Primary Sjögren's syndrome (pSS) is an autoimmune disease that can affect quality of life, cause disability, including progression to systemic complications in patients. In order to evaluate these components, several clinimetric scales have been used in pSS.
MethodsIn order to describe the most commonly used clinimetric scales in pSS, a systematic search of articles was carried out using Google Scholar, Scielo, Embase, Academic Search Ultimate, and Medline databases. Pubmed was used for the search in Medline, with the MeSH terms: ‘Clinimetry’; ‘Clinimetrics’; ‘Quality of life’; ‘Activity Index’; ‘Scales’; ‘Sjögren's syndrome’; linked with the Boolean connector AND. A total of 1081 articles published up to May 2018 were reduced to the 51 of the most relevant after application of inclusion criteria.
ResultsThe most commonly used clinimetric scales in the evaluation of systemic involvement and quality of life in patients with pSS are described.
ConclusionClinimetric methods are very useful from the point of view of follow-up, evaluation of response to treatment, perception of the disease by patients, and objective evaluation of clinical trials in pSS.
El síndrome de Sjögren primario (SSp) es una enfermedad autoinmune, cuyo compromiso se puede ver reflejado en la calidad de vida, incapacidad y progresión a complicaciones en los pacientes. Con el fin de evaluar estos componentes, diversas escalas clinimétricas se han utilizado en el SSp.
MétodosPara describir las escalas de clinimetría más utilizadas en el SSp, se realizó una búsqueda de artículos en las bases de datos Google Scholar, Scielo, Embase, Academic Search Ultimate y Medline. Se empleó Pubmed para la búsqueda en Medline, con los términos MeSH: «Clinimetry»; «Clinimetrics»; «Quality of life»; «Activity index»; «Scales»; «Sjögren's syndrome»; enlazados con el conector booleano AND. Se incluyeron 1.081 artículos publicados hasta mayo de 2018, que luego de la aplicación de los criterios de inclusión, se redujeron a 51 con la mayor relevancia.
ResultadosSe describen las escalas de clinimetría más usadas en la evaluación del compromiso sistémico y de la calidad de vida en los pacientes con SSp.
ConclusiónLos métodos clinimétricos tienen gran utilidad desde el punto de vista de seguimiento, evaluación de respuesta a tratamiento, percepción de la enfermedad por parte de los pacientes y evaluación objetiva de ensayos clínicos en el SSp.
Primary Sjögren's syndrome (pSS) is a systemic autoimmune disease, characterized by involvement mainly of the salivary and lacrimal glands, induced by lymphocytic and neuroendocrine inflammatory mechanisms, which leads to dysfunction and destruction of glandular tissue, resulting in the presentation of dry symptoms.1 However, this inflammatory process can occur in other organs, such as the kidneys, the lungs, the brain and the skin, among others. This syndrome is one of the most prevalent rheumatologic diseases, with a global incidence of 60.8 per 100,000 inhabitants.2 There are 2 forms of the disease, the pSS and the secondary. In the secondary Sjögren's syndrome, another disease coexists with it, especially systemic lupus erythematosus (SLE) (15–36%), rheumatoid arthritis (20–32%) and systemic sclerosis (20–32%), among others.3,4
The main symptoms, which are found in approximately 80% of patients, are: on the one hand, exocrinopathy of lacrimal and salivary glands, which results in dry eye and dry mouth; and on the other hand, fatigue and pain, which are extraglandular manifestations of the disease that have a great impact on the patient's quality of life, as it has been described in the literature that fatigue can become disabling and is associated with loss or decrease of labor productivity.5 Clinically, the characteristics of the pSS can be divided into 2 groups: (1) the benign but disabling symptoms perceived by the patient, such as dryness, pain and fatigue; (2) systemic manifestations, which are potentially severe and affect 30–40% of patients.4
For all the above, it is necessary that the clinician objectively evaluate the involvement of the pathology. Clinimetrics, a basic branch of medicine which, through scales, is responsible for the identification, specification and measurement of the human clinical phenomenon, has been created for this purpose.6,7 In this way, it is possible to evaluate the disease activity, the response to treatment and the perception of the disease in patients with pSS.8,9 Given that the spectrum of affectation of pSS is broad, clinimetric methods that allow to address objectively each aspect have been created since 1996.10,11
This review aims to make a description of the most used instruments for the evaluation of the pSS in clinical practice, both of the activity and severity of the pathology and of the quality of life.
