Dyslipidaemia is the most prevalent metabolic disorder in primary Sjögren's syndrome (PSS) and an association between low HDL cholesterol levels and disease activity has been suggested.
ObjectivesThe purpose of this study is to describe the characteristics of the lipid profile in patients with PSS and explore the correlation between the components of the lipid profile and the activity of the disease.
Materials and methodsA descriptive cross-sectional study. We reviewed the medical records of patients over 18 years of age with criteria for PSS who attended the Hospital Universitario Clínica San Rafael during the period between January 2015 to December 2019. We used R-studio software version 4.0.2 for statistical analysis. A descriptive analysis of the clinical-demographic and serological variables was carried out to evaluate the correlation between them.
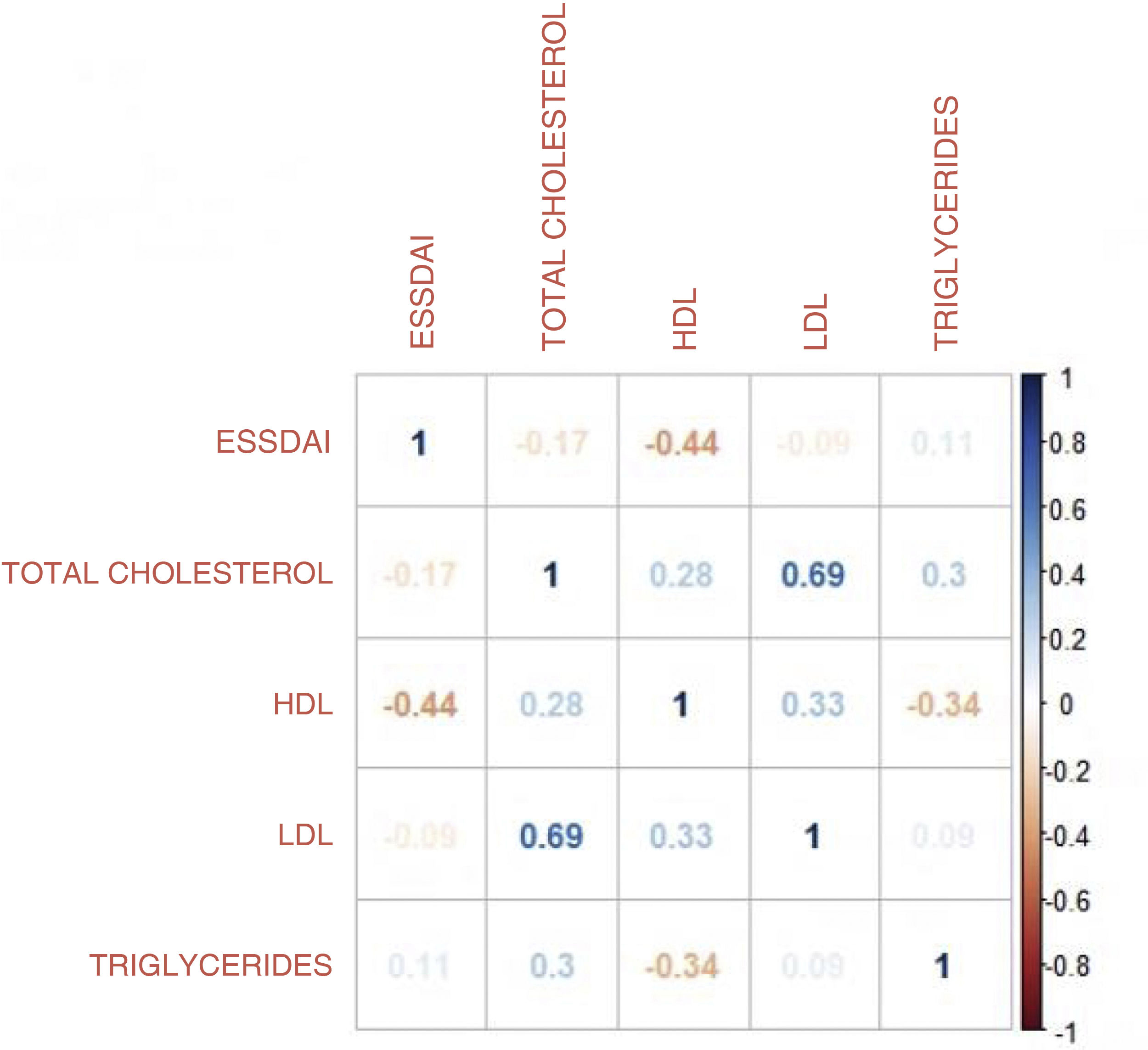
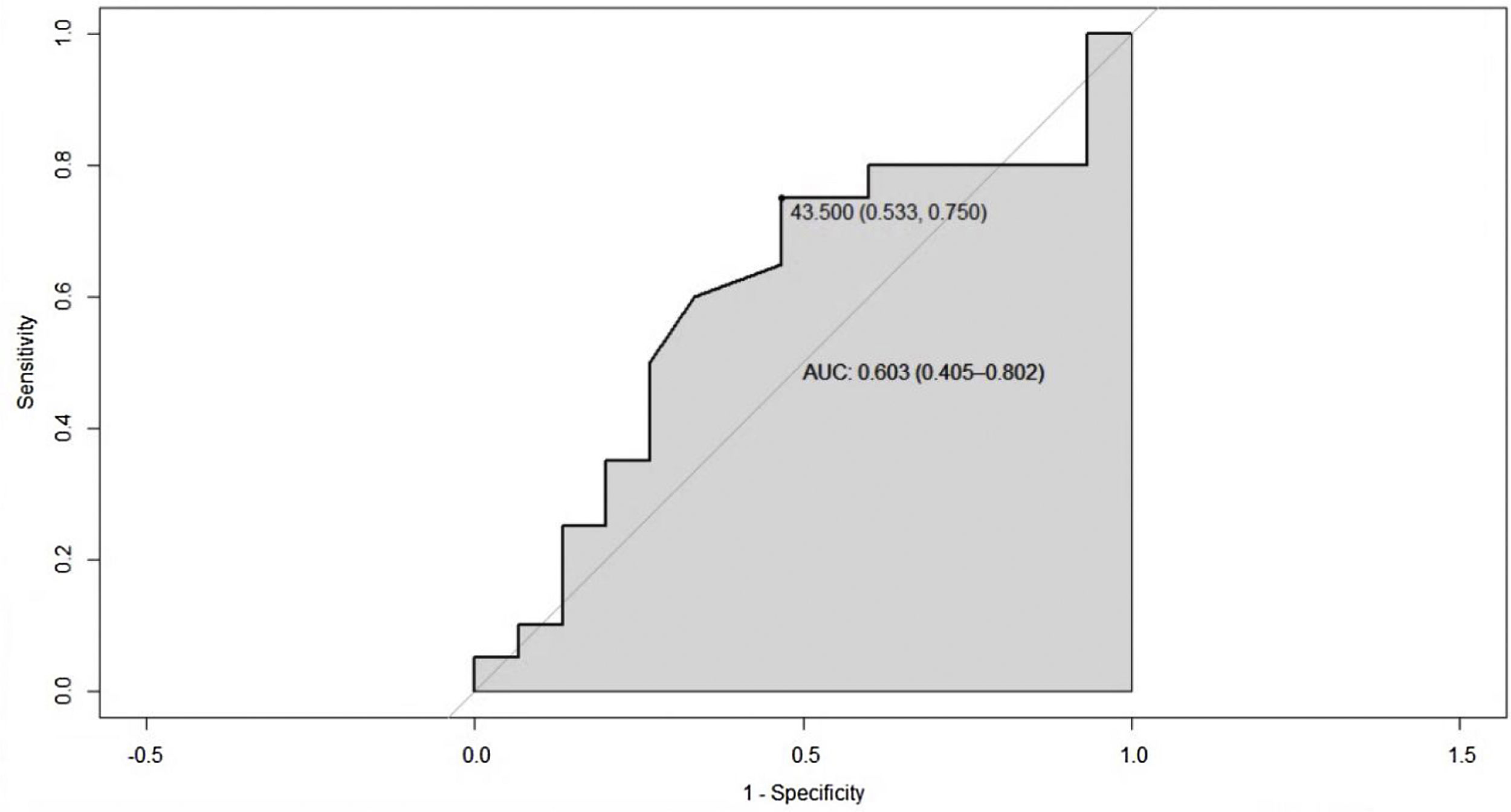
ResultsA total of 250 medical records were reviewed, of which 35 met the inclusion criteria. The average age was 53.4 years and 88.3% were women. The median duration of disease was 42 months. The mean values for total cholesterol, HDL, LDL and triglycerides were 191 mg/dL, 42.6 mg/dL, 118.9 mg/dL and 157 mg/dL respectively. A Pearson correlation coefficient of −0.43 (95% CI −0.67 to −0.12) p-value = 0.008 was found between the ESSDAI activity index and HDL cholesterol. A