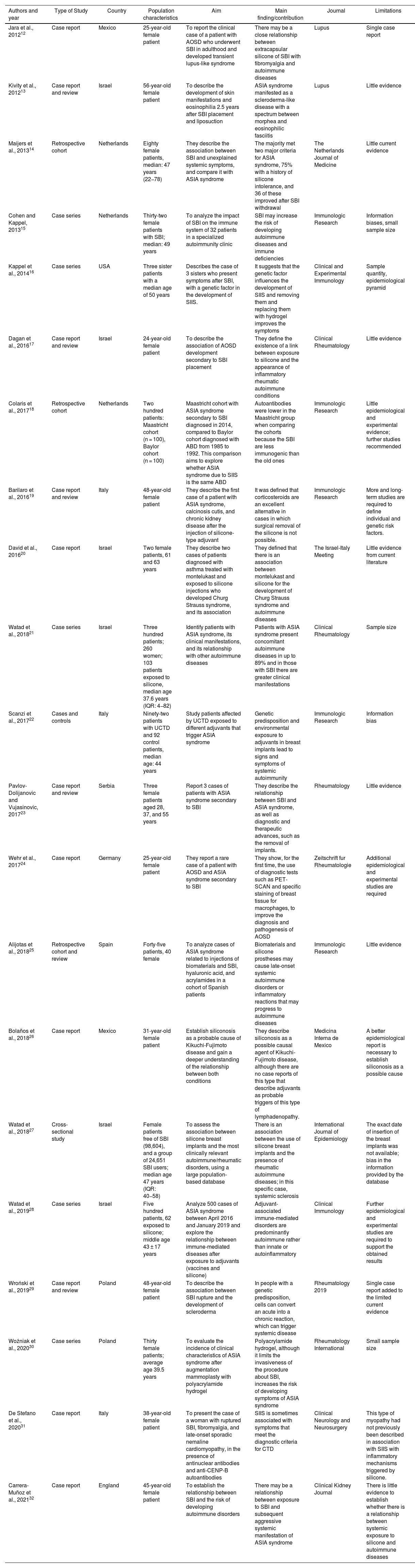
Characteristics of the 21 included publications.
| Authors and year | Type of Study | Country | Population characteristics | Aim | Main finding/contribution | Journal | Limitations |
|---|---|---|---|---|---|---|---|
| Jara et al., 201212 | Case report | Mexico | 25-year-old female patient | To report the clinical case of a patient with AOSD who underwent SBI in adulthood and developed transient lupus-like syndrome | There may be a close relationship between extracapsular silicone of SBI with fibromyalgia and autoimmune diseases | Lupus | Single case report |
| Kivity et al., 201213 | Case report and review | Israel | 56-year-old female patient | To describe the development of skin manifestations and eosinophilia 2.5 years after SBI placement and liposuction | ASIA syndrome manifested as a scleroderma-like disease with a spectrum between morphea and eosinophilic fasciitis | Lupus | Little evidence |
| Maijers et al., 201314 | Retrospective cohort | Netherlands | Eighty female patients, median: 47 years (22−78) | They describe the association between SBI and unexplained systemic symptoms, and compare it with ASIA syndrome | The majority met two major criteria for ASIA syndrome, 75% with a history of silicone intolerance, and 36 of these improved after SBI withdrawal | The Netherlands Journal of Medicine | Little current evidence |
| Cohen and Kappel, 201315 | Case series | Netherlands | Thirty-two female patients with SBI; median: 49 years | To analyze the impact of SBI on the immune system of 32 patients in a specialized autoimmunity clinic | SBI may increase the risk of developing autoimmune diseases and immune deficiencies | Immunologic Research | Information biases, small sample size |
| Kappel et al., 201416 | Case series | USA | Three sister patients with a median age of 50 years | Describes the case of 3 sisters who present symptoms after SBI, with a genetic factor in the development of SIIS. | It suggests that the genetic factor influences the development of SIIS and removing them and replacing them with hydrogel improves the symptoms | Clinical and Experimental Immunology | Sample quantity, epidemiological pyramid |
| Dagan et al., 201617 | Case report and review | Israel | 24-year-old female patient | To describe the association of AOSD development secondary to SBI placement | They define the existence of a link between exposure to silicone and the appearance of inflammatory rheumatic autoimmune conditions | Clinical Rheumatology | Little evidence |
| Colaris et al., 201718 | Retrospective cohort | Netherlands | Two hundred patients: Maastricht cohort (n = 100), Baylor cohort (n = 100) | Maastricht cohort with ASIA syndrome secondary to SBI diagnosed in 2014, compared to Baylor cohort diagnosed with ABD from 1985 to 1992. This comparison aims to explore whether ASIA syndrome due to SIIS is the same ABD | Autoantibodies were lower in the Maastricht group when comparing the cohorts because the SBI are less immunogenic than the old ones | Immunologic Research | Little epidemiological and experimental evidence; further studies recommended |
| Barilaro et al., 201619 | Case report and review | Italy | 48-year-old female patient | They describe the first case of a patient with ASIA syndrome, calcinosis cutis, and chronic kidney disease after the injection of silicone-type adjuvant | It was defined that corticosteroids are an excellent alternative in cases in which surgical removal of the silicone is not possible. | Immunologic Research | More and long-term studies are required to define individual and genetic risk factors. |
| David et al., 201620 | Case report | Israel | Two female patients, 61 and 63 years | They describe two cases of patients diagnosed with asthma treated with montelukast and exposed to silicone injections who developed Churg Strauss syndrome, and its association | They defined that there is an association between montelukast and silicone for the development of Churg Strauss syndrome and autoimmune diseases | The Israel-Italy Meeting | Little evidence from current literature |
