Colchicine is often used in patients with osteoarthritis in which calcium pyrophosphate crystal deposition disease is suspected. Colchicine has also been used by many rheumatologists in clinical practice, and in some trials, on patients with primary osteoarthritis (apparently unrelated to calcium pyrophosphate). However, its role in the treatment of primary osteoarthritis is not clear, and international guidelines have not established recommendations.
ObjectiveTo evaluate the efficacy and safety of colchicine for the treatment of adult patients with primary knee osteoarthritis as well as the form associated with calcium pyrophosphate.
MethodsA structured literature search was conducted using the PubMed, Embase, Cochrane Controlled Trials Register, and LILACS databases. Randomized controlled trials were included in which colchicine was used as intervention in patients with primary or pyrophosphate calcium-associated knee osteoarthritis.
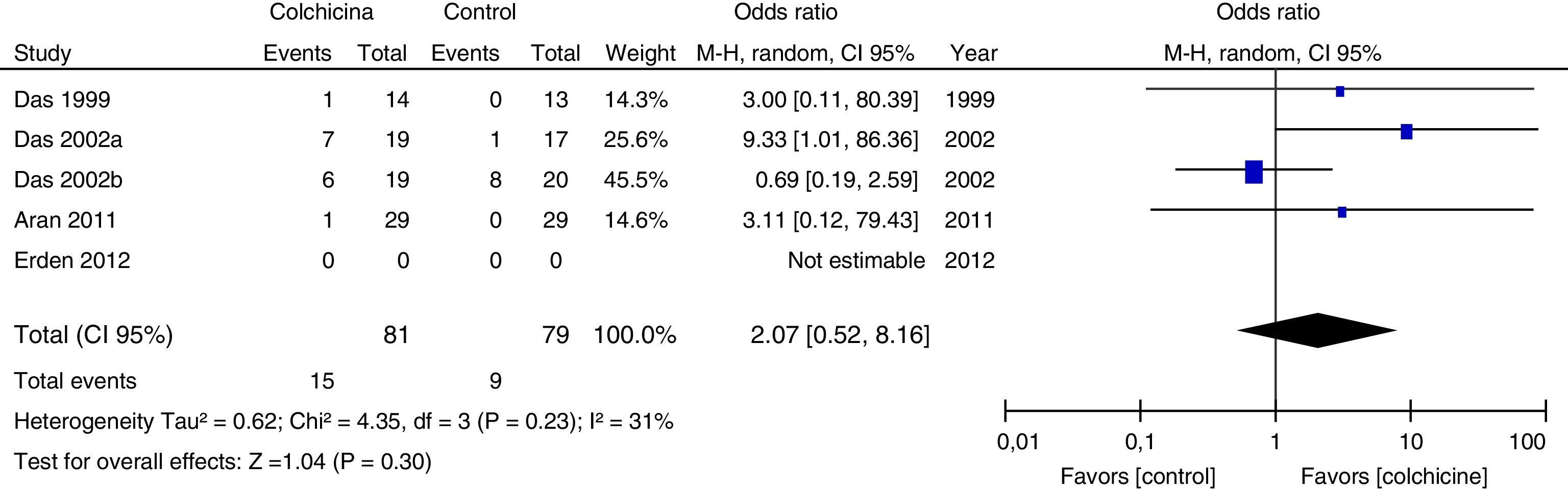
ResultsThe study included 5 randomized controlled trials, all of which showed a common trend in all estimated points of the joint, favoring the use of colchicine for improvement in pain and functionality. Although the effect was not statistically significant in individual studies, there was a greater tendency of gastrointestinal adverse effects with the use of colchicine. None of the studies assessed quality of life.
ConclusionsColchicine appears to be an effective and safe alternative for treatment of adult patients with knee osteoarthritis, either primary or associated with the deposit of calcium pyrophosphate crystals. Its use reduces pain and improves functionality, but it can cause gastrointestinal symptoms in some patients.
En la práctica clínica de muchos reumatólogos y en algunos ensayos clínicos se ha usado colchicina en pacientes con osteoartritis primaria. A pesar de ello, su papel en el tratamiento de la misma no está claro y las guías no establecen recomendaciones al respecto.
ObjetivosEvaluar la eficacia y la seguridad del tratamiento con colchicina en pacientes adultos con osteoartritis de rodilla, tanto primaria como asociada al depósito de cristales de pirofosfato cálcico.