linear model was performed between the total ESSDAI activity index and HDL cholesterol, finding an estimated coefficient of −0.17. A ROC curve was performed with an HDL cholesterol segregation point of 43.5 mg/dL with an area under the curve of 0.603 (95% CI 0.40–0.80). By excluding patients with high BMI, the area under the curve improved with a segregation point of 38 mg/dL.
ConclusionsPatients with low levels of HDL cholesterol showed higher rates of disease activity, with a cut-off point lower than 43 mg/dL being more marked in patients with normal body mass index.
La dislipidemia es la alteración metabólica más prevalente en el síndrome de Sjögren primario (SSP), se sugiere una asociación entre niveles bajos de colesterol HDL y la actividad de la enfermedad.
ObjetivosEl propósito de este estudio es describir las características del perfil lipídico y explorar la correlación entre sus componentes y la actividad del SSP.
Materiales y métodosEstudio descriptivo de corte trasversal, se revisaron las historias clínicas de pacientes mayores de 18 años que asistieron al Hospital Universitario Clínica San Rafael con criterios clasificatorios para SSP durante el periodo de enero del 2015 a diciembre del 2019. Para el análisis estadístico se utilizó el software R studio versión 4.0.2. Se efectuó un análisis descriptivo de las variables clínico-demográficas y serológicas para evaluar la correlación entre ellas.