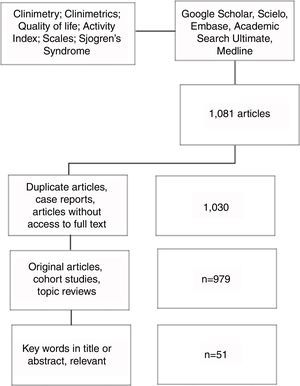
MethodsLiterature searchA search of articles was carried out in Google Scholar, Scielo, Embase, Academic Search Ultimate and Medline databases. Pubmed was used for the search in Medline, with the MeSH terms: «Clinimetry»; «Clinimetrics»; «Quality of life»; «Activity index»; «Scales»; «Sjögren's syndrome»; linked with the Boolean connector AND (Fig. 1).
Article selection and information extractionIn the initial search 1081 articles were found, which were entered into an Excel database. After excluding duplicate articles and selecting those that met the inclusion criteria and that included the keywords in the title or in the abstract, 51 articles that were reviewed by 2 authors were considered relevant. The access to these articles was obtained with the help of the libraries of our University and University Hospital.
Inclusion criteriaOriginal articles, cohort studies, topic reviews; published until 2018; written in English or Spanish.
Exclusion criteriaCase reports; articles without access to full text.
Results51 articles concerning Sjögren's syndrome, clinimetric instruments and their use or applicability in this disease were selected; from these, the following review is carried out.
Instruments that assess clinical/systemic commitmentSSDDI, SSDAI and SSDIIn 2007, 2 indexes were created: the Index of Disease Damage of the pSS (Sjögren's Syndrome Disease Damage Index [SSDDI]) and the Index of Disease Activity of the pSS (Sjögren's Syndrome Disease Activity Index [SSDAI]). For this purpose, data were collected in 12 clinical centers in Italy, from 2004 to 2006. The patients were analyzed to select the individual clinical variables and the combination of some of them that represent the most valid predictors of damage and disease activity. Each symptom, sign and test was precisely defined, as specified by the American College of Rheumatology.10,12
The SSDDI was constructed using variables selected through a multivariate linear regression model, where the score assigned to each element was derived from the weight that the corresponding variable had in the model. The maximum score of 16 points consists of 9 items that are taken into account as predictors of the level of damage of the disease.
The SSDAI consists of a multivariate linear regression model with 11 variables, where the dependent variable was the numerical score for the disease activity (0–10) assigned by the researcher, derived from the weight that the corresponding variable had in the model.
Finally, in the study, the variables of the SSDDI were not or were weakly correlated with the gold standard (Physician's global assessment [PhGA] of the disease status) for the disease activity, while the SSDAI correlated. A score ≥5 points in the SSDAI showed a sensitivity of 86.5% and a specificity of 87.6% to identify patients with active or very active disease, who would be candidates for a more intensive pharmacological therapy.
The geographic limitation (only one country) of the development of the SSDDI and the SSDAI raised doubts about their validity.10 These, like others that were developed later, used tools already validated for the evaluation of activity in other autoimmune diseases (mainly for SLE) based on the European Consensus Lupus Activity Measurement (ECLAM),13 given the systemic commitment observed in both of them. The last measure, however, presents marked disadvantages with respect to the SSDAI, since the characteristics score whether they are present, but it does not establish any distinction between what worsens, improves or is maintained; limiting its application in clinical practice.14
Subsequently, the Sjögren's Syndrome Damage Index (SSDI) was developed in 2008 to assess the longitudinal damage in patients with pSS, using variables similar to the cohort that was used for the SSDAI and the SSDDI. A total of 104 patients from 8 hospitals of the United Kingdom, who met the criteria of the American-European Consensus Group (AECG) of 2002 for pSS,12,15 were assessed by rheumatologists in the first visit and at 12 months. In each occasion, the data regarding damage, disease activity, in addition to biochemical, hematological and immunological tests (Anti-SSA/Ro and Anti-SSB/La) were collected. For this process, the questionnaires SF-36, Sjögren's Systemic Clinical Activity Index (SCAI) and the Profile of Fatigue and Discomfort-Sicca Symptoms Inventory (PROFAD-SSI) were completed.16
The index provided an objective and adequate evaluation of the progression of the disease, for the use either by personnel specialized or not. For example, and advantage of the SSDI over the SSDDI is that it recognizes cardiovascular, gastrointestinal and musculoskeletal diseases, with a methodology based on the damage indexes for SLE (SLICC/ACR).17 This tool consists of 10 domains with a maximum score of 27 points, which is taken into account as a predictor of the level of disease damage in the study population.
