To describe the clinical characteristics and risk factors for uveitis flare in our psoriatic arthritis and axSpA patient cohorts and to describe possible associations of the different disease domains and uveitis flares.
Methodology and methodsRetrospective analysis of patients followed prospectively in the SpA and PsA clinics at the Toronto western hospital (January 2004–December 2020).
ResultsOf the 3306 included, 1724 patients had axSpA and 1582 PsA. 30.2% of the axSpA cohort, and 7.6% of the PsA population were diagnosed with uveitis. The annual rate of flares per person at risk was 12.1% in the axSpA cohort, vs 1.7 in the PsA. In the axSpA patients, 68% were HLA*B27+, compared 16% of the PsA patients. In the axSpA cohort, higher CRP (HR 1.009, p=.004) increased the risk of a uveitis flare, while biologic agents decreased the risk of flare (HR .702, p=.040) in patients with uveitis. In the PsA cohort, being HLA*B27+ (HR 3.084, p=.007) increased the risk of a uveitis flare, while being on a DMARD decreased the risk of a flare in patients with uveitis (HR .262, p=.0088).
ConclusionsUveitis is a common complication seen in inflammatory arthritis, with a prevalence of 30% in the SpA vs 7.6% in PsA. Uveitis is more common in the axSpA population than in PsA. Even though HLA-B27 is more common in the axSpA population, it constitutes a risk for uveitis only in the PsA population.
Describir las características clínicas y los factores de riesgo de las recaídas de uveítis en nuestras cohortes de pacientes con artritis psoriásica (APs) y espondilitis anquilosante axial (axSpA), así como describir las posibles asociaciones de los diferentes dominios de la enfermedad y los brotes de uveítis.
Metodología y métodosAnálisis retrospectivo de pacientes seguidos prospectivamente en las clínicas de espondilitis y artritis psoriásica en el Toronto Western Hospital, entre enero del 2004 y diciembre del 2020.
ResultadosDe los 3.306 pacientes incluidos, 1.724 tenían axSpA y 1.582 APs. El 30,2% de la cohorte de axSpA y el 7,6% de la población con APs fueron diagnosticados con uveítis. La tasa anual de recaídas por persona en riesgo fue del 12,1% en la cohorte de axSpA, frente al 1,7% en la APs. En los pacientes con axSpA, el 68% eran HLA-B27+, en comparación con el 16% de los pacientes con APs. En la cohorte de axSpA, una PCR más alta (HR: 1,009; p=0,004) aumentó el riesgo de brote de uveítis, mientras que los agentes biológicos lo redujeron (HR: 0,702, p=0,040) en pacientes con uveítis. En la cohorte de artritis psoriásica, ser HLA-B27+ (HR: 3,084; p=0,007) aumentó el riesgo de brote de uveítis, mientras que estar en un DMARD convencional disminuyó el riesgo de brote en pacientes con uveítis (HR: 0,262; p=0,0088).
ConclusionesLa uveítis es una complicación frecuente de la artritis inflamatoria, con una prevalencia del 30% en la espondilitis anquilosante axial, frente al 7,6% en la APs. La uveítis es más común en la población con axSpA que en la APs. Si bien HLA-B27 es más común en la población con espondilitis anquilosante axial, constituye un riesgo de uveítis solo en la población con APs.
Spondyloarthritis encompasses a group of medical conditions with musculoskeletal and systemic manifestations which include psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) among others.