ResultadosSe revisaron en total 250 historias clínicas, de las cuales 35 cumplían con los criterios de inclusión. La edad promedio fue 53,4 años; el 88,3% de la población fueron mujeres. La mediana del tiempo de enfermedad fue de 42 meses. Las medias de colesterol total, HDL, LDL y triglicéridos fueron de 191 mg/dl, 42,6 mg/dl, 118,9 mg/dl y 157 mg/dl, respectivamente. Se encontró un coeficiente de correlación de Pearson entre el índice de actividad ESSDAI y el colesterol HDL de −0,43 (IC 95% −0,67–0,12), p valor = 0,008. Se realizó un modelo lineal entre el índice de actividad ESSDAI total y el colesterol HDL, y como resultado se halló un coeficiente estimado de −0,17. La curva ROC, con un punto de segregación de colesterol HDL de 43,5 mg/dl, mostró un área bajo la curva de 0,603 (IC 95% 0,40–0,80). Al excluir los pacientes con IMC alto, el área bajo la curva mejoró, con un punto de segregación de 38 mg/dl.
ConclusionesLos pacientes con niveles bajos de colesterol HDL mostraron mayores índices de actividad de la enfermedad, con un punto de corte menor a 43 mg/dl, siendo más marcado en pacientes con índice de masa corporal normal.
Primary Sjögren's syndrome (PSS) is a chronic systemic autoimmune disease, not associated with another rheumatological process, characterized by a lymphocytic inflammatory infiltrate in the secretory glands, which leads to Sicca syndrome (dry eyes, mouth, pharynx, larynx and/or vagina).1 Extraglandular manifestations have been described in up to 50% of patients, mainly involving the musculoskeletal, neurological, respiratory, and cutaneous systems, as well as metabolic alterations with a higher prevalence of diabetes, hyperuricemia, and dyslipidemia2,3; the latter is the most frequent, with an odds ratio (OR) of 1.42 with respect to the population without a diagnosis of PSS.4,5
So far, the studies of association between dyslipidemia and PSS have made evident an increased cardiovascular risk in this group of patients; however, in recent years the relationship between PSS and high density cholesterol (HDL) has gained importance, as the latter is considered a possible factor of disease activity.6,7 In Colombia, we do not have data regarding the metabolic characteristics of the patients with PSS and their relationship with each of the components of the lipid profile and the immunological activity. The main objective of the present study was to describe the characteristics of the lipid profile in a cohort of patients with PSS and to explore the correlation between the components of this profile and the disease activity. We seek that the results of the study can contribute to a better understanding of the systemic commitment of the disease and that new objectives for its control can be established.
Materials and methodsPopulationPatients over 18 years of age with a diagnosis of PSS according to the 2002 American-European classification criteria, who attended the outpatient and/or hospitalization services of the Hospital Universitario Clínica San Rafael, located in Bogotá (Colombia), between January 2015 and December 2019 were included. Patients with clinical or pharmacological conditions that could alter the lipid profile, such as diabetes mellitus, hypothyroidism, pituitary diseases, hepatocellular disease, alcoholism, human immunodeficiency virus, pregnancy, and consumption of drugs such as steroids, thiazides, and antiretrovirals, among others, were excluded.
Type of study and designA cross-sectional descriptive observational study was conducted. A non-probability convenience sampling was chosen.
Data collectionWith prior authorization of the Medical Ethics Committee of the Hospital Universitario Clínica San Rafael, the clinical records were reviewed in the institutional Health On Line (HEON) system. Patients with the diagnosis by the ICD10 code M350 (Sjögren’s syndrome) were selected and the eligibility criteria and the measurement instrument were applied, with the following variables: age, gender, clinical variables (time of evolution of the disease in months, weight, height, body mass index [BMI], xerophtalmia, xerostomia, xeroderma, arthritis, sialadenitis, Raynaud’s phenomenon, kidney involvement, lung involvement, neuropathy, heart involvement, vasculitis) and serological variables (leukopenia, rheumatoid factor, antinuclear antibodies [ANAs] titer and pattern, anti-Ro/SSA, anti-La/SSB, complement, IgG, vitamin D, total cholesterol, HDL cholesterol, low density cholesterol (LDL), triglycerides, and salivary gland biopsy).
The EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) was the standardized and validated instrument that was used to assess the disease activity. Each of the 12 domains of the instrument that are directly related to the systems compromised by the disease was evaluated: constitutional, central nervous system, pulmonary, renal, muscular, peripheral nervous system, biological, hematological, cutaneous, articular, glandular and lymphatic. An activity score was assigned to each domain, according to what was recorded in the clinical history (0 = no activity, 1 = low activity, 2 = moderate activity, 3 = high activity). Finally, a calculation of the global disease activity was performed by adding the results of the 12 domains and establishing the degree of activity (low activity from 1 to 4, moderate activity from 5 to 13 and high activity higher than 13).8,9
Statistical analysisThe RStudio software, version 4.0.2 (RStudio, Inc.) was used for the statistical analysis. A descriptive analysis of the clinical-demographic and serological variables was performed. To evaluate the correlation between quantitative variables, the Pearson correlation coefficient was used, in accordance with the normality of the data in the bivariate analysis. Subsequently, a simple linear model was performed between the variables with significant correlation to evaluate the association with activity by ESSDAI (dependent variable). For the multivariate analysis, the association between ESSDAI and the predictor variables was evaluated; a polynomial generalized linear model with Poisson distribution canonical function 'log' was executed.