The SSDDI and the SSDI are chronicity indexes that, although can be used in clinical practice, they have limitations such as low external validity and cross-validation; since they are indexes that were developed in cohorts of a single nationality, and therefore they may not completely cover the broad spectrum of the pSS.18 In addition, despite the SSDAI had the advantage of being very simplified, the lack of exhaustiveness caused patients with certain degrees of disease activity to be underestimated.19
SCAIPreviously there were no tools to evaluate the systemic commitment of the pSS, and treatment for severe presentations of the disease was performed empirically with high doses of corticosteroids. Given the need for evaluation tools in clinical trials, the SCAI was created in 2007 using the principles of the BILAG (British Isles Lupus Activity Group) index for SLE, modified for pSS.
This index consists of 8 domains that include: fatigue, constitutional symptoms, arthritis, muscular, cutaneous/vascular, pulmonary, hematologic and glandular inflammation. The majority of items are scored as 0 when it is absent, 1 when there is an improvement, 2 when it remains the same, 3 when it gets worse, or 4 when it appears new, during the last 4 weeks compared with the previous disease activity.
The findings of the study that allowed its implementation suggest that this index is useful in therapeutic studies of pSS.20 However, being extremely exhaustive, it is difficult to apply in daily clinical practice.19
ESSDAIThe ESSDAI (EULAR Sjögren's Syndrome Disease Activity Index) was developed in 2009 by the consensus of a group of European and North American experts, supported by the EULAR (European League Against Rheumatism),21 with the purpose of obtaining a standardized instrument for the evaluation of the systemic disease activity, both in clinical practice and in clinical trials. This index has been validated in multiple studies, being confirmed that it is reproducible.22–24 Its use is increasingly prevalent, to the point that it is currently considered the gold standard for measuring the disease activity. In addition, it has been observed correlation with biomarkers of activity of B lymphocytes,22 as well as with the risk of lymphoma.25,26 Indeed, the decisions regarding the follow-up, treatment and prognosis of patients with a greater degree of disease activity at the time of diagnosis of the pSS are based on the ESSDAI,26 since its wide range of values allows to discriminate between patients with active and inactive disease.27
The ESSDAI includes 12 domains, which make reference to the systems affected by the disease (cutaneous, respiratory, renal, articular, muscular, central nervous, peripheral nervous, hematological, glandular, constitutional, lymphatic and biological) and each is divided into 3 or 4 degrees of activity.28 To ensure a correct score, another pathology different from pSS should be ruled out as the cause of the signs and symptoms, and the characteristics of damage that are irreversible (present for 12 months or more) should also be excluded.
