Psoriatic arthritis (PsA) is an immune-mediated chronic inflammatory disease with a heterogeneous clinical presentation involving joints, entheses, nails, and skin. In addition, it is associated with psychosocial morbidity and decreased quality of life. Up to half of the patients with PsA have associated comorbidities, including mood disorders, which are of increasing interest given their possible association with the inflammatory component of the disease, and impact on outcomes related to treatment goals. In this review we will mention the main aspects (epidemiology, pathophysiology, diagnosis, and treatment) of mood disorders in patients with PsA.
La artritis psoriásica (PsA) es una enfermedad inflamatoria crónica, inmunomediada, con una presentación clínica heterogénea que involucra articulaciones, entesis, uñas y piel. Adicionalmente, se asocia con morbilidad psicosocial y disminución de la calidad de vida. Hasta la mitad de los pacientes con PsA presentan comorbilidades asociadas, entre estas los trastornos del ánimo, que cada día toman más interés dada su posible asociación con el componente inflamatorio de la enfermedad y su impacto en los desenlaces relacionados con las metas de tratamiento. En esta revisión se mencionan los principales aspectos (epidemiología, fisiopatología, diagnóstico y tratamiento) de los trastornos del ánimo en los pacientes con PsA.
The immune system not only produces inflammation in the affected organs, but also intervenes in mood disorders, such as depression and anxiety, among others.1 Psoriatic arthritis (PsA) is a systemic autoimmune disease; its prevalence ranges between 0.3% and 1% of the population,2 and it occurs in 24% of the patients with psoriasis; it causes joint destruction and disability in a large number of patients and is associated with extra-articular manifestations such as uveitis and inflammatory bowel disease, as well as with various comorbidities, including mood disorders.3 In PsA, mood disorders impact disease outcomes and response to treatment, there is little information in the literature about this topic, for this reason that we conducted a narrative review focuses on the main aspects (epidemiology, pathophysiology, diagnosis, and treatment) of mood disorders in patients with PsA.
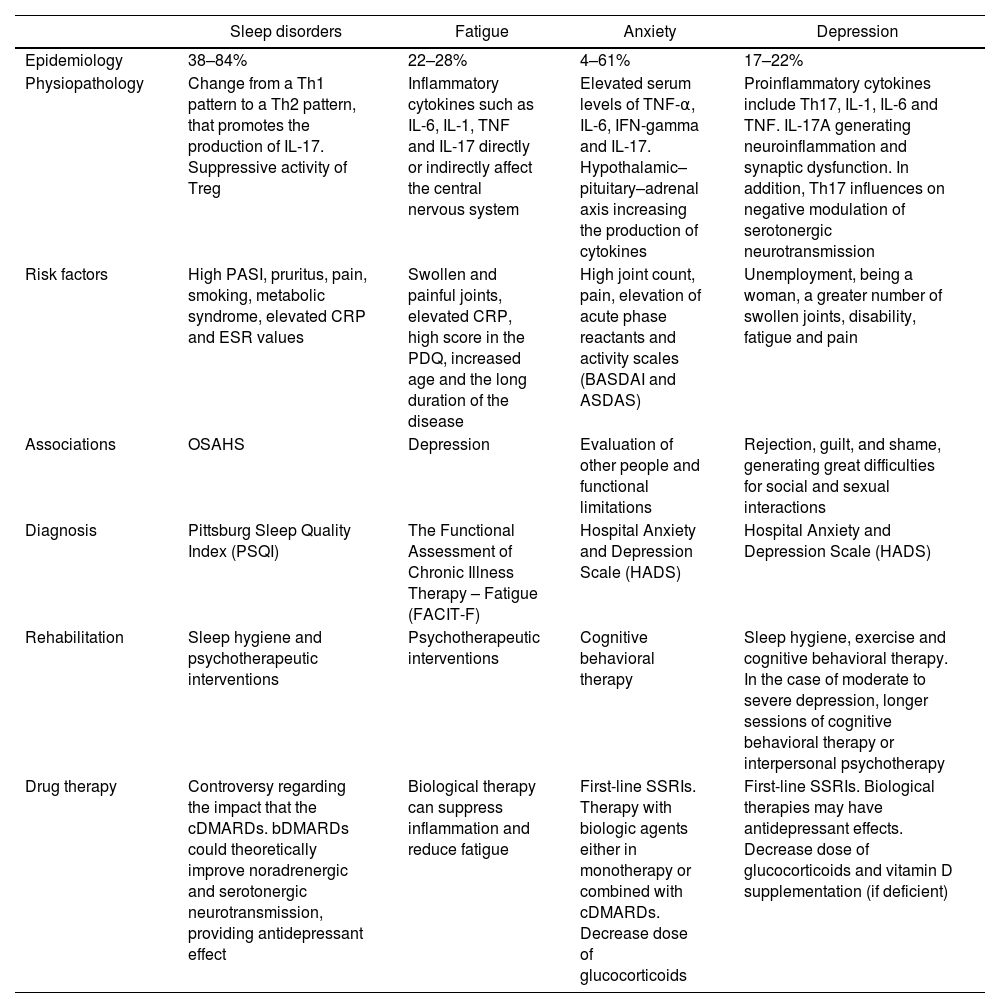
Table 1 summarizes all mood disorders.