Next, the Deviance and Pearson residuals were measured to assess the quality of the model. In order to estimate the cut-off points of the variables with a significant association with respect to the disease activity, ROC curves were created with calculation of the AUC, and sensitivity, specificity, positive predictive value and negative predictive value were calculated in order to assess the accuracy of the test. Finally, to determine the association between the qualitative variables, the Fisher exact test was performed, considering a significant p ≤ 0.05.
Ethical considerationsThis research was classified as without risk, according to Resolution 8430 of 1993 of the Ministry of Health (now of Health and Social Protection), since it is a documentary research that does not intervene or modify the biological or clinical variables of the research subjects; the confidentiality of the information obtained from the clinical records was maintained, according to Resolution 1995 of 1999. Data collection did not begin until approval of the Research Committee of the Hospital Universitario Clínica San Rafael was received.
ResultsA total of 250 clinical records were reviewed in our study, of which 35 met the inclusion criteria. The general characteristics of the population are described in Table 1.
General characteristics of the population.
| (n = 35) | Number (%) | ||
|---|---|---|---|
| Qualitative variables | |||
| Gender | |||
| Female | 31 (88.3%) | ||
| Male | 4 (11.7%) | ||
| Xerophtalmia | 23 (65.7%) | ||
| Xerostomia | 27 (77.1%) | ||
| Xeroderma | 7 (20%) | ||
| Arthritis | 15 (42.9%) | ||
| Sialadenitis | 11 (31.4%) | ||
| Raynaud’s phenomenon | 9 (25.7%) | ||
| Renal involvement | 6 (17.1%) | ||
| Lung involvement | 2 (5.7%) | ||
| Neuropathy | 2 (5.7%) | ||
| Heart involvement | 0 (0%) | ||
| Vasculitis | 2 (5.7%) | ||
| Leukopenia | 4 (11.4%) | ||
| Positive rheumatoid factor | 11 (31.4%) | ||
| Positive ANAs | 22 (62.9%) | ||
| Positive anti-Ro/SSA | 14 (40%) | ||
| Positive anti-La/SSB | 8 (22.9%) | ||
| Cryoglobulinemia | 0 (0%) | ||
| Hypocomplementemia | 7 (20%) | ||
| Polyclonal hypergammaglobulinemia | 7 (20%) | ||
| Salivary gland biopsy | |||
| No report/no biopsy | 22 (62.9%) | ||
| Negative | 6 (17.1%) | ||
| Positive | 7 (20%) | ||
| Quantitative variables | |||
| % | X | IQR | |
| Age | 54 | 48−62 | |
| Time of evolution of the disease (months) | 42 | 15−114 | |
| Total ESSDAI | 2 | 0.1−6.5 | |
| Constitutional | 3 (8.6%) | ||
| Lymphadenopaty | 0 (0%) | ||
| Glandular | 3 (8.6%) | ||
| Articular | 15 (42.9%) | ||
| Cutaneous | 0 (0%) | ||
| Respiratory | 2 (5.7%) | ||
| Biological | 10 (28.6%) | ||
| Renal | 6 (17.1%) | ||
| Muscular | 0 (0%) | ||
| Peripheral nervous system | 2 (5.7%) | ||
| Central nervous system | 0 (0%) | ||
| Hematological | 2 (2%) | ||
| Total cholesterol (mg/dL) | 191 | 167.8−223.5 | |
| HDL cholesterol (mg/dL) | 42 | 34.5−51.5 | |
| LDL cholesterol (mg/dL) | 123,6 | 100.3−141.5 | |
| Triglycerides (mg/dL) | 130,8 | 111−177 | |
| Weight (kg) | 65 | 55.5−71.5 | |
| Height (cm) | 156,5 | 151−160 | |
| Body mass index (BMI) | 26,05 | 22.46−29.16 |
ANA: antinuclear antibodies; ESSDAI: EULAR Sjögren's Syndrome Disease Activity Index.
