Corticosteroids are hormones released by the adrenal gland that act on sodium and glucose metabolism and have anti-inflammatory properties. The first synthetic corticosteroids were prednisolone and prednisone. Deflazacort is the most recent of the synthetic corticosteroids, with molecular differences that bestow benefits such as, for example, in relation to sodium loss, anti-inflammatory potency and immunosuppressive activity, and lower interference on glucose and phosphocalcium metabolism.
ObjectiveWe decided to conduct this study to describe the effect of deflazacort compared to other corticosteroids in rheumatoid arthritis.
Materials and methodsWe conducted a scoping review. A literature search was conducted in Pubmed, Cochrane and BVS databases, and in grey literature, for controlled clinical trials or equivalence studies conducted in adult patients with rheumatoid arthritis and the use of deflazacort versus other corticosteroids. We excluded articles that did not clearly mention the dose of deflazacort, or the comparator used.
ResultsThe search of the 3 databases yielded 166 studies, of which 5 met the eligibility criteria and were included. Four studies evaluated deflazacort versus prednisolone, and one versus methylprednisolone. The results were similar for all 5: less decline in bone mineral density and glucose metabolism.
ConclusionsDeflazacort and prednisolone have pharmacological differences that influence adverse effects at the level of bone and glucose metabolism. However, further studies are required for deflazacort to be used routinely in our practice, especially in diseases such as rheumatoid arthritis.
Los corticoides son hormonas liberadas por la glándula suprarrenal, con actividad sobre el sodio y el metabolismo de la glucosa, así como propiedades antiinflamatorias. Los primeros corticoides sintéticos fueron la prednisolona y la prednisona. El deflazacort es el más reciente de los corticoides sintéticos, con diferencias moleculares que le confieren beneficios como, por ejemplo, en relación con la pérdida de sodio, una potente actividad antiinflamatoria e inmunosupresora y poca interferencia en el metabolismo de la glucosa y el fosfocálcico.
ObjectivoDecidimos llevar a cabo este estudio con el fin de describir el efecto del deflazacort, comparado con otros corticoides en artritis reumatoide.
Materiales y métodosLlevamos a cabo una revisión panorámica de la literatura (del inglés scoping review). Se realizó una búsqueda de la literatura en las bases de datos Pubmed, Cochrane y BVS, como también en la literatura gris, de ensayos clínicos controlados o estudios de equivalencia desarrollados en pacientes adultos con artritis reumatoide y uso de deflazacort, en comparación con otros corticoides. Se excluyeron artículos que no mencionaran claramente la dosis de deflazacort y del comparador utilizadas.
ResultadosLa búsqueda en las 3 bases de datos arrojo 166 estudios, de los cuales se incluyeron 5 que cumplían con los criterios de elegibilidad. Cuatro estudios evaluaron deflazacort comparado con prednisolona, en tanto que uno lo comparó con metilprednisolona. Los resultados fueron similares para los 5: menor caída en la densidad mineral ósea y en el metabolismo de la glucosa.
ConclusionesEl deflazacort y la prednisolona tienen diferencias farmacológicas que influyen en la generación de efectos adversos a nivel del metabolismo óseo y de la glucosa; sin embargo, se requieren más estudios para considerar el uso estándar del deflazacort en nuestra práctica, especialmente en enfermedades como la artritis reumatoide.