Mood disorders in psoriatic arthritis.
| Sleep disorders | Fatigue | Anxiety | Depression | |
|---|---|---|---|---|
| Epidemiology | 38–84% | 22–28% | 4–61% | 17–22% |
| Physiopathology | Change from a Th1 pattern to a Th2 pattern, that promotes the production of IL-17. Suppressive activity of Treg | Inflammatory cytokines such as IL-6, IL-1, TNF and IL-17 directly or indirectly affect the central nervous system | Elevated serum levels of TNF-α, IL-6, IFN-gamma and IL-17. Hypothalamic–pituitary–adrenal axis increasing the production of cytokines | Proinflammatory cytokines include Th17, IL-1, IL-6 and TNF. IL-17A generating neuroinflammation and synaptic dysfunction. In addition, Th17 influences on negative modulation of serotonergic neurotransmission |
| Risk factors | High PASI, pruritus, pain, smoking, metabolic syndrome, elevated CRP and ESR values | Swollen and painful joints, elevated CRP, high score in the PDQ, increased age and the long duration of the disease | High joint count, pain, elevation of acute phase reactants and activity scales (BASDAI and ASDAS) | Unemployment, being a woman, a greater number of swollen joints, disability, fatigue and pain |
| Associations | OSAHS | Depression | Evaluation of other people and functional limitations | Rejection, guilt, and shame, generating great difficulties for social and sexual interactions |
| Diagnosis | Pittsburg Sleep Quality Index (PSQI) | The Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) | Hospital Anxiety and Depression Scale (HADS) | Hospital Anxiety and Depression Scale (HADS) |
| Rehabilitation | Sleep hygiene and psychotherapeutic interventions | Psychotherapeutic interventions | Cognitive behavioral therapy | Sleep hygiene, exercise and cognitive behavioral therapy. In the case of moderate to severe depression, longer sessions of cognitive behavioral therapy or interpersonal psychotherapy |
| Drug therapy | Controversy regarding the impact that the cDMARDs. bDMARDs could theoretically improve noradrenergic and serotonergic neurotransmission, providing antidepressant effect | Biological therapy can suppress inflammation and reduce fatigue | First-line SSRIs. Therapy with biologic agents either in monotherapy or combined with cDMARDs. Decrease dose of glucocorticoids | First-line SSRIs. Biological therapies may have antidepressant effects. Decrease dose of glucocorticoids and vitamin D supplementation (if deficient) |
ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; c/b DMARDs: conventional/biological disease modifying anti-rheumatic drugs; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IL: interleukin; INF: interferon gamma; OSAHS: obstructive sleep apnea–hypopnea syndrome; PASI: Psoriasis Area Severity Index; PDQ: Pain Detect Questionnaire; PsA: psoriatic arthritis; SSRIs: selective serotonin reuptake inhibitors; TNF: tumor necrosis factor; Treg: regulatory T cells.
For the narrative review, an expanded search of the literature was carried out in the different databases (PUBMED, Cochrane, BVS) and in gray literature, including articles published until April 2022 and limited to English and Spanish, with no limits to the type of publication.