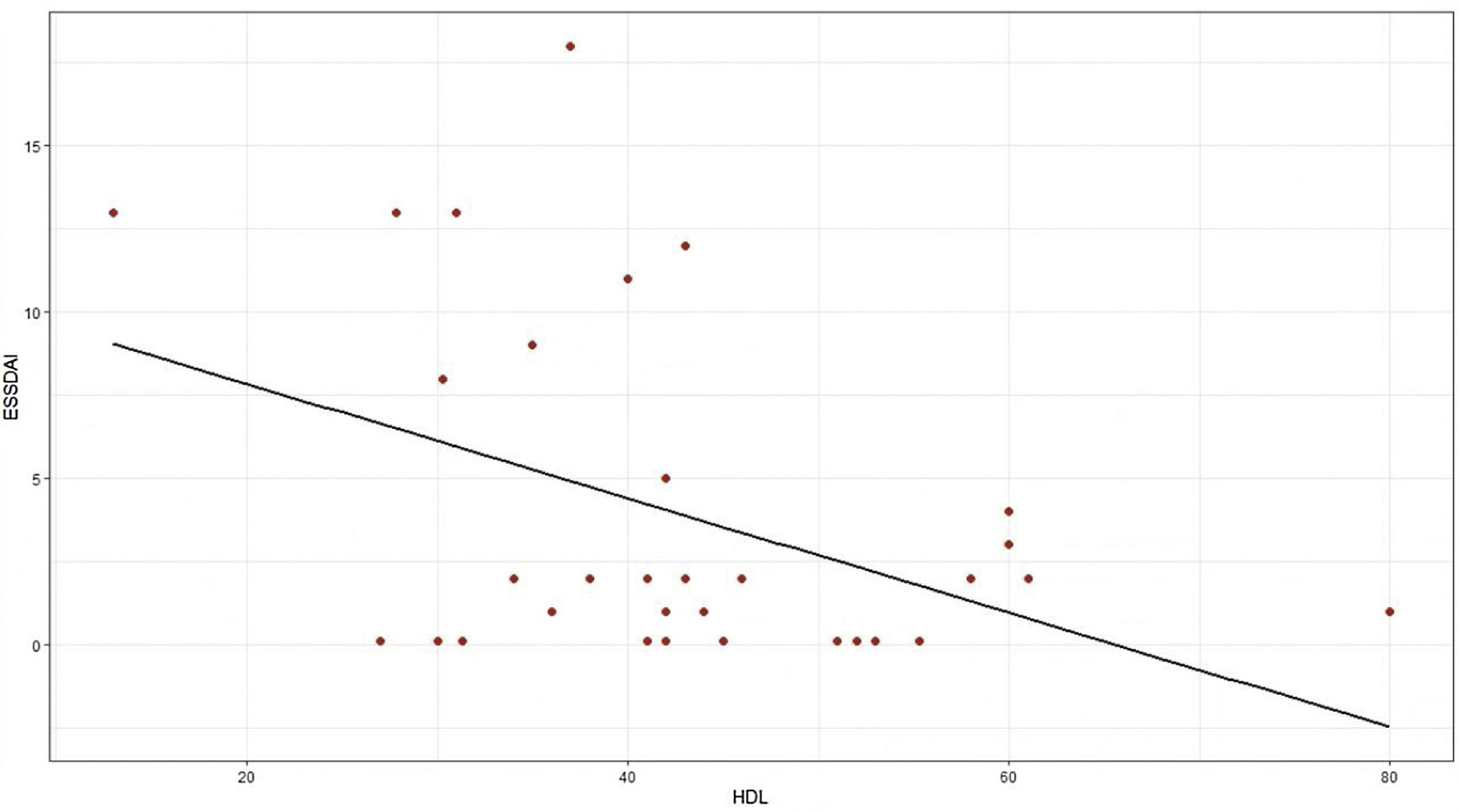
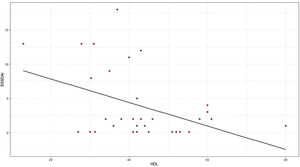
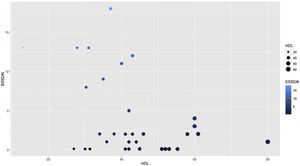
The moderate activity index was 11.5 and no patient presented severe activity. In relation to the BMI, more than 50% had problems of overweight or obesity, with a median of 26 and an interquartile range of 19–38. Pearson’s correlation coefficient between the ESSDAI activity index and HDL cholesterol was −0.43 (95% CI −0.67 to −0.12), p value = 0.008. When exploring the correlation between the ESSDAI activity index and the other components of the lipid profile, no statistical significance was found. Taking the total ESSDAI activity index as the dependent variable and taking into account the significant correlation with HDL cholesterol, a linear model was made between these two variables, and an estimated coefficient of −0.17 was found. (Fig. 1).