Psoriatic arthritis is a chronic inflammatory arthritis that is associated with psoriasis. A systematic review and meta-analysis of 266 studies, deduced that globally, about one in four persons with psoriasis, has psoriatic arthritis (PsA).1 The worldwide average prevalence of PsA is 133 per 100,000 population (95% CI, 107–164 per 100,000) and the incidence is 83 per 100,000 persons per year (95%CI, 41–167 per 100,000).2,3
In the United States the prevalence of psoriatic arthritis ranges from 6 to 25 cases per 10,000 people, depending on the case definition.4 Psoriatic arthritis can affect the peripheral and axial joints, enthesitis and dactylitis, in addition to skin psoriasis, and is associated with extra-musculoskeletal manifestations (EMMs).5,6 In a systematic review and metanalysis of 163–299 PsA patients, the prevalence of these EMMs were as follows: Nail disease was present in 60% (95% CI: 52%, 68%) across 26 studies. Uveitis (3.2%; 95% CI: 1.9%, 5.3%) and IBD (3.3%; 95% CI: 1.5%, 7.1%) were less common.7,8
Axial spondyloarthritis (axSpA) is a rheumatic inflammatory disease that affects the sacroiliac joints and the apophyseal joints of the spine. Like PsA, it can also affect the peripheral MSK system with symptoms such as enthesitis, peripheral arthritis and dactylitis, and moreover they also have EMMs in a similar fashion as PsA, including IBD and uveitis.9,10
Uveitis refers to the inflammation of the uvea, and depending on the area of the eye affected, it can be classified as anterior, intermediate, posterior or pan-uveitis. The differential diagnosis for the combination of arthritis and uveitis is lengthy, and includes ankylosing spondylitis (AS), reactive arthritis (ReA), juvenile rheumatoid arthritis (JIA), inflammatory bowel disease (IBD), Behcet's disease, Lyme disease, Whipple's disease, the vasculitides, Kawasaki disease, familial granulomatous uveitis and sarcoidosis. In each entity, the pattern of ocular involvement is distinctive. i.e.: ankylosing spondylitis is typically associated with a unilateral, sudden onset, recurrent, anterior uveitis, while juvenile rheumatoid arthritis is most often associated with a bilateral, insidious onset, continuous, anterior uveitis.11,12 In psoriatic arthritis, the most common forms of uveitis are anterior (AU) and posterior uveitis.13 Paiba et all reported that in their cohort, uveitis in patients with PsA was more likely to be insidious in onset, continuous, posterior, and active bilaterally. They also concluded that the patients that had uveitis and axial involvement, were more likely to be male, and HLA-B27 positive, compared to patients with uveitis and peripheral arthritis alone.11,14 These results are like other publications where very similar characteristics have been described.12,15,16 As for axSpA, anterior uveitis (AU) is the most common EMM. It presents in an acute fashion, has limited duration, usually is unilateral, and frequently alternates from one eye to the other.9,10 In the search for risk factors and markers for intraocular inflammation in AS, Sun et al.17 were able to correlate increased antistreptolysin levels with the occurrence of intraocular inflammation in AS patients. In this cohort of 390 patients a higher number of peripheral arthritis was associated with an increased risk for uveitis. This has also been confirmed by previous studies, which reported AS patients with peripheral arthritis more likely to develop acute anterior uveitis.18,19
The Spanish SENTINEL study analyzed 798 acute anterior patients with either HLA-B27 positivity or recurrent disease for undiagnosed SpA and found a prevalence of 50% for axSpA and 17.5% for peripheral SpA; in both cases the prevalence was even higher in HLA-B27 positive patients.20
Here we describe the clinical characteristics and risk factors for uveitis flare in our prospectively followed psoriatic arthritis and axSpA patient cohorts in Toronto, Canada, with an established diagnosis of uveitis, during the period between 2004 and 2020.
Materials and methodsThe patients were identified from the axSpA and PsA clinics at the Toronto Western Hospital, Toronto, Ontario, Canada from January 2004 to December 2020 intervals. The patients are followed prospectively at 6–12-month intervals according to a standard protocol that includes: Demographic and clinical variables, including use of cDMARD and bDMARD, ESR, and CRP. All the data are tracked in a web-based database (DADOS), stored on the hospital server.