Related search terms “psoriatic arthritis”, “mood disorders”, “fatigue”, “sleep disturbances”, “anxiety”, “depression” were used. Two reviewers conducted the search independently, reaching to clarify the disagreements by consensus and selecting the articles related to the subject and with subsequent extraction of the data in a database created in the Excel®, mainly evaluating the epidemiology, pathophysiology, diagnosis and treatment of each mood disorder.
Sleep disordersAnother domain compromised in the population with PsA is that of sleep disorders, which negatively affects health-related quality of life (HRQoL), as well as fatigue, anxiety and depression. The prevalence of sleep disorders is very variable, between 38 and 84% in patients with PsA, evaluating the poor sleep quality with a score >5, which has been assessed by the Pittsburgh Sleep Quality Index (PSQI) compared with the population with psoriasis and the control group (PSQI score: PsA 9.24 vs. psoriasis 7.17 vs. control group 5.67; p<0.0001); it affects mostly men (55%), with greater involvement in the 5th and 6th decades of life.4–6
Sleep disorders can be classified into acute or chronic, according to the time of evolution of the sleep deprivation. Acute sleep deprivation makes reference to a lack of sleep or a decrease in the usual total sleep time, which usually lasts 1 or 2 days, and a wakeful time extended beyond the typical 16–18h. Chronic sleep deprivation is defined by excessive daytime sleepiness caused by the routine of sleeping less than the amount required for the optimal functioning and health maintenance, almost every day for at least 3 months; according to what is established by the Third Edition of the International Classification of Sleep Disorders.7
On the other hand, we can classify those disorders using scales in which alteration, duration, latency, and efficiency of sleep are assessed; moreover, that evaluate the need for sleep medications and the quality of sleep perceived by the patient. According to the alteration, we can classify them into insomnia and daytime sleepiness; the latter is defined as an undesired need to sleep during the day, it can be secondary to sleep deprivation, fragmented sleep, which can be nonrestorative, and we can classify it into obstructive sleep apnea–hypopnea syndrome (OSAHS), periodic limb movement disorder, upper airway resistance syndrome, psychogenic hypersomnia, narcolepsy, medically and drug-induced drowsiness, head trauma, recurrent hypersomnia (Kleine–Levin syndrome) and idiopathic hypersomnia. Another alteration, insomnia, can be classified into restless legs syndrome and chronic insomnia; insomnia is considered a symptom that can be identified by: not being able to fall asleep, having too short or unrefreshing sleep, waking up too often, and, during the day: decreased energy, concentration and attention, poor social and professional functioning, physical symptoms such as fatigue and being worried about sleep.8
The risk factors for the sleep disorder related to PsA are high scores in the Psoriasis Area Severity Index (PASI), pruritus and pain; on the other hand, the factors not related to PsA include smoking, consumption of alcohol and anxiety; with an increased risk of developing depression and daytime fatigue.9 When evaluating the long-term outcomes in patients with PsA versus controls, Gezer et al. observed that patients with sleep disorders developed more comorbidities such as metabolic syndrome, cardiovascular disease, diabetes and hypertension.10 In addition, they found negative outcomes in quality of life, with chronic pain, development of anxiety; persistence of positivity for inflammatory markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and enthesitis.11
Insomnia can alter the inflammatory immunological processes through multiple pathways, it can be associated with an alteration in the circulating number and/or activity of leukocytes, mainly neutrophils, monocytes, B lymphocytes, and CD4 T lymphocytes, with a decrease in the phagocytic activity of neutrophils, as well as in the circulating number and cytotoxic activity of natural killer (NK) cells and CD8 T lymphocytes; since there is an increase in systemic and tissue processes with elevation of circulating proinflammatory markers (IL-1, IL-1β, IL-6, CRP, TNF-α), altered presentation of antigens, due to a decrease in dendritic cells. In addition, it promotes the breakdown of immunological self-tolerance with the synergistic participation of cytokines such as IL-6, whose abnormal production results in the activation of polyclonal B cells.12,13
Undisturbed sleep is characterized by a predominance of a Th1 response of CD4 T cells (which express IFN-γ, IL-2 and TNF-α); sleep deprivation leads to a change from a Th1 pattern to a Th2 pattern (IL-4, IL-5, IL-10 and IL-13) that promotes the production of IL-17 and the response of related cells that require IL-6 for activation, which can cause increased production of autoantibodies and formation of immune complexes, or may intensify chronic inflammation by promoting angiogenesis and recruitment of inflammatory cells in the sites of inflammation, as well as cartilage and bone erosion.12