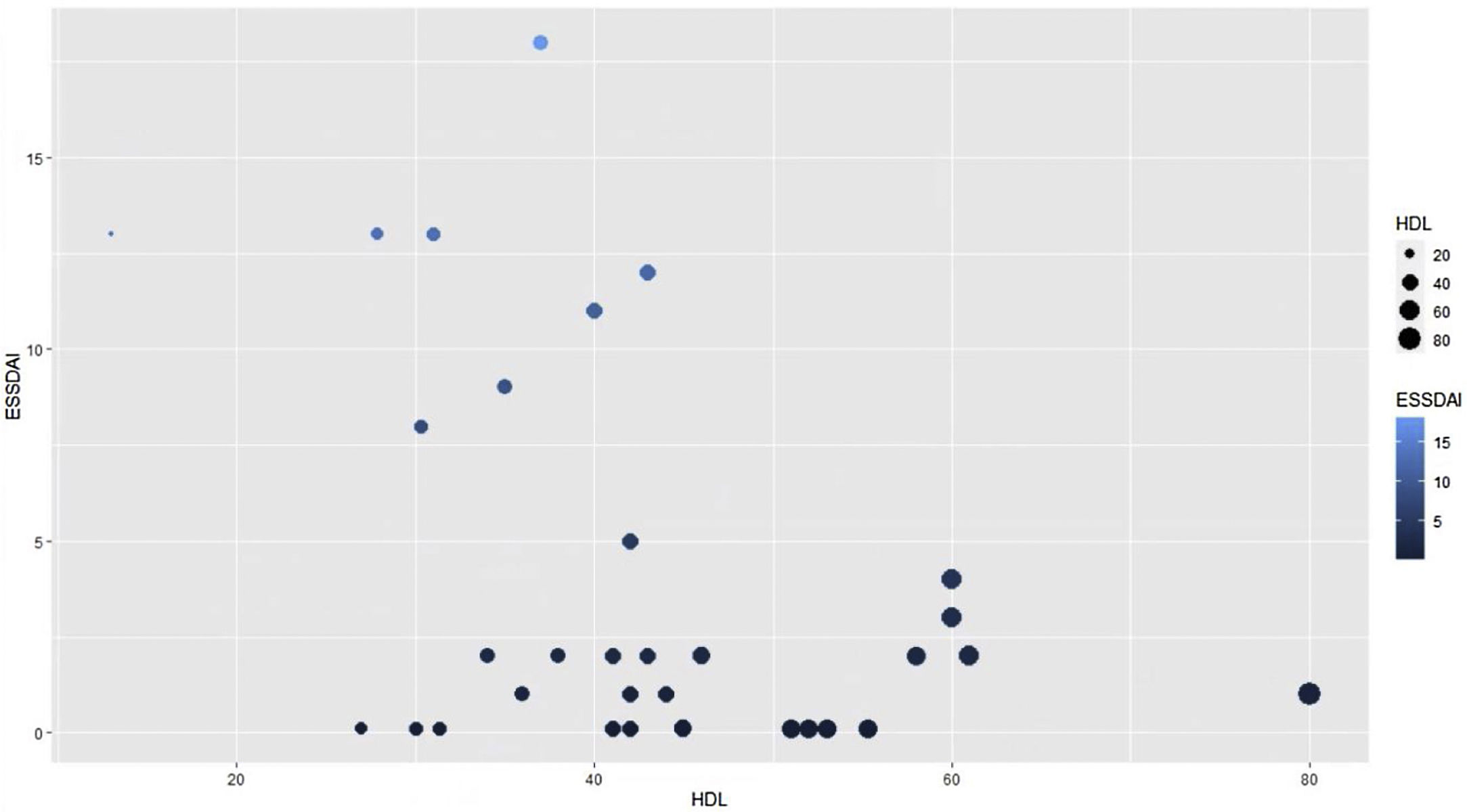
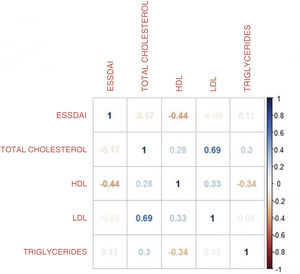
To find the association between the dependent variable ESSDAI, a polynomial generalized linear model was performed with the other components of the lipid profile (total cholesterol, HDL, LDL and triglycerides), using a Poisson distribution function (link = ‘log’), and it was found that HDL cholesterol was preserved as an independent variable with respect to the ESSDAI activity index; in turn, LDL cholesterol also behaved as a predictor variable. The mean of the Deviance residuals was −0.1 and the mean of the Pearson residuals was 0.3. When representing the relationship between the ESSDAI activity index and the HDL cholesterol level, it was observed that the higher the HDL cholesterol values, the lower the disease activity (Figs. 2 and 3).
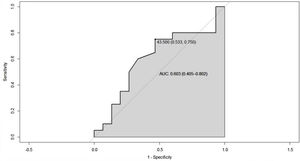
Since the lower the HDL cholesterol value, the higher the disease activity, the total ESSDAI variable was dichotomized into actives and non-actives, with the purpose of establishing a cut-off point for HDL cholesterol that would allow segregating the two states of the disease. In this sense, it was created the ROC curve, in which a segregation point of HDL cholesterol of 43.5 mg/dL was found, with an AUC of 0.603 (95% CI 0.40−0.80), which reflects a moderate discrimination capacity (Fig. 4).