Ethical considerationsAll patients part of the SpA and PsA clinics signed a consent to enroll in the clinic and to participate and use their data in the research activities carry out by the team. This present research work has been approved by the ethical committee group of the University health Network, in Toronto, Ontario, Canada. No patient has been harmed in any way with the present study using their medical data.
Statistical analysisA descriptive analysis on baseline demographic variables and clinical factors was done for both cohorts (Tables 1 and 2). Then, patients with and without uveitis were compared using T- test for continuous variables and chi-squared for dichotomous variables, at baseline visits on demographic and clinical factors. Cox proportional-hazard models with time-dependent covariates were performed on patients with uveitis, with their first instance of a uveitis flare as the outcome event. Univariate analysis was done for each factor, controlling for age, sex, and disease duration. Significant factors (<0.1) in the univariate analysis were included in a multivariate analysis, controlling for age, sex, and disease duration.
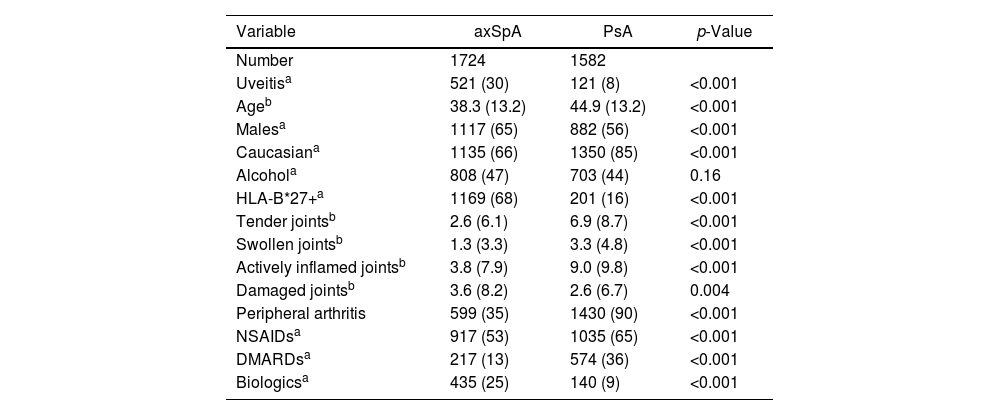
Demographic characteristics of the entire cohort.
| Variable | axSpA | PsA | p-Value |
|---|---|---|---|
| Number | 1724 | 1582 | |
| Uveitisa | 521 (30) | 121 (8) | <0.001 |
| Ageb | 38.3 (13.2) | 44.9 (13.2) | <0.001 |
| Malesa | 1117 (65) | 882 (56) | <0.001 |
| Caucasiana | 1135 (66) | 1350 (85) | <0.001 |
| Alcohola | 808 (47) | 703 (44) | 0.16 |
| HLA-B*27+a | 1169 (68) | 201 (16) | <0.001 |
| Tender jointsb | 2.6 (6.1) | 6.9 (8.7) | <0.001 |
| Swollen jointsb | 1.3 (3.3) | 3.3 (4.8) | <0.001 |
| Actively inflamed jointsb | 3.8 (7.9) | 9.0 (9.8) | <0.001 |
| Damaged jointsb | 3.6 (8.2) | 2.6 (6.7) | 0.004 |
| Peripheral arthritis | 599 (35) | 1430 (90) | <0.001 |
| NSAIDsa | 917 (53) | 1035 (65) | <0.001 |
| DMARDsa | 217 (13) | 574 (36) | <0.001 |
| Biologicsa | 435 (25) | 140 (9) | <0.001 |
Demographic characteristics of the patients with uveitis diagnosis.