The follow-up of patients with pSS who are clinically stable has a limited clinical value with this tool.29 However, its cumulative use has been described, that is, when applied in these patients, the clinical value is added when describing the severity of the disease and it is calculated by adding the maximum score per domain that the individual has reached at any time of his/her life.29
Although it has been seen that the biological domain is affected in two thirds of patients24,26,30 and that the high scores on the scale correlate with this domain and not with the other manifestations of activity, this falsely induces the association between these 2 aspects. For this reason, in 2017 was created the ClinESSDAI, in which the biological domain was eliminated,31 and it was determined that it is capable of detecting the changes with a sensitivity close to that of the ESSDAI, and therefore it provides an accurate evaluation of the disease activity independent of the biomarkers of activation of the B lymphocytes.31,32
Instruments that evaluate the symptoms perceived by the patientPhGAPhGA is a scoring system performed by the specialist to compile data on the clinical results and estimate the severity of the disease.33 It is part of the basic set of measures of the disease activity of the American College of Rheumatology, including both the Patient Global Assessment (PGA/PtGA) and the PhGA.34
In clinical studies, the physicians evaluate the patients using the PhGA in 2 scales: one numerical and other nominal. For the first, each patient receives a score for the damage and disease activity from 0 (no damage or activity) to 10 (maximum damage or disease activity). For the second, the change in disease activity is evaluated, answering questions such as: «Compared with the previous visit, how is the activity of the pSS of this patient now?». The answers are classified into 5 categories (inactive disease, low, moderate, high and very high activity) and the evaluation process is carried out in at least 2 visits at 3 or 6 months.10,24,21
It has been evidenced that the opinion of the physician regarding the disease state may differ from the opinion of the patient,35,36 leading to a low correlation between PhGA and PGA, where it becomes apparent that the variables that are important for the physicians are not the same as those that are important for the patients.37
Assuming that PhGA or PGA can represent the “gold standard” for assessing an aspect of the disease, such as damage or activity, can introduce several methodological biases since, regardless of how the questions are formulated, it always implicitly incorporates his/her own opinion in the evaluation of the systemic activity and the symptoms of the patient.38
PGAEULAR constantly insists on the need to work together with patients, particularly in rheumatic diseases;39 this is why PGA arises as a measure to reduce bias in scales that are only evaluated by clinicians.40
As with the PhGA, the PGA is assessed by means of numerical scales of 0–10 points or with visual analog scales where higher results indicate a worse disease status. However, other PGA formulations that are frequently used in the consultation are: “How do you calculate the activity of your disease today?” and are classified and evaluated in the same way as the PhGA.36
Different studies have compared the effectiveness of these evaluations in diseases other than pSS, finding significant discrepancies in the variables considered to establish the overall evolution of the disease by patients and physicians41–43; however, the evidence in pSS is limited.
For example, patients with prolonged disability may report a good quality of life (despite what may seem externally paradoxical) due to factors such as adaptability to disability, which contributes to the physician-patient discordance.36
PROFAD-SSIFatigue in pSS is considered a highly disabling symptom, present in up to 75% of patients and with the ability to substantially affect the quality of life.44 Therefore, the Profile of Fatigue and Discomfort – Sicca Symptoms Inventory (PROFAD-SSI) and the most recent: EULARSjögren's Syndrome Patients Reported Index (ESSPRI) were created.45,46
In 2004 was published the PROFAD-SSI; for which data were collected of patients with a diagnosis of pSS who met the AECG classification criteria,12 recruited from different rheumatology clinics in the United Kingdom.47 It was created as a self-report of the patients about their symptoms of fatigue, discomfort and pain.48,49
This score consists of 64 questions scored on an 8-point Likert scale (0–7). Its disadvantage is that being so long it becomes difficult for patients, particularly when used as an evaluation of results in clinical trials. For this reason, the PROFAD-SSI-SF (SF: short form), which consists of 19 questions grouped into the same 8 domains with a score for the PROFAD and another for the SSI was developed.18 Thus, the PROFAD has 9 elements divided into 4 domains: physical fatigue, mental fatigue, arthralgia and vascular; and the SSI has 10 elements divided into 4 domains: ocular, oral, vaginal and cutaneous dryness. Its advantage is the distinction between physical and mental fatigue, as well as of the sites of dryness, which not always appear concomitantly and with the same intensity.49 When used together in several studies, a profile that captures the symptomatic component of the pSS is established.14
ESSPRIThe EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) was developed in 2011 with a multinational collaboration of patients from countries of Europe, North America and South America.47 To carry out the study, the PGA, the gold standard at this time, was used as comparator index. It was developed with the purpose of evaluating the characteristics of dryness, fatigue and pain that affect the quality of life; and to be used both in clinical trials and in routine clinical practice.