| Watad et al., 201821 | Case series | Israel | Three hundred patients; 260 women; 103 patients exposed to silicone, median age 37.6 years (IQR: 4−82) | Identify patients with ASIA syndrome, its clinical manifestations, and its relationship with other autoimmune diseases | Patients with ASIA syndrome present concomitant autoimmune diseases in up to 89% and in those with SBI there are greater clinical manifestations | Clinical Rheumatology | Sample size |
| Scanzi et al., 201722 | Cases and controls | Italy | Ninety-two patients with UCTD and 92 control patients, median age: 44 years | Study patients affected by UCTD exposed to different adjuvants that trigger ASIA syndrome | Genetic predisposition and environmental exposure to adjuvants in breast implants lead to signs and symptoms of systemic autoimmunity | Immunologic Research | Information bias |
| Pavlov-Dolijanovic and Vujasinovic, 201723 | Case report and review | Serbia | Three female patients aged 28, 37, and 55 years | Report 3 cases of patients with ASIA syndrome secondary to SBI | They describe the relationship between SBI and ASIA syndrome, as well as diagnostic and therapeutic advances, such as the removal of implants. | Rheumatology | Little evidence |
| Wehr et al., 201724 | Case report | Germany | 25-year-old female patient | They report a rare case of a patient with AOSD and ASIA syndrome secondary to SBI | They show, for the first time, the use of diagnostic tests such as PET-SCAN and specific staining of breast tissue for macrophages, to improve the diagnosis and pathogenesis of AOSD | Zeitschrift fur Rheumatologie | Additional epidemiological and experimental studies are required |
| Alijotas et al., 201825 | Retrospective cohort and review | Spain | Forty-five patients, 40 female | To analyze cases of ASIA syndrome related to injections of biomaterials and SBI, hyaluronic acid, and acrylamides in a cohort of Spanish patients | Biomaterials and silicone prostheses may cause late-onset systemic autoimmune disorders or inflammatory reactions that may progress to autoimmune diseases | Immunologic Research | Little evidence |
| Bolaños et al., 201826 | Case report | Mexico | 31-year-old female patient | Establish siliconosis as a probable cause of Kikuchi-Fujimoto disease and gain a deeper understanding of the relationship between both conditions | They describe siliconosis as a possible causal agent of Kikuchi-Fujimoto disease, although there are no case reports of this type that describe adjuvants as probable triggers of this type of lymphadenopathy. | Medicina Interna de Mexico | A better epidemiological report is necessary to establish siliconosis as a possible cause |
| Watad et al., 201827 | Cross-sectional study | Israel | Female patients free of SBI (98,604), and a group of 24,651 SBI users; median age 47 years (IQR: 40−58) | To assess the association between silicone breast implants and the most clinically relevant autoimmune/rheumatic disorders, using a large population-based database | There is an association between the use of silicone breast implants and the presence of rheumatic autoimmune diseases; in this specific case, systemic sclerosis | International Journal of Epidemiology | The exact date of insertion of the breast implants was not available; bias in the information provided by the database |
| Watad et al., 201928 | Case series | Israel | Five hundred patients, 62 exposed to silicone; middle age 43 ± 17 years | Analyze 500 cases of ASIA syndrome between April 2016 and January 2019 and explore the relationship between immune-mediated diseases after exposure to adjuvants (vaccines and silicone) | Adjuvant-associated immune-mediated disorders are predominantly autoimmune rather than innate or autoinflammatory | Clinical Immunology | Further epidemiological and experimental studies are required to support the obtained results |