Corticosteroids are hormones released by the adrenal gland, which are divided into 2 groups according to their biological activity: glucocorticoids (regulation of the metabolism of carbohydrates) and mineralocorticoids (balance of fluids and electrolytes, mainly sodium). The potency of these hormones is given by their activity on sodium, glucose metabolism and their anti-inflammatory properties; the latter 2 are closely related since they share the same receptor. Although these concepts are important when talking about synthetic corticosteroids, it should be taken into account that they share characteristics of one group or another. For example, although prednisone is considered a glucocorticoid, it also has mineralocorticoid activity. This property of acting on different pathways depends on the structure of the drug and the metabolism carried out by the enzyme 11-beta-hydroxydehydrogenase.1
The first synthetic corticosteroids developed were prednisolone and prednisone, which, as mentioned above, have a predominantly glucocorticoid effect. Subsequently, other drugs were developed such as dexamethasone and more recently deflazacort, a derivative of prednisolone with a methyl-oxazoline ring that differentiates it from the original compound and gives it its attractive pharmacological properties: lack of activity on loss of sodium, potent anti-inflammatory and immunosuppressant activity and little interference with glucose and phosphocalcic metabolism.2,3 This explains why, when compared with corticosteroids such as prednisolone or methylprednisolone, it exhibits fewer adverse effects: less decrease in bone mineral density (BMD), no alteration of the lipid profile and prevention of fat accumulation.4 Ganapati et al.5 conducted a study in which they compared the adverse effects of deflazacort and prednisolone in patients with systemic lupus erythematosus, and confirmed these benefits: lower weight gain, less hirsutism and low cushingoid severity index, as well as less impact on blood glucose.
It has been demonstrated that deflazacort inhibits the inflammatory exudative phase, as well as the development of chronic granulomatous inflammation. It has been seen in experimental models that it also exerts its effect on the joints and, although the majority of patients treated with deflazacort are those with muscular dystrophies,3,6,7 there are several disorders in which its use has been tested with satisfactory results, such as sarcoidosis, juvenile idiopathic arthritis, polymyalgia rheumatica, systemic lupus erythematosus, and rheumatoid arthritis (RA).3,8,9 However, its use is infrequent in rheumatological practice and there are some gaps such as the dose ranges and de-escalation.
