The relationship between adverse events and the application of biological drugs in patients with a diagnosis of rheumatoid arthritis has been documented worldwide, but with little evidence of the situation in Colombia. If adverse events and/or drug reactions with relevant clinical findings in health because of this therapeutic treatment affect the patient's quality of life and influence health indicators at the national level and system resources, it is important to assess their impact.
ObjectivesTo determine the frequency of adverse events and/or adverse reactions related to the use of biological drugs in a cohort of patients diagnosed with rheumatoid arthritis from a national insurer, in the period from 2000 to 2019.
MethodologyA descriptive, cross-sectional, and retrospective study with analytical scope was carried out in patients diagnosed with rheumatoid arthritis, on biological therapy, under a nationwide insurer, with records in their medical records from 2000 to 2019.
Results252 clinical records of users with a rheumatoid arthritis diagnosis and biological therapy were analysed; 62.7% had at least one adverse reaction; nine drugs were evaluated in this study: Tocilizumab, Etanercept, Adalimumab, Abatacept, Certolizumab, Golimumab, Infliximab, Rituximab, and Tofacitinib. Tofacitinib was included in this study at the request of the insurer providing the information.
ConclusionsAdverse reactions with biological therapy in patients with rheumatoid arthritis are frequent and were severe in 27.3%. This is a situation previously unknown in Colombia.
La relación entre eventos adversos y aplicación de medicamentos biológicos en pacientes con diagnóstico de artritis reumatoide ha sido documentada a escala mundial, pero con escasa evidencia en Colombia. Si se asume que los eventos adversos o reacciones medicamentosas con hallazgos clínicos relevantes en la salud, como consecuencia de este tratamiento terapéutico, recaen sobre la calidad de vida del paciente e influyen en los indicadores de salud a escala nacional y en los recursos del sistema, se hace importante evaluar su impacto.
ObjetivosDeterminar la frecuencia de eventos adversos o reacciones adversas relacionados con el uso de medicamentos biológicos en una cohorte de pacientes con diagnóstico de artritis reumatoide de una aseguradora nacional, en el periodo comprendido entre los años 2000 y 2019.
MetodologíaSe realizó un estudio descriptivo, transversal y retrospectivo, con alcance analítico, en pacientes diagnosticados con artritis reumatoide, con terapia biológica, en una aseguradora a escala nacional, con registros en historias clínicas del año 2000 al 2019.
ResultadosSe analizaron 252 registros clínicos de usuarios con diagnóstico de artritis reumatoide y terapia biológica; el 62,7% presentó al menos una reacción adversa; se evaluaron 9 fármacos: tocilizumab, etanercept, adalimumab, abatacept, certolizumab, golimumab, infliximab, rituximab y tofacitinib. Este último es un fármaco incluido en este estudio por solicitud de la aseguradora fuente de la información.
ConclusionesEn la terapia biológica de pacientes con artritis reumatoide, las reacciones adversas son frecuentes, y en un 27,3% resultan severas, lo cual describe un panorama situacional previamente desconocido en Colombia.
Rheumatoid arthritis (RA) is considered a chronic inflammatory disease characterized by progressive destruction of the synovial joints, with typical clinical signs that allow, on occasions, high suspicion based on a diligent semiological examination1. RA is an autoimmune disease due to its pathophysiological behavior determined by autoantibodies (rheumatoid factor and anti-citrulline antibodies), which have special clinical utility: they are diagnostic, preceded by more complex clinical manifestations, and have prognostic value in both treatment and disease progression.
RA is closely related to a permanent disability; the Pan American Health Organization estimates that 34 million people suffer from it2, with a direct impact on work ability and functional performance; therefore, early diagnosis and timely treatment are essential pillars to improve disease prognosis and reduce severity1. RA management can be carried out with multiple care interventions3, disease-modifying anti-rheumatic drugs or biologics, according to the treating clinical specialist criteria4; however, this alternative is expensive and increasingly related to effects on patients’ health and adverse events (AEs), defined by the World Health Organization as any unintentional injury or damage caused by healthcare intervention, but not by the underlying disease5.
In addition to the clinical and public health impact, the AEs associated with the management of autoimmune diseases with biological agents represent an economic burden equivalent to 1% of the total annual cost of treatment6, an underestimated value, since global studies with greater accuracy are lacking7. In developed countries, scientific literature has reported an increase in the frequency of AEs associated with biologics in various autoimmune diseases, such as Crohn’s disease and ankylosing spondylitis, and RA, especially neurological (multiple sclerosis activation), cardiovascular (heart failure flare), and increased risk of opportunistic infections (tuberculosis reactivation)8. This has led to the construction of consensus arguments that question the safety of these drugs in the treatment of these diseases.