| Variable | axSpA | PsA | p-Value |
|---|---|---|---|
| Number* | 521 | 121 | |
| Age** | 42.2 (12.5) | 48.1 (13.6) | <0.001 |
| Males* | 341 (65) | 57 (47) | <0.001 |
| Caucasian* | 348 (67) | 114 (94) | <0.001 |
| Alcohol | 249 (48) | 37 (31) | <0.001 |
| HLA-B*27+* | 435 (83) | 32 (31) | <0.001 |
| Tender joints** | 2.4 (6.0) | 6.0 (8.2) | <0.001 |
| Swollen | 1.1 (3.0) | 2.3 (4.1) | <0.001 |
| Active | 3.4 (7.3) | 7.5 (9.2) | <0.001 |
| Axial d | 426 (95) | 80 (68) | <0.001 |
| Peripheral arthritis | 180 (35) | 97 (80) | <0.001 |
| NSAIDs | 288 (55) | 82 (68) | 0.012 |
| DMARDs | 72 (14) | 48 (40) | <0.001 |
| Biologics | 140 (27) | 23 (19) | 0.07 |
A total of 3306 patients were included, 1724 patients with axSpA and 1582 patients with PsA. Five hundred and twenty-one patients (30.2%) of the axSpA cohort had uveitis, and only 121 patients (7.6%) of the PsA population were diagnosed with uveitis. The annual rate of flares per person years at risk was 12.1% in the axSpA cohort, vs 1.7% in the PsA.
The general demographic and clinical features are depicted in Table 1. Here we can see that the predominant sex in the total cohort was male (axSpA 65% and PsA 56%), most of the patients were caucasian (axSpA 66%, and 85% for the PsA group), followed by a small proportion of Indians, middle eastern and African Americans. Among axSpA patients, 1169 (68%) were HLA*B27+, whereas a much smaller proportion of PsA patients, 201 (16%) were positive. In general, a much larger proportion of patients in the PsA cohort had peripheral arthritis (90%, p<0.0001) compared to 35% in the axSpA group. More patients in the PsA group were on cDMARDs 36%, and 25% of the axSpA on bDMARDs Vs 9% of the PsA cohort.
When looking only at the patients with uveitis diagnosis (Table 2), in the axSpA cohort the main characteristic were as follow: more men (65%, p<0.0001), caucasian (67%, p<0.0001) and 83% were HLA-B*27+ (p<0.001). Less of these patients had peripheral disease (35%, p<0.001)), tender joints (6%, p<0.001) and were taking cDMARDs (14%, p<0.001). In contrast, in the PsA cohort 47% were men (p<0.001), only 31% were HLA-B*27+ (p<0.001), more patients had peripheral disease (80%, p<0.001) and were taking cDMARDs (40%, p<0.001) and less were on bDMARD.