After considering the previous points and with the purpose of examining the extent and nature of the existing literature in this area, summarizing and disseminating its results and identifying gaps in the current literature around the use of deflazacort in RA, we have decided to carry out this panoramic literature review (scoping review), which aims to describe the effect of deflazacort compared with other corticosteroids in RA.
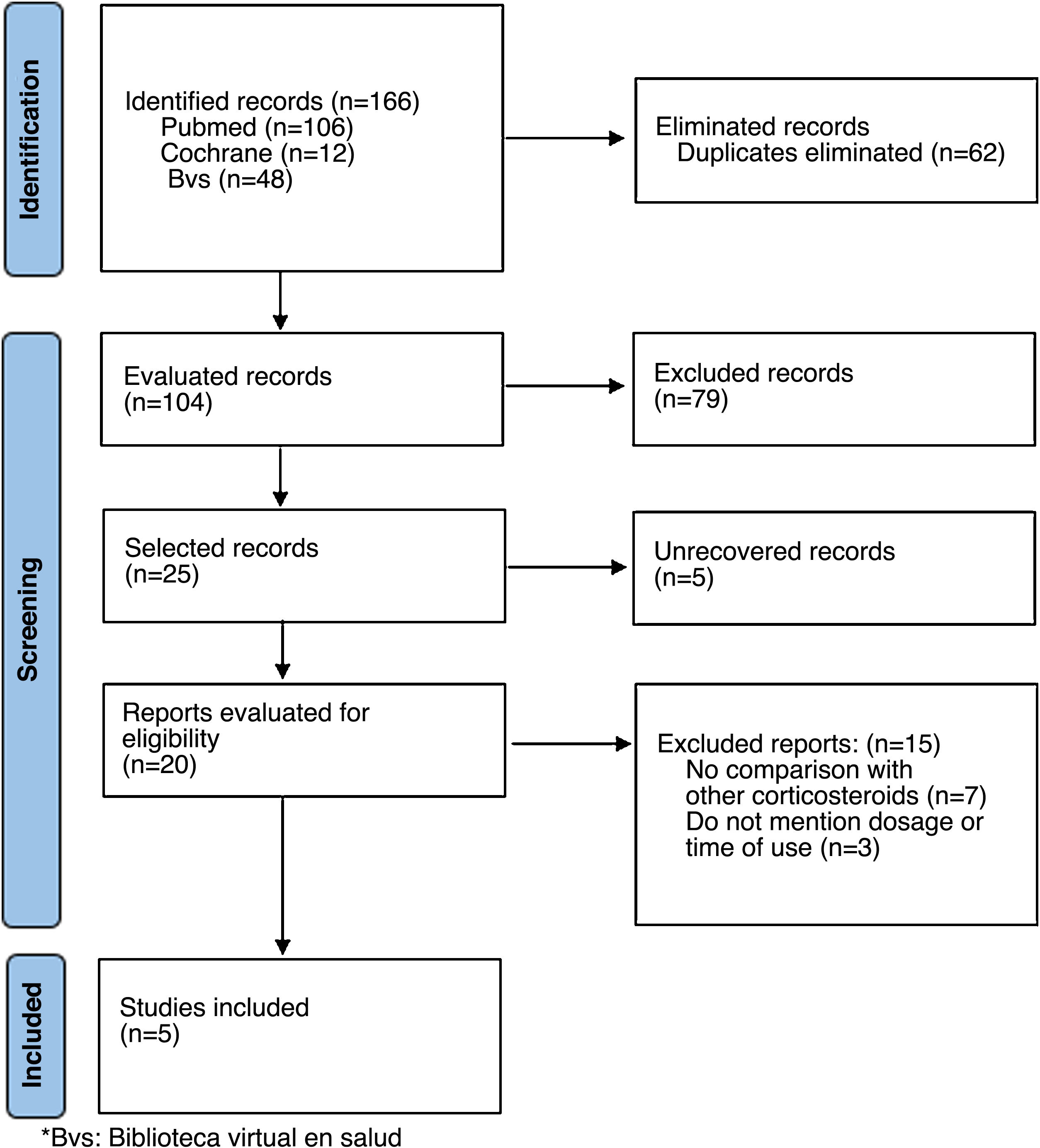
Materials and methodsFor the scoping review, a literature search was conducted in the different databases (PubMed, Cochrane, BVS) and in the grey literature as sources of information, including articles published until June 2021 and limited to English and Spanish languages, with no limits on the type of publication. The PRISMA extension for scoping reviews [PRISMA-ScR] guideline was followed (Fig. 1)10,11 (Appendix A Supplementary material 1 Appendix annex 1).
The related search terms «deflazacort», «rheumatoid arthritis» were used. In the construction of the search equations, each of the MeSH terms was crossed with the respective keywords using different Boolean operators (OR, AND). The full search strategy is presented in the Appendix B Supplementary material 2 (Appendix B annex 2). Two reviewers conducted the search independently and clarified disagreements by consensus. The following eligibility criteria were considered: controlled clinical trials or equivalence studies with a comparator, developed in adult patients with RA (confirmed according to validated criteria) and use of deflazacort compared with other corticosteroids for control of the disease. The articles that did not clearly mention the dose of deflazacort and of the comparator used were excluded. The articles related to the topic were selected and the data were subsequently extracted from a database created in the Excel® software, evaluating as main variables: type of study, sample size, age of the participants in both arms (years), disease (in the case of others evaluated in addition to RA) dose of deflazacort and comparator, and follow-up time.
In accordance with Colombian regulations (Resolution 008430 of 1993 of the Ministry of Health) this systematic review is considered a risk-free investigation, because no intervention or intentioned modification of the biological, physiological, psychological or social variables of the individuals was made.
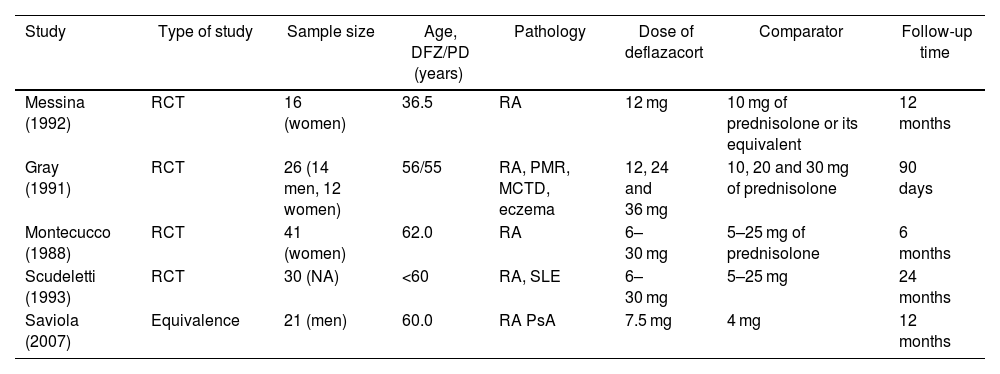
ResultsThe initial search yielded a total of 166 studies coming from the 3 databases. After excluding duplicates in the identification, screening and assessing eligibility through the review of the abstract and the full text, 5 studies (Fig. 1) that met the eligibility criteria were included, which are summarized in Table 1. The results are exposed in detail below.