In Colombia, RA has a prevalence of 0.56%; biological therapy (BT) represents an alternative for its management6,9. The Ministry of Health and Social Protection adopts the international definition of BT as those therapeutic procedures without genetic information use10. The National Institute for Food and Drug Surveillance (Invima) regulates the use and marketing of this type of medication, which must be registered, with mandatory reporting of documented adverse events. Until 2019, Invima had approved the following biologics for the treatment of RA: infliximab, rituximab, etanercept, abatacept, certolizumab pegol, golimumab, adalimumab, and tofacitinib11. The latter was included in this study after considering that it is an alternative for the management of patients with RA; however, it is not classified as a biologic, since it is part of the category of specific targeted synthetic disease-modifying antirheumatic drugs.
Even though in Colombia there is a clinical practice guideline for the management of RA with BT, the reports and evidence of AEs in RA are still limited12, and there is no baseline that allows the follow-up of these patients. Likewise, the safety of BT and its effect on improving health, disease status, and functional disability of patients are recognized13. The objective of this research was to identify AEs or adverse drug reactions (ADRs) in subjects diagnosed with RA who were managed with BT, between 2000 and 2019.
Population and study areaMaterials and methodsType of studyOperational research was carried out through a descriptive, cross-sectional study, with an analytical scope, and retrospective data collection.
Source of informationThe clinical records of a cohort of patients with RA under BT in a benefit plan administrator entity (BPAE) that operates nationwide, through its health service provider institutions (HPI) and a specialized HPI, were reviewed, from 2000 to 2019. The Aranson classification of severity11,14 was used for the study of Adverse Drug Reactions (ADRs), making use of their degrees and variations according to the source of information; this is described in detail in the results.
Eligibility criteriaInclusion: Records of users with a confirmed diagnosis of RA, who had received a single dose, several doses, dose suspension, or partial or definitive change of BT were included. There had to be at least 2 assessments in the previously defined interval.
Exclusion: Records of users with a diagnosis of psoriatic RA, juvenile RA, difficulties in drug dispensing, or incomplete medical history were excluded.
Sample size252 medical records of patients who met the selection criteria defined above were included. These records were analyzed from 2 sources: specialized HPI and primary care HPI; Information was collected from January 1, 2000, to June 31, 2019, as shown in Fig. 1. A data collection instrument was developed that enabled the achievement of information, the construction of the studied features, and its subsequent statistical analysis. For the quality of data, double reviews of records were conducted; thirty registries already documented were taken randomly and the obtained data were audited.
Data management and statistical analysisUnivariate analysis was performed using Stata14® software (StataCorp, College Station, Texas, USA). Descriptive statistics were applied, and the Shapiro-Wilk test was used for numerical variables. The distribution and quality of data of clinical and demographic characteristics were assessed; means with their respective standard deviation were calculated, while the nominal variables were summarized using means of proportions; for all, statistically significant differences were considered with p values ≤0.05.
For bivariate analysis, the calculation of the odds ratio (OR) was used for those who presented and those who did not present AE or ADR. Considering that all users included in the registries were exposed to one or several biologics, significant differences were sought through the odds ratio (OR), with their respective 95% confidence intervals and their significant P values ≤.05, based on the Fisher’s t verification test and the Shapiro-Wilk tests for the normal distribution of variables. The estimation of the prevalence ratio was also used, using 2 × 2 tables to explore the possible associations against the different types of exposure and their clinical findings. Lastly, a multivariate analysis was performed using logistic regression to review the variables that were correlated with AE or ADR through bivariate analysis.
Ethical considerationsThis research was approved as risk-free research by the ethics committees of the BPAE, the specialized IPS, and Universidad Libre, according to the national regulations outlined in Resolution 8430 of 1993 of the Colombian Ministry of Health, included in Act 010-2019.
ResultsSelection criteria were applied to a population of 586 users diagnosed with RA, exposed to BT, and belonging to a cohort of a BPAE with national coverage, for which medical records from 2000 to the first semester of 2019 were reviewed. According to this, 57% (334/586) of the records were excluded and the information of the remaining 43% (252/586) that fully met inclusion criteria was analyzed.