On the other hand, it has been observed in experimental studies an alteration in the suppressive activity of CD4 regulatory T cells (Treg), which have greater activity at night and less activity in the day. The suppressive function of Treg toward an excessive immune response is an important homeostatic mechanism, since its deterioration is implicated in the pathogenesis of autoimmune diseases.12
It is important to emphasize that this is a condition that is not properly diagnosed in clinical practice, and as already noted, it is generally underdiagnosed and not adequately addressed or recognized in the consultation, for which these symptoms do not receive appropriate attention or management in the vast majority of cases.5 The medical staff who cares for patients with a diagnosis of psoriasis and psoriatic arthropathy should be sensitized to routinely investigate and address these symptoms in the consultation, since they affect the quality of life and are related to poor control of the inflammatory activity. Questions should be asked about sleep quality, duration and latency, using scales such as the Pittsburg Sleep Quality Index (PSQI), widely used in different clinical trials, which subjectively assesses sleep quality in the last month taking into account 7 domains; a value higher than or equal to 6 is indicative of poor sleep quality, with high specificity.11
There is controversy regarding the impact that the use of conventional disease-modifying drugs and biological therapies can have on the control of these symptoms. In some series, no favorable relationship has been found, but in others it did; the explanation for this discrepancy lies in the quality of the studies and in the heterogeneity of the scales used to assess sleep quality.11,14 As previously noted, it is defined that inflammatory joint activity and elevated CRP and ESR values have a direct relationship with sleep disorders in these patients, and an adequate control of the disease has been related to benefits in the control of these symptoms. Some cohorts have documented better quality of sleep and improvement in insomnia of up to 47% with the use of continuous biological therapies at 2-year follow-up, both with tumor necrosis factor (TNF) inhibitors and with ustekinumab, changes that have not been found with the use of conventional disease-modifying drugs (cDMARDs),13,15 establishing that the use of this type of drugs, the adequate control of the disease and the maintenance in continuous inflammatory remission are priority aspects in the control of sleep disorders. The explanation for this fact lies in the control of anxiety and the tranquility that guaranteeing the maintained control of the symptoms generates in the patient; furthermore, the use of biological therapies could theoretically improve noradrenergic and serotonergic neurotransmission, providing antidepressant effects.13,14
Although with the current information it is not possible to define a causal relationship between the different modifying drugs used for the control of the disease and the risk of developing sleep disorders, the use of methotrexate has been associated with the development of anxiety, depression and sleep disorders; while the use of cyclosporine has been associated with control of these symptoms; this finding, despite the poor evidence, is an aspect to be taken into account in the treatment of patients who develop insomnia or who use biological therapy combined with methotrexate.14
In addition to the adequate control of the inflammatory activity and to the probable benefit of using biological therapies for its treatment, multidisciplinary management with the support of specialties related to these conditions is important, since there are multiple factors related to the presence of insomnia that must also be controlled, without leaving aside the importance of always offer the patients non-pharmacological options such as sleep hygiene and psychotherapeutic interventions.16,17
FatigueThe incidence and prevalence of fatigue in patients with PsA has not been fully characterized in large cohorts. The study conducted by Lai et al. (2020), assessed fatigue using a self-administered FACIT-F (The Functional Assessment of Chronic Illness Therapy – Fatigue), questionnaire finding a prevalence of severe fatigue (defined as a FACIT-F score <30) of 22.1% of the patients.18 Another study determined the presence of fatigue in 28% and 16% of the patients with PsA and psoriasis, respectively. The severity of the fatigue was significantly greater in the patients with PsA compared with the patients with psoriasis (p<0.0001).19
The DANBIO registry determined the components associated with fatigue.19 The first component, which contributed to 31% of the fatigue, was made up of inflammatory factors that included swollen and painful joints, physician global assessment, elevated CRP and high score in the Pain Detect Questionnaire (PDQ); the second component, which contributed to 17%, consisted of the increased age and the long duration of the disease. The third component, which contributed to 15%, consisted of high PDQ score, tender joint count, older age, and concomitant low CRP.20