| Wroński et al., 201929 | Case report and review | Poland | 48-year-old female patient | To describe the association between SBI rupture and the development of scleroderma | In people with a genetic predisposition, cells can convert an acute into a chronic reaction, which can trigger systemic disease | Rheumatology 2019 | Single case report added to the limited current evidence |
| Woźniak et al., 202030 | Case series | Poland | Thirty female patients; average age 39.5 years | To evaluate the incidence of clinical characteristics of ASIA syndrome after augmentation mammoplasty with polyacrylamide hydrogel | Polyacrylamide hydrogel, although it limits the invasiveness of the procedure about SBI, increases the risk of developing symptoms of ASIA syndrome | Rheumatology International | Small sample size |
| De Stefano et al., 202031 | Case report | Italy | 38-year-old female patient | To present the case of a woman with ruptured SBI, fibromyalgia, and late-onset sporadic nemaline cardiomyopathy, in the presence of antinuclear antibodies and anti-CENP-B autoantibodies | SIIS is sometimes associated with symptoms that meet the diagnostic criteria for CTD | Clinical Neurology and Neurosurgery | This type of myopathy had not previously been described in association with SIIS with inflammatory mechanisms triggered by silicone. |
| Carrera-Muñoz et al., 202132 | Case report | England | 45-year-old female patient | To establish the relationship between SBI and the risk of developing autoimmune disorders | There may be a relationship between exposure to SBI and subsequent aggressive systemic manifestation of ASIA syndrome | Clinical Kidney Journal | There is little evidence to establish whether there is a relationship between systemic exposure to silicone and autoimmune diseases |
ASIA: Autoimmune/inflammatory Syndrome Induced by Adjuvants; ABD: Adjuvant-associated breast disease; UCTD: Undifferentiated Connective Tissue Diseases; AOSD: Adult-Onset Still disease; CTD: Connective Tissue Diseases; SBI: Silicone Breast Implants; SIIS: Silicone Implant Incompatibility Syndrome.
Colaris et al.18 compared a first Maastricht cohort of 99 women and a second Baylor cohort of 100 female patients, both with symptoms and criteria for ASIA syndrome. The Maastricht cohort had a median age of SBI placement of 33 years (IQR: 14–56), the onset of symptoms at 41 years on average (IQR: 20–68), and a latency period between aesthetic procedure and symptoms of for 4 years (IQR: 1–39). At the time of diagnosis, subjects were an average age of 49 years (IQR: 27–72), and 13 years (IQR: 2–43) elapsed from the aesthetic procedure to diagnosis. Among the local adverse reactions reported were capsular contractures and implant ruptures, while 70% of the population presented lymphadenopathy. The most frequent clinical manifestations were arthralgia and chronic fatigue. Eighteen individuals presented undifferentiated connective tissue disease (UCTD), 5 vasculitis, 4 RA, and 3 granulomatous disease. Fifty percent of the patients showed improvement in symptoms after SBI withdrawal.
In the Baylor cohort,18 97 SBI users were assessed, 3 of whom had received liquid silicone injections, 76 had capsular contracture associated with sensitivity, pain, and infections in the breasts, and 58 had lymphadenopathy. The most frequent clinical manifestations were chronic fatigue and myalgia. More than 95% of the cohort had their implants removed, but it was not reported whether there was an improvement in symptoms, while 33 individuals had positive antinuclear antibodies (ANA). In both cohorts, 80% were users of SBI as a purely aesthetic procedure and the symptoms were similar. The authors commented that the presence of lower antibody positivity in the Maastricht cohort is probably due to less immunogenicity to the current SBI.