Sociodemographic characterization of the study population revealed that women constitute most users, with 91% (227/252); the median age was 57 years (IQR: 26–88). Most of them came from the Colombian Caribbean region: 44% (111/252), and 54% (136/252) of the cases were actively employed (Table 1).
Sociodemographic characteristics of the population with RA in BT in an insurer in Colombia between 2000 and 2019.
| Characteristic | Description | Summary measure |
|---|---|---|
| Age | Years | Mean: 56.6; SD: 12.7 |
| Sex | Men | 25 (9%) |
| Woman | 227 (91%) | |
| Origin | Caribbean | 111 (44%) |
| Southwest | 84 (33.3%) | |
| Northeast | 35 (13.9%) | |
| Central East | 16 (6.3%) | |
| Coffee Axis | 6 (2.4%) | |
| Work activity | Active | 136 (54%) |
| Inactive | 116 (46%) | |
| Membership Type | Contributory | 175 (69.4%) |
| Beneficiary | 77 (30.6%) | |
| Socioeconomic income | A minimum wage | 187 (74%) |
| More than one minimum wage | 65 (26%) |
RA: Rheumatoid arthritis; BT: Biological therapy; SD: Standard Deviation.
RA occurs more frequently between 56 and 57 years; 54% found themselves active at jobs.
In the conducted reviews, 30 different types of clinical findings were identified and classified as ADRs, according to their literature support, clinical relevance, and their persistent reference within the group of subjects evaluated, such as adynamia and asthenia, commonly referenced by patients with several drugs and found repeatedly (Table 2).
Frequency of expected and unexpected adverse reactions in patients with RA under BT reported in clinical records from 2000 to 2019.
| Expected adverse reactions | No. | % |
|---|---|---|
| Myalgia/arthralgia | 89 | 24.2 |
| Severe headache (migraine) | 53 | 14.4 |
| Recurring flu syndromes | 51 | 13.9 |
| Sjogren’s syndrome | 39 | 10.6 |
| Dyslipidemia | 22 | 6.0 |
| TB | 19 | 5.2 |
| Urticaria at the puncture site | 15 | 4.1 |
| Herpes zoster | 13 | 3.5 |
| COPD flare | 12 | 3.3 |
| Elevation of transaminases | 9 | 2.4 |
| Adynamia and asthenia | 8 | 2.2 |
| Psoriasis | 5 | 1.4 |
| Heart failure flare | 6 | 1.6 |
| Hepatitis B | 2 | 0.5 |
| Recent diagnosis of SLE | 2 | 0.5 |
| Vasculitis | 1 | 0.3 |
| Upper limb demyelination | 1 | 0.3 |
| Itching at the puncture site | 1 | 0.3 |
| Unexpected adverse reactions | N | % |
|---|---|---|
| Hepatic steatosis | 4 | 1.1 |
| Fever during administration | 3 | 0.8 |
| Dizziness/vertigo | 3 | 0.8 |
| Dermatitis | 2 | 0.5 |
| Thyroid cancer | 1 | 0.3 |
| Alopecia | 1 | 0.3 |
| Bradycardia during administration | 1 | 0.3 |
| Diarrhea | 1 | 0.3 |
| Recurrent UTI | 1 | 0.3 |
| Puncture site bruising | 1 | 0.3 |
| Chest pain and dyspnea during administration | 1 | 0.3 |
| Tachycardia and chest pain during administration | 1 | 0.3 |
The expected adverse reactions refer to those documented in the references, while the unexpected adverse reactions are documented as clinical findings from the post-application case material of biological therapy, in which myalgia/arthralgia are the most frequent in the first group, while hepatic steatosis is the most frequent in the second group.
RA: Rheumatoid arthritis; BT: Biological therapy; TB: Tuberculosis; COPD: chronic obstructive pulmonary disease; SLE: Systemic lupus erythematosus; UTI: urinary tract infection.