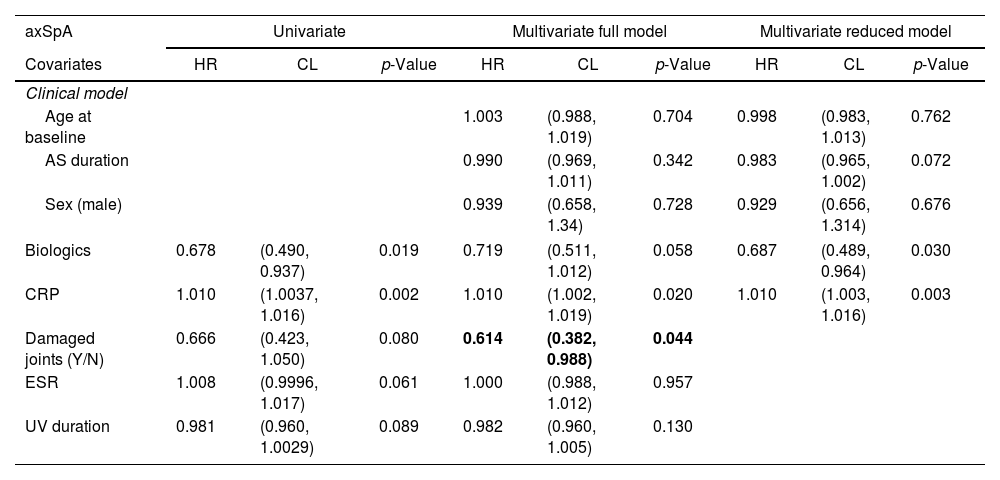
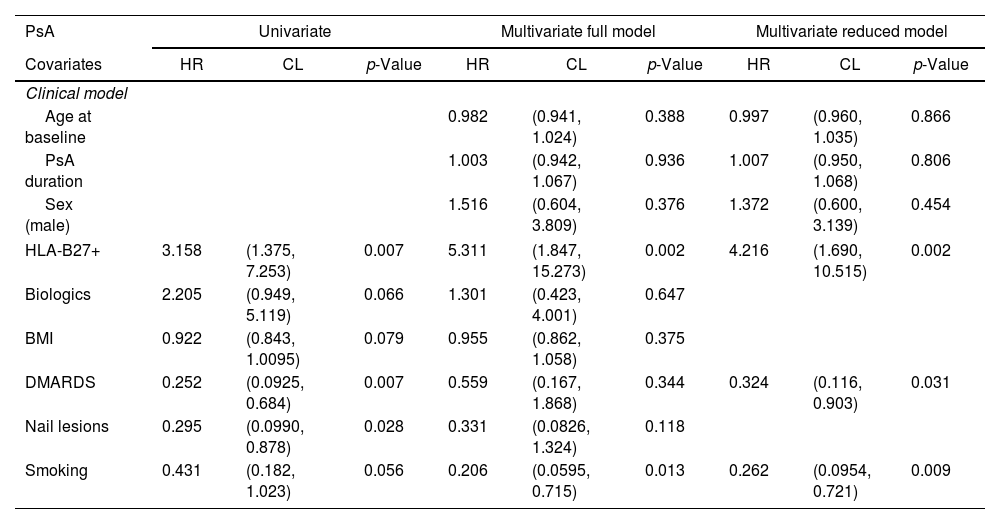
In the multivariate analysis (Tables 3 and 4) we found that in the axSpA cohort, higher CRP (HR 1.010, p=0.003) increased the risk of a uveitis flare, while biologic agents decrease the risk of flare (HR 0.687, p=0.030). In the axSpA cohort these patients had longer disease duration (which seemed to have a protective effect on future flares) and were mostly male. In the PsA cohort, being HLA*B27+ (HR 4.216, p=0.002) increased the risk of a uveitis flare, while being on a cDMARD decreased the risk of a flare (HR 0.324, p=0.031). Disease duration seemed to be a risk factor (but did not have a significative p-value) for future flares.
Univariate and multivariate analysis of the relevant characteristics of the axSpA cohort with UV.
| axSpA | Univariate | Multivariate full model | Multivariate reduced model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariates | HR | CL | p-Value | HR | CL | p-Value | HR | CL | p-Value |
| Clinical model | |||||||||
| Age at baseline | 1.003 | (0.988, 1.019) | 0.704 | 0.998 | (0.983, 1.013) | 0.762 | |||
| AS duration | 0.990 | (0.969, 1.011) | 0.342 | 0.983 | (0.965, 1.002) | 0.072 | |||
| Sex (male) | 0.939 | (0.658, 1.34) | 0.728 | 0.929 | (0.656, 1.314) | 0.676 | |||
| Biologics | 0.678 | (0.490, 0.937) | 0.019 | 0.719 | (0.511, 1.012) | 0.058 | 0.687 | (0.489, 0.964) | 0.030 |
| CRP | 1.010 | (1.0037, 1.016) | 0.002 | 1.010 | (1.002, 1.019) | 0.020 | 1.010 | (1.003, 1.016) | 0.003 |
| Damaged joints (Y/N) | 0.666 | (0.423, 1.050) | 0.080 | 0.614 | (0.382, 0.988) | 0.044 | |||
| ESR | 1.008 | (0.9996, 1.017) | 0.061 | 1.000 | (0.988, 1.012) | 0.957 | |||
| UV duration | 0.981 | (0.960, 1.0029) | 0.089 | 0.982 | (0.960, 1.005) | 0.130 | |||
Univariate and multivariate analysis of the relevant characteristics of the PsA cohort with uveitis.