In a retrospective study, Alijotas et al.25 reported 45 cases of patients who presented symptoms of systemic inflammatory response, of which 88.8% were female and 42% were SBI users. The main symptoms for which they consulted were arthralgia, muscle weakness, myalgia, palmar erythema, and arthritis. The autoimmune disorders that occurred were UCTD, Sjögren syndrome, SSc, seropositive polyarthritis, vasculitis, sarcoidosis, and Crohn’s disease. Regarding the laboratory results, positive ANA, hypergammaglobulinemia, and anti-Ro antibodies were obtained. Of the total population, 55.5% was HLA-B*8 positive. SBI and biomaterials were removed in 10 patients, of which 6 showed clinical improvement and immunomodulatory management was initiated mainly with prednisone and hydroxychloroquine. The researchers concluded that biomaterials used in aesthetic procedures, cosmetics, dentistry, and plastic surgery can trigger local symptoms and subsequently develop autoimmune disorders.
Cases and controls studiesScanzi et al.22 evaluated 92 subjects with UCTD and 92 healthy controls using a questionnaire, for which they inquired about exposure to adjuvants and their autoimmune profile. Patients with UCTD showed increased exposure to hepatitis B virus vaccines, tetanus toxoid, metal implants, smoking, and pollution, compared to controls. Individuals exposed to vaccines or SBI associated with UCTD presented with symptoms such as chronic fatigue and others suggestive of autoimmunity. Therefore, the authors considered that ASIA syndrome and UCTD may be related and that genetic factors and environmental conditions may induce signs and symptoms of systemic autoimmune diseases.
Cross-sectional studiesWatad et al.27 described the association between the use of SBI and autoimmune disorders in a group of 24,651 patients and a control group of 98,604 healthy subjects. The group of patients with SBI had a higher prevalence of smoking and breast cancer compared to the group without implants. A multivariate model showed an association between SBI users and autoimmune diseases (adjusted OR: 1.22; 95% CI: 1.18–1.26), the most frequent being sarcoidosis (adjusted OR: 1.98; 95% CI: 1.26–1.97). The authors reported an association between SBI and autoimmune diseases such as sarcoidosis, SSc, and Sjogren's syndrome.
Case seriesKappel et al.16 reported a series of 3 cases of sisters carrying a BRCA-1 gene mutation, for which they all underwent preventive mastectomy and reconstruction with textured SBI in 2004, 2005, and 2006, respectively. After the reconstruction, the 3 patients developed symptoms of fatigue, arthralgia, myalgia, and sleep disorders that were attributed to SBI. In 2009, their breast implants were replaced with silicone-free Monobloc Hydrogel ones. After this procedure, the symptoms improved significantly. Regarding laboratory test findings, the presence of ANA with a fine-speckled nuclear staining pattern was evident in all three patients. The authors postulated that silicone implant incompatibility syndrome may be part of ASIA syndrome and that when implant rupture occurs, the immune system is stimulated, which would promote the appearance of these syndromes.
Watad et al.21 reported the results of 300 cases of ASIA syndrome, of whom 86.7% were female and 13.3% male, with a mean age of onset of the disease of 37.6 years (IQR: 4−82). The database registry was analyzed by physicians and rheumatologists to validate the case reports and ensure that they met ASIA syndrome criteria. Symptoms such as arthralgia, myalgia, chronic fatigue, and sleep disturbances were the most frequent. Another autoimmune condition was diagnosed in 89%; of these, the most common was UCTD. ANA were positive in 51.7% of subjects. It was concluded that clinical manifestations vary depending on the type of adjuvant used, the gender of the individual, and the intrinsic conditions of the person.