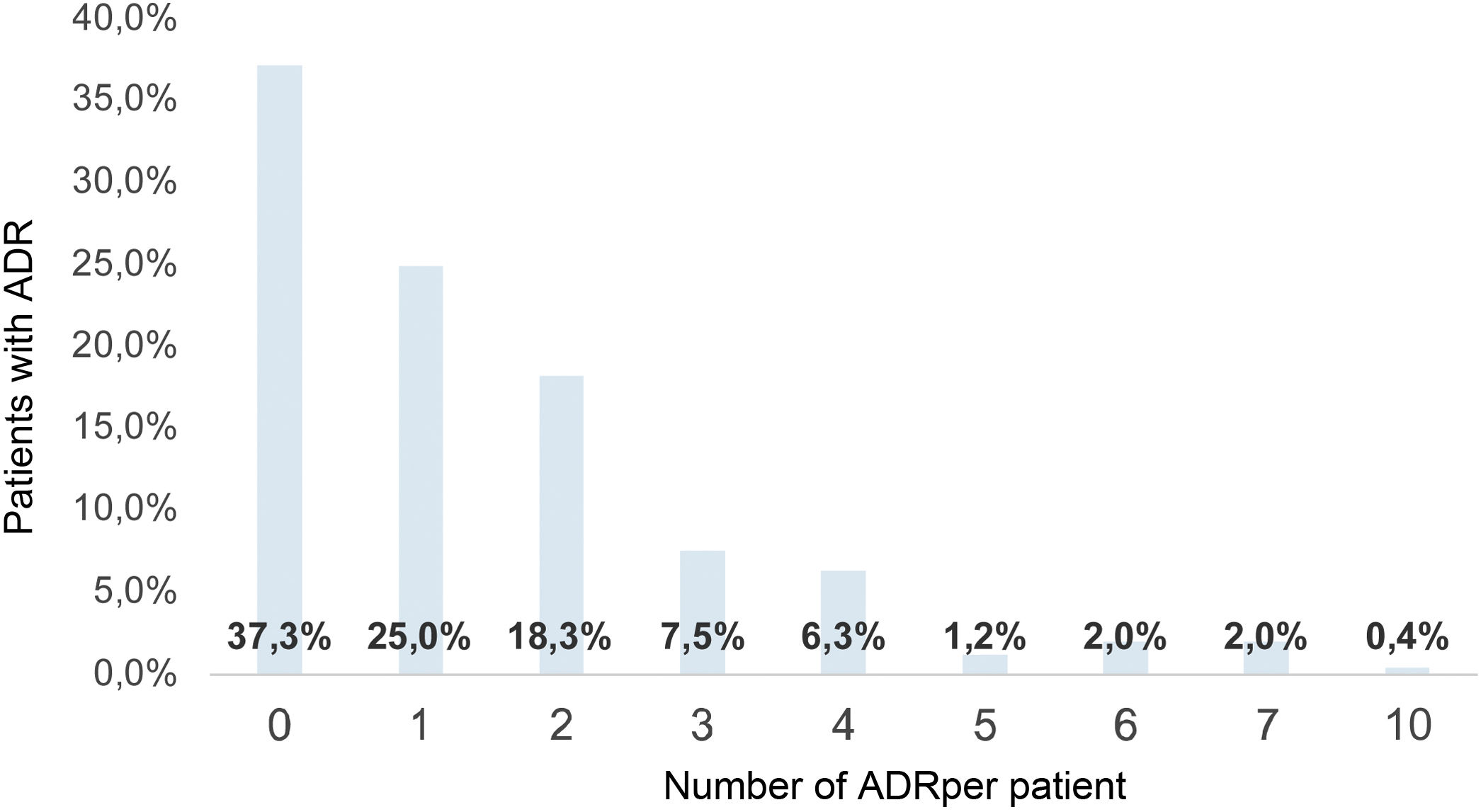
Etanercept was the drug with the most ADRs identified (20), and infliximab had the least (eight); the average number of ADRs per patient was 1.5 (σ: 1.71; 0–10); therefore, each patient with RA exposed to BT had at least one ADR during treatment (Fig. 2).
Bivariate analysis showed a relationship between exposure to biologics and clinical findings classified in our study as ADR. In this sense, significant differences were found: male gender (OR: 2.5; 95% CI: 1.02–6.67; P = .02), time exposure to BT for more than two years (OR: 3.1; 95% CI: 1.6–5.56; P = .0001), and receiving one biologic vs. more than one (OR: 0.3; 95% CI: 0.17–0.62; P = .0002) as a protective factor (Table 3).