It consists of 3 domains: dryness, pain or discomfort and fatigue. Dryness includes 6 components that afflict the patient with pSS: ocular, oral, cutaneous, nasal, tracheal and vaginal. Each one is rated from 0 to 10, according to severity.47
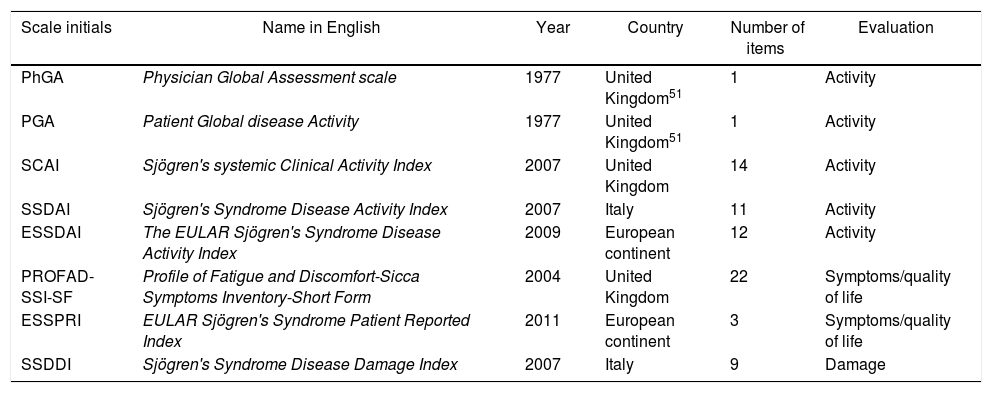
Compared with previous clinimetric methods, ESSPRI is a short and easy to use tool that allows measuring the severity of each symptom without losing the validity. In addition, it takes into account the sicca features in order of importance since in their study oral and ocular dryness are more important than nasal, tracheal or vaginal.47,50 It has a sensitivity to detect changes much higher than other questionnaires on quality of life, such as SSI and PROFAD. Table 1 shows the clinimetric tools most commonly used in pSS and the items that each one evaluates. Table 2 summarizes the scales.
Items evaluated by the clinimetric tools in pSS and how they are scored.
| SSDDI | SSDAI | SSDI | PROFAD-SSI-SF | ESSDAI | ESSPRI | |
|---|---|---|---|---|---|---|
| EVALUATED DOMAINS | Lymphoproliferative disease | Constitutional symptoms | Ocular domain | Need for rest | Constitutional domain | Dryness |
| Renal damage | Articular symptoms | Oral domain | Difficulty to start an activity | Domain of lymphadenopathy and lymphoma | Fatigue | |
| Ocular damage | Hematological characteristics | Systemic domain | Difficulty to continue an activity | Glandular domain | Pain | |
| Oral/salivary damage | Pleuropulmonary symptoms | Difficulties due to lack of muscle strength or feeling or weakness | Articular domain | |||
| Lung damage | Leukopenia/lymphopenia | Difficulties to think clearly or to concentrate | Cutaneous domain | |||
| Neurological damage | Vasculitis changes | Difficulties due to forgetting things of making mistakes | Lung domain | |||
| Active renal involvement | Limbs discomfort | Renal domain | ||||
| Peripheral neuropathy | Discomfort in fingers or wrists | Muscular domain | ||||
| Discomfort due to cold hands | Domain of the peripheral nervous system | |||||
| Discomfort due to dry skin | Domain of the central nervous system | |||||
| Difficulties due to vaginal dryness | Hematological domain | |||||
| Difficulties due to sensitive eyes | Biological domain | |||||
| Difficulties due to irritable eyes | ||||||
| Visual discomfort | ||||||
| Feeding difficulties | ||||||
| Discomfort due to dry throat or nose | ||||||
| Discomfort due to bad breath | ||||||
| Need for liquids to moisten the mouth | ||||||
| Discomfort from other problems | ||||||
| SCORE | It consists of 6 domains and 9 items distributed among them. Each item has a specific score of 1, 2 or 5 according to the corresponding weight. | It consists of 8 domains and 11 items distributed among them. Each item has a specific score of 1, 2, 3 or 4 according to the corresponding weight. | It consists of 27 items that are scored as 0 if it is absent or 1 if it is present. | Each question is scored from 1 to 10, the result is an average. | Each domain has different scores depending on the degree of activity. In turn, each domain has a specific weight. The result is obtained by multiplying the degree of activity by the weight of each domain. | Each question is scored from 1 to 10, the result is an average. |
ESSDAI: EULARSjögren's Syndrome Disease Activity Index; ESSPRI: Sjögren's Syndrome Patient Reported Index; EULAR: European League Against Rheumatism; PROFAD-SSI-SF: Profile of Fatigue and Discomfort-Sicca Symptoms Inventory Short Form; SSDAI: Sjögren's Syndrome Disease Activity Index; SSDDI: Sjögren's Syndrome Disease Damage Index; SSDI: Sjögren's Syndrome Damage Index; pSS: Primary Sjögren's syndrome.