Pavlov-Dolijanovic and Vujasinovic23 reported 3 cases of female patients aged 28 (patient A), 37 (patient B), and 55 years (patient C), who were users of SBI, who consulted for symptoms related to the procedure and who complied with ASIA syndrome criteria. Patient A had a history of 2 spontaneous abortions, was an active smoker, and presented with small joint arthralgias one year after SBI placement. In this case, management was done with non-steroidal anti-inflammatory drugs and immunosuppressive therapy. The patient underwent C-reactive protein (CRP) studies and cryoglobulin tests, which were positive; the rest of the autoimmune tests were negative. Patient B consulted 10 years after the aesthetic procedure due to fatigue, severe Raynaud's phenomenon, and arthralgias in the lower limbs. She had no family history of autoimmune disease; the antibody and capillaroscopy studies were normal, so she was treated for symptoms with angiotensin II receptor antagonist, aspirin, vitamin C, and vitamin E. Patient C was an active smoker and had an allergy to pipemidic acid. Ten years after the placement of SBI, he began to develop Raynaud's phenomenon, and after 5 years, arthralgia in small joints, dysphagia for solids, telangiectasias in the hands, positive anticentromere antibodies and capillaroscopy with a pattern suggestive of SSc, for which he was treated with methotrexate and cyclophosphamide. Additionally, 2 years later, she developed a left breast infection that was treated with successful intravenous antibiotic therapy. The researchers concluded that the symptoms associated with SBI begin years later, during which the rupture of these implants or migration of the silicone occurs, which triggers different rheumatological diseases.
Case reportsJara et al.12 reported the case of a 25-year-old woman with a history of juvenile idiopathic arthritis successfully treated with aspirin and prednisone since she was 11 years old. She presented with a clinical picture of 2 years of evolution of peripheral polyarthritis and predominantly evening fever. The patient reported that the onset of symptoms occurred after undergoing augmentation mammoplasty with SBI at the age of 22. In the laboratory tests requested during this hospitalization, ANA of 1:160 drew attention. The authors confirmed a diagnosis of SLE with joint involvement and began management with prednisone and chloroquine, with adequate evolution.
Dagan et al.17 reported the case of a 24-year-old patient who consulted with a 6-week history of fever, arthralgia, pleuritic pain, and odynophagia. The patient had a history of tonsillectomy at age 18 and breast augmentation with SBI at age 21. Physical examination revealed dyspnea and decreased breath sounds in the left base. Laboratory tests on admission reported leukocytosis with neutrophilia, CRP at 13,973 mg/dl, elevated liver enzymes, hyperferritinemia at 1178 ng/mL, and elevated lactate dehydrogenase. Given the persistence of symptoms, ANA, extractable nuclear antibodies, and rheumatoid factor were requested, which were negative. A positron emission tomography scan reported the presence of little periprosthetic fluid. The patient was diagnosed with AOSD, complying with Yamaguchi criteria, and immunomodulatory treatment was initiated with prednisone until the systemic inflammatory response was controlled. During the following year, she presented several episodes of fever, pleuritic pain and arthralgias managed with systemic corticosteroids, methotrexate, and hydroxychloroquine without symptom control, for which management with biological medications was initiated. The authors proposed a relationship between exposure to SBI and the appearance of rheumatic or autoimmune conditions.
Wehr et al.14 reported the case of a 25-year-old woman who presented with clinical symptoms lasting one week consisting of abdominal pain, fever of 40 °C, myalgias, and skin rash. Physical examination depicted axillary lymphadenopathy and associated moderate pain; laboratory tests revealed leukocytosis, elevated CRP, a 4-fold increase in transaminases, and hepatosplenomegaly detected in abdominal ultrasound. Infectious, neoplastic causes and polyarteritis nodosa were ruled out. The authors considered that these results could be associated with an autoimmune component. The patient had undergone SBI placement one year earlier, so a systemic response syndrome against them was suspected, and she additionally met the Yamaguchi criteria for AOSD. It was decided to remove the breast implants, after which there was improvement.