Factors related to the presence of ADRs in patients diagnosed with RA under BT documented in clinical records from 2000 to 2019.
| Characteristic | Description | No. | ADR | OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Sex | Men | 25 | 15 | 10 | 2.5 | 1.02–6.65 | .02 |
| Woman | 227 | 84 | 143 | ||||
| Age | More than 60 years | 111 | 71 | 40 | 1.1 | 0.7–1.9 | .7 |
| Under 60 years | 141 | 87 | 54 | ||||
| Membership Type | Contributory | 175 | 107 | 68 | 0.8 | 0.43–1.45 | .4 |
| Beneficiary | 77 | 51 | 26 | ||||
| Socioeconomic income | A minimum wage | 187 | 120 | 67 | 1,3 | 0.69–2.36 | .4 |
| More than a minimum wage | 65 | 38 | 27 | ||||
| Work activity | Inactive | 136 | 85 | 51 | 1 | 0.57–1.70 | .94 |
| Active | 116 | 73 | 43 | ||||
| Number of biological drugs received | One | 168 | 92 | 76 | 0.3 | 0.17–0.62 | .0002 |
| More than one | 84 | 66 | 18 | ||||
| Time of use of biological therapy | More than 2 years | 177 | 125 | 52 | 3.1 | 1.6–5.56 | .0001 |
| Less than 2 years | 75 | 33 | 42 | ||||
The most characteristic risk factors for causing ADRs were male sex (OR: 2.5), exposure to BT for more than 2 years (OR: 3.1), and use of more than one biologic (OR: 0.3).
RA: rheumatoid arthritis; BT: Biological therapy; CI: confidence interval; OR: odds ratio; ADR: adverse drug reaction.
However, when running the logistic regression model, the adjustment of the crude ORs was achieved and two effect-modifying variables were found: exposure time to the biologic for more than two years and BT with one drug vs. more than one. This reversal of the OR values is an exotic finding that raises new questions, such as the assessment of different time ranges against the exposure proposed by each drug, which was not possible for this study because the specific duration data for each biologic could not be obtained from the source of information, but the start and ending date of BT, in general, was clear. Concerning the other effect modifier variable (BT with one medication vs. more than one), further evaluation is also required.
In this way, emphasis is placed on the need for analytical studies that evaluate these two variables with better control of information bias. Male gender was determined as a confounding variable in the adjustment with this same model. No significant differences were found between the sociodemographic characteristics and the presence of ADR. The logistic regression model used was automated (stepwise), which prevented the manual operation of these variables (Table 4).