The pathophysiological mechanisms of fatigue in PsA are still poorly understood. In PsA, the central nervous system is directly or indirectly affected by the inflammatory disease process; this may contribute to fatigue in inflammatory disorders in PsA. It has been demonstrated that the treatment with biological therapy effectively suppresses inflammation and reduce fatigue both in patients with psoriasis and with PsA, suggesting that the inflammatory state of the disease itself and the role of different proinflammatory cytokines play an important role in the generation of fatigue.9 The relationship between IL-17 and fatigue has also been demonstrated in some cohorts,21 as well as the participation of other inflammatory cytokines such as IL-6, IL-1 and TNF, involved in the permeability of the blood–brain barrier, as it occurs in depression (see the section “Depression”).22
There are no objective methods for measuring the severity of the fatigue associated with chronic diseases, and all available measurement instruments are based on self-assessment.22,23 The simplest and most frequently applied scale for assessment of fatigue is a horizontal visual analogue scale (VAS) of 100mm, which allows the patient to determine the severity by indicating the space between the outermost points, where 0 indicates absence of fatigue and 100 indicates a total lack of strength and energy. The simplicity of the measurement using the VAS is an unquestionable advantage of this method. Another instrument for the evaluation of fatigue is the FACIT-F, which consists of questions about general, physical, mental fatigue and the desire to live, already validated for PsA.23
The therapy for fatigue is mainly based on the treatment of the underlying disease and in non-pharmacological methods; however, even an effective treatment reduces, but does not eliminate fatigue. It is necessary to remember that fatigue can be associated with the adverse effects of the immunomodulatory medications.22
AnxietyAnxiety is a subjective state of discomfort and excessive worry, the latter generally associated with 3 or more of the following symptoms: restlessness, easy fatigue, difficulty concentrating, irritability, muscle tension, and sleep disturbances.24 There are other affine definitions such as the state of anxiety that is defined as an unpleasant emotion in the face of a threat and can be normal; and the trait anxiety, which refers to a predisposition to experience anticipatory anxiety, and if it occurs at high levels, it is a problematic condition.25 Anxiety is not a single condition; it is made up of a series of disorders classified in the DSM-IV manual such as: panic disorder, agoraphobia, specific phobias, social phobia, obsessive compulsive disorder, post-traumatic stress disorder, acute stress disorder and generalized anxiety disorder. However, the majority of patients do not meet the criteria for an anxiety disorder since the spectrum of manifestations is mild. It should also be taken into account that in the context of rheumatic diseases such as PsA, there is usually a combination with other pathologies, especially with depressive disorder, this is reflected in the studies carried out on this subject, where anxiety is rarely referred to as the only disorder and the results are combined with depression.26
To talk about prevalence, it should be highlighted that it is different and lower when it comes to anxiety only in skin involvement, which is around 24.4%. In the context of PsA, the prevalence can vary according to the study, in some studies it has been reported in 43% and in others in 36.6%.27,28 In 2020, Zhao et al. published a systematic review with a meta-analysis of 24 studies in which they sought to estimate the prevalence of mental disorders in patients with PsA and compare them with the disease activity. For anxiety, they documented a prevalence between 4% and 61%, depending on the cut-off point of the Hospital Anxiety and Depression Scale (HADS). We emphasize that the fact of having the combination of joint and skin involvement increases the risk of anxiety.27
In terms of pathophysiology, there are biological and non-biological causes. For anxiety it is not as clear, nor as studied. Pro-inflammatory cytokines have been found as precipitators in mood disorders. Some studies conducted in healthy patients but with high levels of anxiety have detected elevated serum levels of TNF-α, IL-6 and IFN-gamma. In addition, anxiety itself can activate the hypothalamic–pituitary–adrenal axis, increasing the production of cytokines. There are case series in other rheumatic pathologies such as rheumatoid arthritis (there are no specific data for PsA) in which elevated serum levels of IL-17 have been documented.26,29 As associated factors, the female gender, unemployment, chronic pain, fear of the disease or of the evaluation of other people and functional limitations have been identified as associated factors.27,29