Clinimetric tools in pSS.
| Scale initials | Name in English | Year | Country | Number of items | Evaluation |
|---|---|---|---|---|---|
| PhGA | Physician Global Assessment scale | 1977 | United Kingdom51 | 1 | Activity |
| PGA | Patient Global disease Activity | 1977 | United Kingdom51 | 1 | Activity |
| SCAI | Sjögren's systemic Clinical Activity Index | 2007 | United Kingdom | 14 | Activity |
| SSDAI | Sjögren's Syndrome Disease Activity Index | 2007 | Italy | 11 | Activity |
| ESSDAI | The EULAR Sjögren's Syndrome Disease Activity Index | 2009 | European continent | 12 | Activity |
| PROFAD-SSI-SF | Profile of Fatigue and Discomfort-Sicca Symptoms Inventory-Short Form | 2004 | United Kingdom | 22 | Symptoms/quality of life |
| ESSPRI | EULAR Sjögren's Syndrome Patient Reported Index | 2011 | European continent | 3 | Symptoms/quality of life |
| SSDDI | Sjögren's Syndrome Disease Damage Index | 2007 | Italy | 9 | Damage |
EULAR: European League Against Rheumatism; pSS: primary Sjögren's syndrome.
For several years work has been done to develop clinimetric tools that allow to make a global assessment of patients with pSS regarding their pathology and that at the same time are valid, replicable and easy to perform. Within these approaches, the clinimetric methods for pSS have been divided into 2 large groups: those that evaluate the clinical component of the disease as damage or activity and those that evaluate the quality of life of the patient, which implies the use of at least 2 questionnaires for the comprehensive evaluation of the patient in medical controls and in research studies.
However, in clinical practice one of the big limitations for the regular use of these scales is the time it takes their completion, as in the case of the SCAI,19,20 for this reason, many of them have been used only in the research context.
From a practical point of view, the main interest of the clinician during the follow-up of patients with pSS should be the objective evaluation of the disease activity. For this, the ESSDAI behaves as a rigorous tool, which covers the manifestations of the disease in a systemic and detailed way by subdividing each domain evaluated in different degrees of activity, which makes the result for each individual a true reflection of the behavior of the disease at the time of assessment.28 Although it is reliable, it is extensive and the qualification of each item is subject to a series of conditions that must be taken into account,22 in order to facilitate its application in the clinic and without the need for laboratory results, the ClinESSDAI modification, which allows to obtain interpretations similar to the ESSDAI in relation to the disease activity was made.31 The importance of the ESSDAI in clinical practice has led to include in research studies aimed at finding effective treatments for pSS, only patients with ESSDAI above 5 and below 14, indicating an active disease.32
On the other hand, it is common in clinical practice that there is a discrepancy between the objective findings, given by the commitment of organs or immunological findings (complement consumption, lymphopenia, among others) and the symptoms expressed by the patient, where fatigue can be one of the main complaints. Given this aspect, it is essential to evaluate at the same time the impact of the disease on the quality of life of the patient, being the ESSPRI a questionnaire that is easy to apply in the medical consultation and that facilitates a specific focus on aspects of the pSS (fatigue, dryness and pain),47 unlike others such as PROFAD, in which there are questions that could be attributed to causes other than this syndrome.18
In this regard, for an objective assessment, the ESSDAI is a very useful scale; while from the subjective point of view, the ESSPRI, self-applied by the patient, is a very useful tool due to its easy completion and the information of interest on the aspects to be improved with medical interventions.
ConclusionIt is important to know the usefulness of these tools and the need to reduce the limitations of each one, since their application facilitates and guarantees the comprehensive study of patients with pSS.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Posso-Osorio I, Méndez-Rayo T, Soto D, Nieto-Aristizábal I, Cañas CA, Tobón GJ, et al. Clinimetría en el síndrome de Sjögren. Rev Colomb Reumatol. 2019;26:262–269.