Logistic regression applied to the start time of the biologic and number of biologics received in the clinical records of patients with RA between 2000 and 2019.
| Characteristic | Description | N. | ADR | OR | CI (95%) | P-value | Adjusted OR | CI (95%) | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||||
| Number of biologicals received | One | 168 | 92 | 76 | ||||||
| 0.3 | 0.17–0.62 | .0002 | 2.02 | 1.31–3.14 | .002 | |||||
| More than one | 84 | 66 | 18 | |||||||
| Use time of BT | More than 2 years | 177 | 125 | 52 | 0.81– | |||||
| 3.1 | 1.6–5.56 | .0001 | 0.89 | 0.98 | .020 | |||||
| Less than 2 years | 75 | 33 | 42 | |||||||
The number of biologics received, and the time of BT use are features that make it possible to predict a tendency to present ADRs.
RA: rheumatoid arthritis; CI: confidence interval; OR: odds ratio; ADR: Adverse drug reaction; BT: Biological therapy.
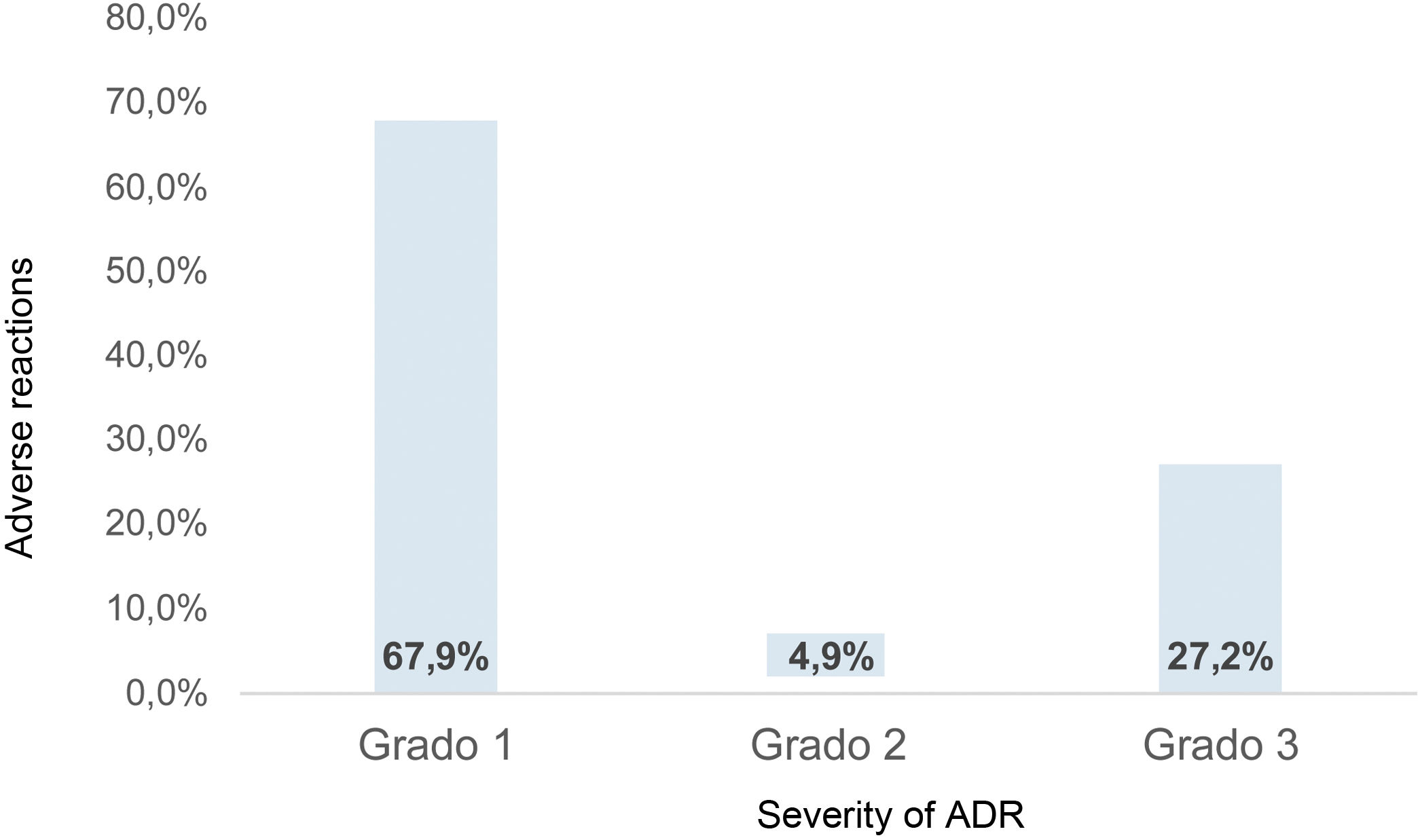
Under the Aranson classification of severity for the study of ADRs11,14, (grade 1:no change in dosage; grade 2: a change in dose; grade 3: suspension of treatment), the conduct taken by the treating physician was determined. Due to the limitations of the medical records, subtypes A, B or C were not used. In this way, 368 ADRs occurred in this population, of which 67.9% (250/368) were classified as grade 1 (Fig. 3).
Distribution of the severity of the total ADRs reported in the medical records of patients with RA between 2000 and 2019. In summary, 27.2% of ADRs were classified with the most severe grade according to Aranson’s classification. Clinical records prevented establishing a trend in the number of ADRs per year.
RA: Rheumatoid arthritis; ADR: adverse drug reaction.
The most frequent ADRs were myalgia/arthralgia in 24.2% (89/368), headache (migraine) in 14.4% (53/368), and recurrent flu syndromes in 13.9% (51/368).
The ADRs that occurred with all biologics included Sjögren's syndrome, migraine, myalgia/arthralgia, and recurrent flu syndromes. Regarding the ADR prevalence ratio (PR) compared between drugs, it was found that psoriasis is five times more prevalent in rituximab (PR = 5.0); chronic obstructive pulmonary disease flare was 4.2 times more prevalent with infliximab (PR = 4.2), and elevation of transaminases was 3.6 times more prevalent with tocilizumab (Table 5).
Detail of the ADRs according to the biological drug received and reported in the medical records of patients with RA between the years 2000 and 2019.