Summary of articles included.
| Study | Type of study | Sample size | Age, DFZ/PD (years) | Pathology | Dose of deflazacort | Comparator | Follow-up time |
|---|---|---|---|---|---|---|---|
| Messina (1992) | RCT | 16 (women) | 36.5 | RA | 12 mg | 10 mg of prednisolone or its equivalent | 12 months |
| Gray (1991) | RCT | 26 (14 men, 12 women) | 56/55 | RA, PMR, MCTD, eczema | 12, 24 and 36 mg | 10, 20 and 30 mg of prednisolone | 90 days |
| Montecucco (1988) | RCT | 41 (women) | 62.0 | RA | 6–30 mg | 5–25 mg of prednisolone | 6 months |
| Scudeletti (1993) | RCT | 30 (NA) | <60 | RA, SLE | 6–30 mg | 5–25 mg | 24 months |
| Saviola (2007) | Equivalence | 21 (men) | 60.0 | RA PsA | 7.5 mg | 4 mg | 12 months |
RA: rheumatoid arthritis; DFZ: deflazacort; RCT: randomized clinical trial; MCTD: mixed connective tissue disease; SLE: systemic lupus erythematosus; mg: milligrams; NA: not available; PD: prednisone; PMR: polymyalgia rheumatica; PsA: psoriatic arthritis.
Source: own elaboration.
The effects of deflazacort and prednisone are usually diverse, considering the dose, the underlying disease, and concomitant medications. There are particular modifications that may influence their choice based on the clinical benefit to the patients. These differences are related to the chemical transformation that prednisone undergoes, which gives way to the constitution of the C-17 nanoxazoline group, which reduces its solubility in lipids and exerts an effect on blood components that limits or excludes the tissues, particularly the osteoblasts, without interfering in the clinical response.12
Regarding the use of prednisolone in patients with RA, the harmful effects on bone metabolism, with induction of osteoporosis, have been widely described, and the use of deflazacort has been proposed as a therapy that could reduce this effect. In a study conducted by Messina et al.13 both drugs were compared for 12 months — deflazacort 12 mg/day vs. prednisolone 10 mg/day — finding equal rates of synovitis and body bone mineral count. However, at the level of Ward’s triangle, the drop in BMD was lower with deflazacort, which suggests a possible lower impact on the bone with the use of this drug in patients with RA. In the study conducted by Gray et al.14 in patients with RA, participants received titratable doses of prednisolone and deflazacort for 2 months. At 90 days, the reduction in the erythrocyte sedimentation rate and activity indices were similar, with an increase in urinary calcium excretion and greater inhibition of endogenous secretion of cortisol in the deflazacort group, and it was demonstrated a therapeutic potency of 83% with respect to prednisolone, so that it may be better tolerated, in addition to showing fewer adverse effects in the long term. Montecucco et al.15 supported this statement in their study, in which they compared the serum levels of osteocalcin (a marker of osteoblastic activity) after management with prednisone or deflazacort. At 6 months, the serum concentration of osteocalcin was reduced in the 2 groups, but the decrease was more significant with prednisolone, even more so when the doses were higher than 10 mg/day.