| PsA | Univariate | Multivariate full model | Multivariate reduced model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariates | HR | CL | p-Value | HR | CL | p-Value | HR | CL | p-Value |
| Clinical model | |||||||||
| Age at baseline | 0.982 | (0.941, 1.024) | 0.388 | 0.997 | (0.960, 1.035) | 0.866 | |||
| PsA duration | 1.003 | (0.942, 1.067) | 0.936 | 1.007 | (0.950, 1.068) | 0.806 | |||
| Sex (male) | 1.516 | (0.604, 3.809) | 0.376 | 1.372 | (0.600, 3.139) | 0.454 | |||
| HLA-B27+ | 3.158 | (1.375, 7.253) | 0.007 | 5.311 | (1.847, 15.273) | 0.002 | 4.216 | (1.690, 10.515) | 0.002 |
| Biologics | 2.205 | (0.949, 5.119) | 0.066 | 1.301 | (0.423, 4.001) | 0.647 | |||
| BMI | 0.922 | (0.843, 1.0095) | 0.079 | 0.955 | (0.862, 1.058) | 0.375 | |||
| DMARDS | 0.252 | (0.0925, 0.684) | 0.007 | 0.559 | (0.167, 1.868) | 0.344 | 0.324 | (0.116, 0.903) | 0.031 |
| Nail lesions | 0.295 | (0.0990, 0.878) | 0.028 | 0.331 | (0.0826, 1.324) | 0.118 | |||
| Smoking | 0.431 | (0.182, 1.023) | 0.056 | 0.206 | (0.0595, 0.715) | 0.013 | 0.262 | (0.0954, 0.721) | 0.009 |
The PsA patients had more peripheral active disease, therefore, were taking more NSAIDs, and cDMARDs, compared to the SpA group, that had more axial involvement. The chi-squared analysis revealed no difference in uveitis between peripheral and non-peripheral disease in AS, and patients without peripheral disease were less likely to have uveitis.
DiscussionIn our cohort of patients, uveitis was more common in the axSpA than in the PsA population, with a prevalence of 30% in axSpA vs. 7.6% in PsA. In general, both cohorts had higher proportion of men, and there was no major difference in age and ethnicity (most of the patients were caucasian and over the age of 35).
When looking at the patients with uveitis diagnosis, we found that the main risk factors for flare were having an elevated CRP (HR 1.009, p=0.004) in the axSpA group, and even though HLA-B*27 was more common in the axSpA population, it was a risk factor for uveitis in the PsA population when present.
It seems that biologics were protective for uveitis flare in patients with axSpA – perhaps in this patient population with active axial disease, biologics work for uveitis as well. Since most axSpA patients are not treated with conventional DMARDs there is no way for us to know whether they would be protective. On the other hand, the PsA patients that have been treated with conventional DMARDs showed significant protective effect over uveitis in the multivariate analysis. Those PsA patients treated with biologics seem to have more severe disease, that could constitute a risk factor for uveitis flare, but despite a HR>1, both multivariate analyses showed no significance at the end. We found that patients without peripheral disease are less likely to have uveitis, that could indicate a less severe disease and less EMM.
For the future, we believe a detailed analysis of the type of conventional and biologic DMARDs used in our population with uveitis would be helpful to elucidate if there is correlation with the type mechanism of action and the uveitis protection found this time.
FundingKrembil Research Institute. Centre for Prognosis Studies in the Rheumatic Diseases, Toronto Western Hospital.
Conflict of interestThe authors declare they have no conflict of interest.