Mood disorders, including anxiety, have an impact on the disease activity. It has been documented an association with high joint count, pain, elevation of acute phase reactants and activity scales such as BASDAI (Bath Ankylosing Spondylitis Disease Activity Index), BASFI (Bath Ankylosing Spondylitis Functional Index), and ASDAS (Ankylosing Spondylitis Disease Activity Score). In another study, there was increased pain and global assessment of the disease by patients and physicians, but with no impact on swollen joints or acute phase reactants. When there was a combination of anxiety and depression, the impact on the disease activity was considerable.16
There are several questionnaires to assess the symptoms of anxiety. One of the most widely used is the Hospital Anxiety and Depression Scale (HADS), which is a tool that responds to the patient by detecting symptoms of anxiety and depression; it has a subscale for depression and another for anxiety, the latter contains 7 questions assessing the symptoms of the previous week. Each question is scored from 0 to 3 for a total of 21; up to 7 points is considered normal, between 8 and 10 as a possible mood disorder and values higher than 10 make it probable.30 The short-form 36 (SF-36) uses anxiety items but does not provide anxiety subscales. Other scales that assess anxiety are: GAD-Q-IV (Generalized Anxiety Disorder Questionnaire for DSM-IV) inventory of symptoms according to DSM-IV, ASI (Anxiety Sensitivity Index), among others.29
Unfortunately, mood disorders are rarely diagnosed in the consultation, which prevents to do interventions, especially psychosocial, that have a positive impact on improving quality of life and reducing the burden of the disease. In addition, it should be emphasized that anxiety, like depression, influence adherence to treatment and the perception of health of those who suffer from PsA, which is ultimately reflected in the scales of disease activity.27
Regarding the treatment, the first line of management of anxiety disorders are serotonin reuptake inhibitors (SSRIs). Of non-pharmacological therapies, cognitive behavioral therapy appears to be the preferred first-line treatment for anxiety disorders, employing a variety of techniques that include cognitive restructuring, exposure, and behavioral experiments.29 With regard to biological therapy, in the study conducted by Freire et al., therapy with biologic agents either in monotherapy or combined with conventional DMARDs was associated with a lower prevalence of anxiety.31
DepressionOne-third of patients have depression at the time of the diagnosis of PsA. The prevalence in different literature reports ranges from 17% to 22%,2 being much higher compared with that of psoriasis (9.6%). The factors related to depression include: unemployment, being a woman, a greater number of swollen joints, disability, fatigue and pain.27
In PsA, the risk of depression is higher than in rheumatoid arthritis, due to the addition of multiple extra-articular comorbidities; for example, cutaneous manifestations that can leave sequels and stigmatization, giving rise to symptoms of rejection, guilt and shame, generating great difficulties for social and sexual interaction32; therefore, the predisposition to depressive disorders, including suicidal ideation, is high. Unfortunately, these aspects are poorly evaluated and intervened in the consultation of rheumatology or dermatology and are associated with lower rates of remission or response to conventional treatment.3,32
The neurobiological basis for the development of depression began to emerge after studies that demonstrated an imbalance between pro-inflammatory and anti-inflammatory cytokines. The proinflammatory cytokines that represent the key element in depression and rheumatic diseases such as PsA include Th17, interleukin-1 (IL-1), IL-6 and tumor necrosis factor (TNF). Th17s have been considered probable inducers of brain damage, they induce neuronal cell death and promote neuronal toxicity.33
IL-17A is capable of reducing the expression of genes associated with tight junctions and of altering the integrity of the monolayer of cells in the blood–brain barrier, promoting the passage of proinflammatory cytokines, generating neuroinflammation and synaptic dysfunction. The IL-17A produced by resident cells of the central nervous system appears to be localized and not sufficient to induce depression, however, it has been hypothesized that in diseases such as PsA, the production of IL-17A is increased by a pathogenic subpopulation of Th17 at the level of tissues and entheses, generating sufficient stimulus to promote the appearance of depressive symptoms.32
TNF, IL-1 and IL-6 allow the differentiation of Th17 cells, but in addition to this function they seem to have two other mechanisms involved in depression. The first, the negative modulation of serotonergic neurotransmission due to the degradation of tryptophan, this being an essential amino acid for the synthesis of serotonin. During inflammation, the TNF is capable of inducing an increase in the serum enzymes responsible for the degradation of tryptophan. The other mechanism is the hyperactivation of the hypothalamic-pituitary-adrenal axis associated with the corticotropin-releasing factor (CRH); proinflammatory cytokines promote the expression of the glucocorticoid receptor β isoform, resulting in decreased inhibition.