A double-blind study compared the efficacy of deflazacort and prednisolone in patients with systemic lupus erythematosus and RA. The 2 drugs induced clinical remission within one month, which was maintained until the sixth month. Likewise, immunological modifications persisted with deflazacort for 1–1.5 months, including the significant reduction in T lymphocytes and CD4/CD8 ratio, with an apparent lower effect on calcium and glucose metabolism compared with prednisone.8
In addition, del Rosso et al.16 evaluated the effects of the administration of deflazacort on the synoviocytes of healthy and RA patients, and documented in vitro that the expression and activity of the fibrinolytic system are reduced. This system plays a leading role in the invasion and proliferation of synoviocytes in RA.
Deflazacort vs. methylprednisoloneMethyl prednisolone, administered orally or intravenously, is a member of the group of glucocorticoids that has a great anti-inflammatory activity, which is why it has been used in several inflammatory and autoimmune disorders, such as inflammatory bowel disease, vasculitis, systemic lupus erythematosus and RA, among others. Likewise, it shares the undesirable effects generated by the use of prednisolone.17
Regarding the use of methylprednisolone in patients with RA, Grassi et al.12 evaluated its frequency of use and found that it was the main corticosteroid prescribed in patients with RA (63.2%), with an average dose of 5.7±3.6 mg.
Saviola et al.18 evaluated the equivalence of correct clinical efficacy in low doses of deflazacort and methylprednisolone in a 12-month clinical trial, and as a secondary objective they considered the bone metabolic effects of such doses in the treatment of RA and psoriatic arthropathy. There were no significant differences in achieving ACR20 response at 6 and 12 months. Regarding bone metabolism, measurements of bone-specific alkaline phosphatase, osteocalcin and osteoprotegerin (OPG) were made, without finding statistically significant differences, even though there was a trend towards a more marked decrease in OPG levels in the methylprednisolone group at 3 months (6% with deflazacort vs. 24% with methylprednisolone). Although it was not the main objective of the research and the conclusions in favor of the use of deflazacort are not strong, it does open a path to conduct studies in which the formulated hypothesis is evaluated.
DiscussionIn the rheumatology setting, glucocorticoids are frequently used, however, unfortunately, they are not innocuous drugs and can trigger side effects, such as loss of bone mineral density, osteoporosis, weight gain, hypertension, diabetes mellitus, or neuropsychiatric alterations, among others.19 Deflazacort and prednisolone are corticosteroids with the same therapeutic effects, used in different clinical scenarios, mainly in muscular dystrophies in the case of deflazacort,20 with limited use in systemic autoimmune diseases, perhaps due to lack of evidence.
The drugs studied show pharmacological differences, such as the methyl-oxazoline ring in the chemical structure of deflazacort, which influence the generation of adverse effects, as we have observed in our narrative review.3 The majority of the studies had prednisolone as comparator, in relation to which there appear to be no difference in terms of therapeutic efficacy, as documented by Montecucco et al.,15 Gray et al.14 and other authors. However, the negative impact of deflazacort on bone and glucose metabolism is lower than that of its comparator.
Studies comparing deflazacort and methylprednisolone were also found, with results similar to those that compared it with prednisolone.
The present study has some limitations, since as it is a panoramic literature review (scoping review) it has been focused on 3 main search databases, without doing a quantitative analysis of the data of meta-analysis type. In addition, due to the type of studies obtained, it was not possible to perform a quantitative analysis thereof. However, in order to avoid biases, the extension of the PRISMA guide for panoramic reviews has been followed, thanks to which more objective results have been obtained. Like all panoramic reviews, the results of the present review could serve as input for decision-making and to determine the need and feasibility of conducting a systematic review subsequent to the present panoramic review.
Deflazacort is a promising drug in rheumatology clinical practice, due to its apparent safety for long-term use and comparable efficacy to that of prednisolone; However, questions remain to be answered, such as the scheme of use and, specifically, de-escalation. Studies with a broader statistical power and that include a larger sample of patients with rheumatological diseases are required to consider the standard use of deflazacort in our practice.
FundingThe study did not receive any funding from agencies in the public, commercial or nonprofit sectors.
Conflict of interestThe authors declare that they have no conflict of interest.