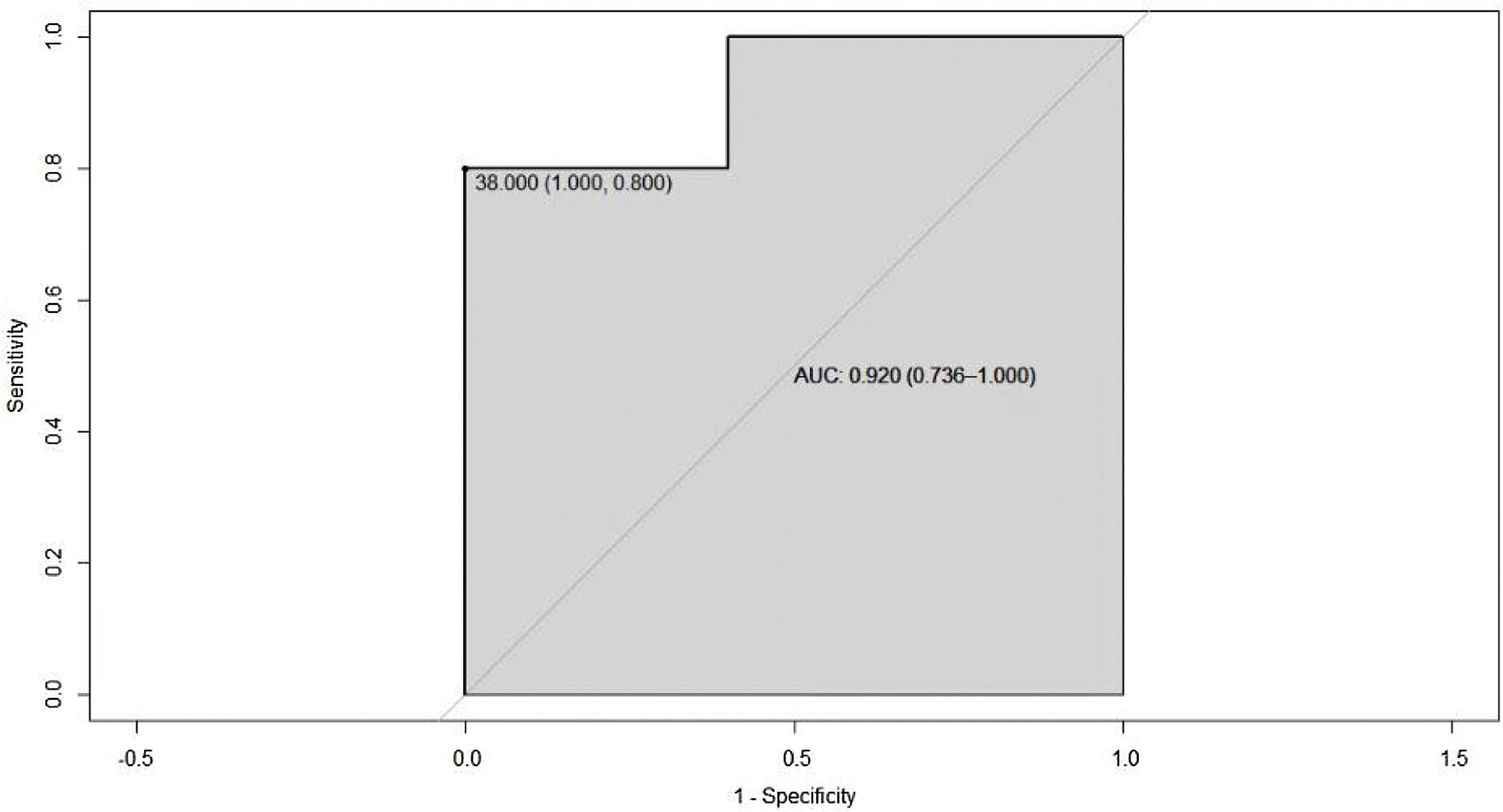
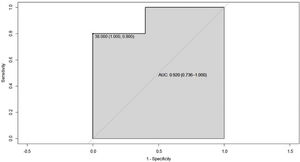
Since overweight or obesity can interfere in the definition of the HDL cholesterol cut-off point, only the group of patients with normal BMI was analyzed and it was found a ROC curve with a segregation point of HDL cholesterol of 38 mg/dL and AUC of 0.92 (95% CI 0.73–1), which shows an improvement in the ability to classify this group of patients (Fig. 5).
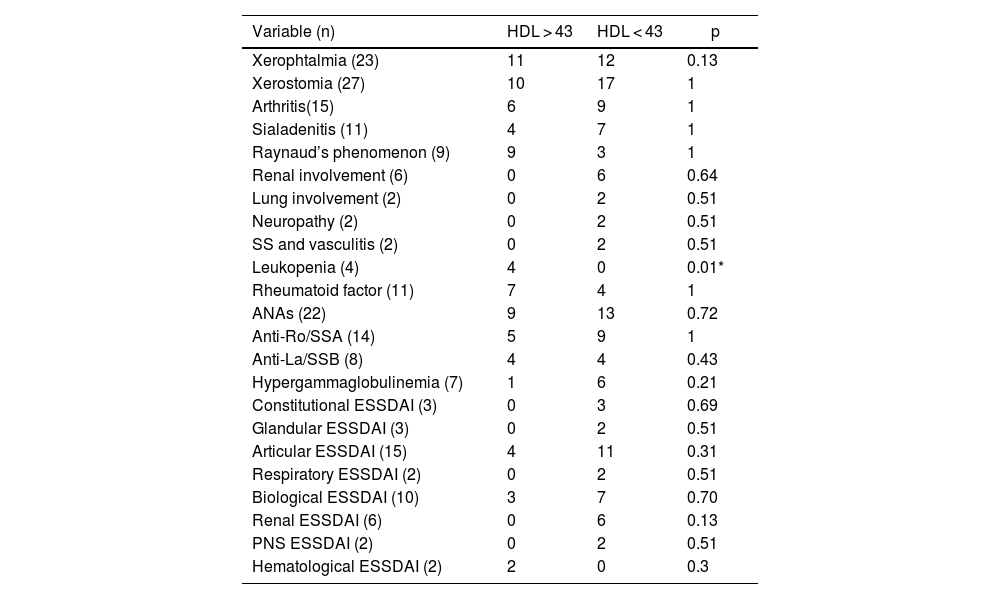
Finally, a significant association was found between leukopenia and HDL cholesterol levels with a cut-off point of 43 mg/dL (Table 2).