Regarding IL-1, it has been found that IL-1β activates the kynurenine pathway and decreases neurogenesis in the human hippocampal progenitor cells, which is a common finding in depression.34–36
Different scales have been used for the objective identification of depression in patients with AS, the HADS (already mentioned previously for anxiety)37 is the most widely used. It has a sensitivity and specificity close to 80%, when compared with the structured interview for DSM-IV.27,37
However, there is no specific tool for the diagnosis of depression in patients with PsA. In the outpatient consultation, the NICE group proposes screening for depression with two questions: during the last month, how often have you been bothered by feeling sad, depressive or hopeless? And, during the last month, how often have you been bothered by little interest or lack of pleasure in doing things?38 Other tools used in the diagnosis are the health-related quality of life questionnaire SF-36, in which a score of less than 38 in the mental component or less than 56 in the mental health subscale is suggestive of depression. In other studies, the diagnosis was made clinically by psychiatry or psychology,3 in the majority of occasions following the DSM criteria.
Other scales found for the assessment of patients with PsA are the Beck Depression Inventory (BDI) and the short-form version of the Depression Anxiety Stress Scale (DASS-21). The BDI has 21 questions, each with a score from 0 to 3, according to the severity of the symptoms, where cut-off points between 0 and 13 are minimal depression, 14–19 mild, 20–28 moderate, and 29–63 severe depression.39,24 The Patient Health Questionnaire-9 (PHQ-9), was validated in Spanish and has 9 items with a score from 0 to 27, where higher scores indicate greater depression.40,41 Finally, within the most used tools found in this narrative review, is the Hamilton Depression Rating scale (HAM-D), in which values lower than 7 do not suggest depression and cut-off points between 8–13, 14–18 and higher than 19, classify the patient with mild, moderate and severe depression, respectively.42
The management of patients with PsA and depression is multidisciplinary, especially because of the comorbidities that afflict them, where it is essential to achieve the control of joint and eye pathology, cutaneous lesions, obesity and metabolic disorders, always accompanied by specialties such as psychology and psychiatry. In mild depression, vegetative symptoms such as sleep disorders, appetite changes and fatigue may occur, so measures of sleep hygiene, exercise and cognitive behavioral therapy are recommended in these cases, the latter guided and supervised by self-help groups. Management with antidepressants is not effective in these patients.29
In the case of moderate to severe depression, it may be necessary to add longer sessions of cognitive behavioral therapy or interpersonal psychotherapy to the measures described above, which, if they are not effective, the addition of antidepressant drugs should be assessed, being the first-line serotonin reuptake inhibitors. Likewise, the production of proinflammatory cytokines such as TNF, IL-6 and IL-17, with a negative modulation of serotonergic neurotransmissions (as previously described), has a role in the presence of depressive symptoms; therefore, the use of biological therapies may have antidepressant effects.29,32
In the study conducted by Wu et al.,14 in patients with psoriasis and PsA who presented depression and insomnia, and in whom biological therapy with Anti-TNF was started, a reduction in the need to use of antidepressants was observed. In the case of therapies like anti IL-17, the benefit in the management of comorbidities such as anxiety and depression is not clear, and in the same way that the anti-TNFs, it could be presumed a possible peripheral antidepressant effect, rather than central, due to its questionable passage through the blood–brain barrier.33
Other considerations to keep in mind are the use of glucocorticoids, which can exacerbate or produce different mood disturbances at high doses, or sustained over time, so they should be titrated down as soon as possible.32 Vitamin D supplementation is also necessary in cases of deficiency and for the management of anxiety, pain or frequent somatization that occurs in patients with depression.27,32
ConclusionThe prevalence of mood disorders associated with PsA and their impact on the disease outcomes and the response to treatment are important. There is increasing evidence that explains the association between inflammation and mood disorders, especially depression. Pharmacological and non-pharmacological treatment for this type of mood disorders is important, since there are already studies on the benefit of biological therapy in these disorders.
Ethical considerationsThis is a research without risk, is a narrative review without patients intervention.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interestThe authors declare no conflict of interest.