DiscussionIn the current study, the literature about autoimmune diseases in patients using SBI with ASIA syndrome was described. UCTD, Sjögren's syndrome, autoimmune thyroiditis, SSc, and AOSD were the most common autoimmune diseases in the SBI user population.12–32 Most subjects presented symptoms of chronic fatigue, arthralgia, fever, and myalgia, which began one to ten years after SBI placement.12,20,28,30 Active smoking and allergy/atopy were reported in the past medical history. Regarding the immunological profile, ANA were positive (n = 477 patients) in most of the included studies.12–17,28,31 Immunosuppressive management and surgical removal of the implants were related to adequate clinical evolution and even complete remission of symptoms in some cases.15,19,32
It has been reported that exposure to silicone, infectious agents, vaccine components, aluminum salts, and squalene triggers an uncontrolled immune response, which improves once these agents are removed from the human body.33,34 These adjuvants enhance the responses of CD4 T lymphocytes and macrophages, which causes the inflammatory reaction to be perpetuated by forming capsular tissue around the implant, thereby increasing the risk of capsular contracture leading to rheumatological diseases.34 Silicone, being highly immunogenic, can cause autoimmune diseases such as RA, SLE, polymyositis, and SSc. However, those individuals with a genetic predisposition and exposure to environmental factors such as tobacco smoke are more susceptible to suffering from these concomitant diseases with ASIA syndrome.7,23,33 Regarding the genes that have been studied in recent years in SBI users and ASIA syndrome, positive results have been found for the PTPN22 gene and HLA class II types DR4, DR53, DR51, DQ2, DQB1, DRB1, and DQA1 *0102.2,3,7,33 Risk factors, background, and genetic markers must be considered by health professionals and institutional protocols for adequate stratification of the risk of developing autoimmune diseases in the population undergoing augmentation mammoplasties with SBI.2,7
Since the creation of SBI, the performance of purely aesthetic surgical procedures such as augmentation mammoplasty has been in great demand; however, it is important to highlight that a significant number of individuals underwent the placement of these devices for breast reconstructions secondary to a history of breast cancer, due to the development of rheumatic disorders in cancer patients.16,35,36 Although adjuvants are considered foreign bodies in the body, capable of triggering uncontrolled immune responses, it cannot be excluded that activation of the immune system occurs in breast cancer patients using SBI, favored by the interaction between carcinogenic and immunogenic processes, or by chemotherapy or radiotherapy therapies.31 To date, the available evidence that establishes the association between the development of autoimmune diseases and populations undergoing breast reconstruction for neoplastic processes is limited, which is why a greater number of studies are necessary that describe the pathophysiological and clinical findings.
De Boer et al.37 conducted a systematic review in PubMed, Medline, Embase, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews and assessed the effectiveness of withdrawal of SBI as a treatment for patients with associated symptoms. The authors reported that the removal of these implants can reduce symptoms and improve the quality of life in up to 75% of the population, data like those reported in the current review. Among the main pathophysiological processes responsible for the decrease in symptoms at the time of removal of foreign bodies is the diminution in the secretion of proinflammatory cytokines, the proliferation of fibroblasts, and lymphocytic infiltration to the breast tissue, effects enhanced by immunosuppressive medical treatment.38,39
UCTD are a heterogeneous group of autoimmune diseases that share clinical manifestations and paraclinical tests of SLE, SSc, Sjögren syndrome, dermatomyositis, polymyositis, and RA but do not meet the diagnostic criteria.40 There is a significant risk of developing autoimmune disorders in subjects with ASIA syndrome who use SBI,27 although the non-specificity in the characterization of the manifestations of this disease may be a limitation when making a simultaneous diagnosis with ASIA syndrome. A greater number of studies are needed that evaluate clinical characteristics and laboratory tests for a timely diagnostic and therapeutic approach in breast implant users.41
LimitationsMost of the studies found were descriptive, case-report type, so the sample studied is small. Additionally, in the present exploratory systematic review, an assessment of the quality of the included studies was not carried out, because it is not part of the objectives described in the PRISMA-ScR guideline.11
ConclusionThe existing scientific literature reports an association between the use of silicone breast implants and the development of ASIA syndrome and rheumatic diseases such as undifferentiated connective tissue disease, Sjögren syndrome, systemic sclerosis, and adult-onset Still disease. The main clinical features reported are chronic fatigue, arthralgia, fever, and myalgia. Management with immunomodulators and removal of the implants usually allow adequate control of the disease. A greater number of studies are required to evaluate clinical characteristics and risk factors in patients who use silicone breast implants that may predispose the risk of developing autoimmune diseases.
FinancingThe authors declare that they have not received funding for this research work.
Conflict of interestsNone.