| ADR clinical finding | ABA | ADA | CTZ | ETN | GOL | IFX | RTX | TCZ | TOF |
|---|---|---|---|---|---|---|---|---|---|
| Myalgia/arthralgia | 16.7% | 19.4% | 27.9% | 20.8% | 36.0% | 25.0% | 27.3% | 32.0% | 44.4% |
| Severe headache (migraine) | 16.7% | 19.4% | 9.3% | 9.4% | 24.0% | 12.5% | 18.2% | 20.0% | 22.2% |
| Recurring flu síndromes | 13.6% | 12.9% | 7.0% | 13.4% | 16.0% | 25.0% | 13.6% | 16.0% | 33.3% |
| Sjögren’s syndrome | 10.6% | 3.2% | 4.7% | 11.4% | 4.0% | 25.0% | 13.6% | 8.0% | 11.1% |
| Dyslipidemia | 4.5% | 6.5% | 4.7% | 6.0% | 12.0% | 0.0% | 0.0% | 12.0% | 11.1% |
| TB | 1.5% | 3.2% | 23% | 6.7% | 4.0% | 0.0% | 4.5% | 16.0% | 0.0% |
| Urticaria at the puncture site | 4.5% | 9.7% | 23% | 3.4% | 0.0% | 12.5% | 4.5% | 4.0% | 0.0% |
| Herpes zoster | 4.5% | 3.2% | 23% | 5.4% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| COPD flare | 4.5% | 0.0% | 0.0% | 2.7% | 4.0% | 12.5% | 0.0% | 12.0% | 0.0% |
| Elevation of transaminases | 1.5% | 6.5% | 0.0% | 1.3% | 0.0% | 0.0% | 4.5% | 12.0% | 0.0% |
| Adynamia and asthenia | 0.0% | 3.2% | 23% | 2.7% | 0.0% | 0.0% | 4.5% | 4.0% | 11.1% |
| Heart failure flare | 0.0% | 0.0% | 23% | 2.0% | 4.0% | 0.0% | 0.0% | 4.0% | 0.0% |
| Psoriasis | 0.0% | 3.2% | 0.0% | 1.3% | 0.0% | 0.0% | 9.1% | 0.0% | 0.0% |
| Hepatic steatosis | 1.5% | 0.0% | 0.0% | 1.3% | 0.0% | 0.0% | 4.5% | 0.0% | 0.0% |
| Fever during administration | 1.5% | 3.2% | 23% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Dizziness/vértigo | 0.0% | 3.2% | 0.0% | 1.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Hepatitis B | 0.0% | 0.0% | 23% | 0.7% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Recent diagnosis of SLE | 0.0% | 0.0% | 0.0% | 0.7% | 0.0% | 12.5% | 0.0% | 0.0% | 0.0% |
| Dermatitis | 0.0% | 0.0% | 0.0% | 0.0% | 4.0% | 0.0% | 4.5% | 0.0% | 0.0% |
| Vasculitis | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 12.5% | 0.0% | 0.0% | 0.0% |
| Upper limb demyelination | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 11.1% |
| Thyroid cancer | 0.0% | 3.2% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Alopecia | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 11.1% |
| Bradycardia during administration | 1.5% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Diarrhea | 0.0% | 0.0% | 0.0% | 0.7% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Recurrent UTI | 0.0% | 0.0% | 0.0% | 0.7% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Itching at the puncture site | 0.0% | 0.0% | 23% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Puncture site bruising | 0.0% | 0.0% | 0.0% | 0.7% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Chest pain and dyspnea during administration | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 4.0% | 0.0% |
| Tachycardia and chest pain during administration | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 4.5% | 0.0% | 0.0% |
368 RAMs were registered in the sample. Rituximab is evidenced in trend with the ADR psoriasis, as well as COPD flare with infliximab, and elevation of transaminases with tocilizumab.
RA: Rheumatoid arthritis; TB: Tuberculosis; ABA: abatacept; ADA: adalimumab; CTZ: certolizumab; ETN: etanercept; GOL: golimumab; IFX: infliximab; RTX: rituximab; TCZ: tocilizumab; TOF tofacitinib; COPD: chronic obstructive pulmonary disease; SLE: Systemic lupus erythematosus; UTI: urinary tract infection.
In Colombia, the prevalence of RA and its sociodemographic characteristics have been established9; however, evidence regarding patient safety with therapeutic options, including BT, is still scarce15. This research revealed a situational and contextual scenario in an BPAE with national coverage of ADRs related to biologics for the management of this disease.
The considerations analyzed are based on the study of other sociodemographic aspects such as age, territorial location, health system affiliation, and work activity, which did not show any link with the effect on the user's health during exposure to BT16.
Among the clinical features, it was found that 37.5% of the ADRs were related to the application of etanercept, which despite being the most frequent, did not establish a trend concerning severe ADRs; this finding was also referenced in the literature when compared with other drugs within the analyzed sample7.