Association between clinical-serological variables and HDL cholesterol levels with cut-off point 43 mg/dL.
| Variable (n) | HDL > 43 | HDL < 43 | p |
|---|---|---|---|
| Xerophtalmia (23) | 11 | 12 | 0.13 |
| Xerostomia (27) | 10 | 17 | 1 |
| Arthritis(15) | 6 | 9 | 1 |
| Sialadenitis (11) | 4 | 7 | 1 |
| Raynaud’s phenomenon (9) | 9 | 3 | 1 |
| Renal involvement (6) | 0 | 6 | 0.64 |
| Lung involvement (2) | 0 | 2 | 0.51 |
| Neuropathy (2) | 0 | 2 | 0.51 |
| SS and vasculitis (2) | 0 | 2 | 0.51 |
| Leukopenia (4) | 4 | 0 | 0.01* |
| Rheumatoid factor (11) | 7 | 4 | 1 |
| ANAs (22) | 9 | 13 | 0.72 |
| Anti-Ro/SSA (14) | 5 | 9 | 1 |
| Anti-La/SSB (8) | 4 | 4 | 0.43 |
| Hypergammaglobulinemia (7) | 1 | 6 | 0.21 |
| Constitutional ESSDAI (3) | 0 | 3 | 0.69 |
| Glandular ESSDAI (3) | 0 | 2 | 0.51 |
| Articular ESSDAI (15) | 4 | 11 | 0.31 |
| Respiratory ESSDAI (2) | 0 | 2 | 0.51 |
| Biological ESSDAI (10) | 3 | 7 | 0.70 |
| Renal ESSDAI (6) | 0 | 6 | 0.13 |
| PNS ESSDAI (2) | 0 | 2 | 0.51 |
| Hematological ESSDAI (2) | 2 | 0 | 0.3 |
ANAs: antinuclear antibodies; ESSDAI: EULAR Sjögren's Syndrome Disease Activity Index; PNS: peripheral nervous system; SS: Sjögren’s syndrome.
Alterations in the lipid profile are common in various autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis and Sjögren’s syndrome, and entail a high risk of cardiovascular complications. In recent years, the role of HDL cholesterol in the inflammatory response has been demonstrated in animal models, since its inhibition is associated with the proliferation of hematopoietic stem cells and favors the expression of receptors on the cell surface, promoting the activity of T lymphocytes and the formation of autoantibodies.10–12 It has been described the PSS as an independent predictor of atherosclerotic disease, as an increase in the thickness of the arterial wall was described in this group of patients, in the presence of traditional cardiovascular risk factors, including alterations in the lipid profile OR 2.8 (95% CI 1.04–7.54).13
Other similar investigations not only found the same association, but they also observed that values of HDL cholesterol lower than 39 mg/dL were associated with other immune-based diseases such as type 1 diabetes mellitus, primary immune purpura and celiac disease.15 A variable that was observed in common with other published studies is overweight, however, when performing the statistical analyzes it was demonstrated that this variable does not alter the previously established relationship; in fact, when a sub-analysis of the patients who had a normal BMI was performed, a higher negative correlation was found between disease activity and HDL levels.4,14,16 The ROC curve in this subgroup of patients shows an AUC of 0.92 (95% CI 0.73–1), which suggests a good ability to classify between patients with and without disease activity.
The variables of the lipid profile of LDL cholesterol, total cholesterol and triglycerides had a behavior similar to that described in other studies.13,17,18 Regarding the values of HDL cholesterol, those obtained in this study (42.6 mg/dL) are lower than the majority of those registered in other studies, whose mean value ranges between 49 and 69 mg/dL.17,18 Likewise, the percentage of patients with levels of HDL cholesterol lower than 40 mg/dL was higher in this study compared to others (34 vs. 10–13%).
The foregoing raises the possibility of establishing a possible therapeutic objective in the comprehensive management of patients with PSS, in relation to the control and follow-up of the lipid profile, not only because of the high cardiovascular risk that is already established, but also because of the possibility that this could be a marker of immunological activity. Likewise, it is worth to consider in future studies whether the strategies focused on the increase in the levels of HDL cholesterol have an impact on the control of the disease, which encourages the routine analysis of the lipid profile in patients with PSS, given the possible impact on the disease activity.
LimitationsThe limitations of this study are related to its retrospective nature and the absence of temporal follow-up, which prevents determining the evolution of the variables evaluated. On the other hand, when reviewing the clinical records, there is a large percentage with incomplete clinical data, which entails a significant decrease in the sample size.
ConclusionsIn this study, it was found that patients with PSS with low levels of HDL cholesterol showed higher indexes of disease activity, with a cut-off point lower than 43 mg/dL, and when patients with a high BMI were excluded, a high improvement in the AUC of 0.92 was observed, with a segregation point of 38 mg/dL, which should be explored in further studies to determine if it might be useful for assessing the disease activity.
Conflict of interestThe authors declare that they have no conflict of interest.