Determining the most frequent ADRs disputes the true impact of the use of BT for treatment of RA, since myalgia and arthralgia were the most frequent in this study, considering that it could be an important determinant for users’ quality of life and their work potential, in terms of temporary and permanent disability, which is associated with the numbers mentioned by health organizations on an international scale2,17.
Another relevant ADR after BT was tuberculosis18,19, with a proportion of 5.2% (252/19), which, although rare, should have control and surveillance by the treating physicians, due to a high impact on public health in Colombia; these findings were also reported in Canada and European countries18,19.
Among the ADRs with low statistical impact, but with clinical relevance in this study, we mention the case of demyelination of the upper limbs20, like one reported in Ireland, where the relationship between rituximab and demyelinating diseases is suggested21. In addition, a single case of thyroid cancer was described22, which may be related to immune response alteration that may favor activation of oncogenesis.
ADRs described in this research considerably impact the quality of life and well-being of BT users, considering that 63% of them presented at least one that compromised their safety under the application of this technology. Review of evidence is controversial regarding the presence of ADRs and the use of BT since the conflicts of interest mediate in showing objective and impartial results; even in ADR reviews in other diseases susceptible to being treated with this therapy, precise data on different outcomes were not found.
On the other hand, a relationship between the time of exposure to BT and receiving one or more biologics and the presence of ADRs may be feasible; the statistical analyses obtained only allow us to propose these relationships as new hypotheses that must be verified with future studies. In the literature reviewed, no follow-up of complete cohorts of patients with all types of BT was evidenced; mainly isolated case reports23 or economic evaluations of up to 3 drugs7 were found, which strengthened the need for this study.
Grade-3 severity in the ADR classification had a significant proportion (27%), which may influence the safety outlook of these medications; therefore, these results are an initial approximation for patient safety adjustments in healthcare institutions, as recommended by the Ministry of Health guidelines24.
LimitationsThe secondary analysis of a database limited the information on the definitions of clinical outcomes or ADRs or AEs, since, in the analyzed clinical records, the quality of data (information bias) was variable and heterogeneous, which made it difficult to determine an EA or ADR and its classification. Moreover, the reduction of clinical records of medical specialists and lack of knowledge on this topic by the service provider network to inquire in-depth on symptoms and signs presented by patients after BT, as well as the absence of a continuous and judicious follow-up of these events, were common.
It was not possible to quantify the specific exposure time characteristic for each drug, since some of the clinical records were not clear about the start and ending date of each exposure to biologics, which significantly reduced the population intervened, considering that the records had a quality control from the analysis of data in specialized HPI and primary care institutions. For the analysis of the information, the crude OR presented an unusual condition, for which the discussion and conclusions are exposed based on trends in the interpretation of measurement of variables, leaving the precedent of using other tools for other studies, projecting changes in sample size or generating an analytical research design.
ConclusionsADRs produced by exposure to BT in RA are frequent and severe; this study achieved the projection of a previously unknown scenario, inquiring about the readjustment of the well-known concept of risk-benefit in clinical practice. Going deeper into the investigation on patient safety will allow warnings for early detection by medical specialists and experts, given that the current ignorance of the subject may be part of the problem since the patient is not investigated clinically searching for possible negative effects on their biopsychosocial well-being derived directly and indirectly from therapy.
The fact of highlighting the most serious degree of severity (which implies the suspension of the drug due to ADRs), as a large proportion within the classified groups, confirms the previously stated importance of delving into the issue of patient safety about BT of the therapy for RA, considering that the patient exposed to this indication during his treatment must be assessed in a routine, systematic, and diligent manner to timely identify the presence of ADRs.
RecommendationsIt is considered that the BPAE, HPI and medical specialists related to RA care should have an easily accessible technological tool that enables them to measure the risk of presenting ADRs during BT, strictly monitoring these users through clinical registries with optimal information, allowing safe clinical practices, mitigating risk, and benefiting the quality of life of users. The effective and constant communication of the entire healthcare network of the safety programs would provide favorable support for the prevention of ADR.
This study promotes research related to conditions described in public health clinical findings, such as tuberculosis and hepatitis B, emphasizing their impact on the community. It is also recommended to include in detail the cost of ADRs within economic evaluations of this type of therapy to reach comprehensive conclusions that allow taking sound clinical decisions. With the advent of new types of biologics each year for different diseases, teamwork of professionals specialized in patient safety is recommended for each of the actors in the system with the goal of surveillance and prevention of ADRs.
Conflict of interestsThe authors declare the absence of any conflict of interest.
To the health institutions for allowing access. To the patients for being an anonymous source of information.