MétodosSe llevó a cabo una búsqueda estructurada de la literatura utilizando las bases de datos Pubmed, Embase, Cochrane Controlled Trials Register y LILACS. Se incluyeron ensayos clínicos controlados, aleatorizados, en donde se haya usado colchicina como intervención en pacientes adultos con osteoartritis de rodilla, primaria o relacionada con pirofosfato de calcio.
ResultadosSe incluyeron 5 ensayos clínicos controlados. Se observó una tendencia común en todos los estimados puntuales de los artículos a favorecer el uso de la colchicina para la mejoría del dolor y de la funcionalidad. Se observó una mayor tendencia de efectos adversos gastrointestinales con el uso de la colchicina, sin embargo, el efecto no fue estadísticamente significativo en los estudios individuales. Ninguno de los estudios evaluó calidad de vida.
ConclusionesLa colchicina parece ser una alternativa eficaz y segura para el tratamiento de pacientes adultos con osteoartritis de rodilla, tanto primaria como asociada al depósito de cristales de pirofosfato de calcio. Su uso reduce el dolor y mejora la funcionalidad, aunque puede producir síntomas gastrointestinales en algunos pacientes.
Osteoarthritis is a highly prevalent chronic disease which is associated with severe pain and functional disability.1 From the medical point of view, it is a frustrating condition for which multiple pharmacological and non-pharmacological interventions have been attempted. The usual practice is to use analgesics as a single drug or in combination, especially non-steroidal anti-inflammatory drugs, acetaminophen at high doses and weak opioids, all of which pose risks of potentially serious adverse effects, especially in the elderly.2,3
Osteoarthritis is the most common form of joint pathology. The term osteoarthritis, possibly rather than representing a disease can be the final common route of a great variety of conditions with different etiologies, but with similar morphological expression and clinical outcomes. Although the classifications of osteoarthritis into primary and secondary could be useful in primary care and research, such distinctions are artificial and ignore the most common scenario of overlapping etiologies in the same patient.4
Although the synovial inflammation within the joints affected by osteoarthritis is often less intense than in the traditional inflammatory arthritis (rheumatoid arthritis, gout, etc.), activation of inflammatory responses also occurs in joints with osteoarthritis, both in synoviocytes and chondrocytes. The abnormal cartilage may contain a great variety of calcium crystals, especially calcium pyrophosphate, which stimulate multiple intracellular mechanisms of inflammation. The presence of crystals can be demonstrated in up to 70% of the specimens of synovial fluid of patients with osteoarthritis.5
An interesting phenomenon frequently observed in the degenerated joint tissues is the deposition of inorganic crystals. Pseudogout due to pyrophosphate is strongly associated with the cartilage degeneration seen in osteoarthritis, and it can be detected in radiographs or through the study of the synovial fluid. In general, the cause and the consequence of the presence of chondrocalcinosis in osteoarthritis remain ambiguous, since it is difficult to establish what happened first: the cellular and matrix degradation or the formation of the crystals. It is most likely that they promote each other and that the metabolic disorder leads to cell damage and vice versa.6,7 Osteoarthritis and aging are strongly associated with calcium pyrophosphate arthropathy.7,8 The strong interrelationship existing between the crystals of pyrophosphate and osteoarthritis has led several authors to consider the pyrophosphate arthropathy simply as a subgroup within the osteoarthritis.9
Like in the osteoarthritis classified as primary, in that associated with calcium pyrophosphate the affected patients are mainly elderly women, being the knee the site most frequently involved.9 The arthropathy linked with crystals of calcium pyrophosphate may be associated with slightly more inflammatory characteristics such as more pain, stiffness, joint effusion and disability, however, such associations are marginal and not useful for the diagnosis. Further studies are required to determine whether or not there are significant differences in symptoms, articular distribution or clinical outcomes between the patients with osteoarthritis with or without associated calcium pyrophosphate.7
Since crystals of calcium pyrophosphate8 and uric acid are demonstrated in patients with primary osteoarthritis, as well as activation of the innate immune system,10 there is a support for the extrapolation of the use of colchicine. This drug has multiple mechanisms of action that lead to the attenuation of different inflammatory pathways and modulate the innate immunity. In addition, it has been demonstrated that it has antifibrotic properties and diverse effects on endothelial function.11
The same treatment modalities to improve pain and symptoms in primary osteoarthritis are frequently used in patients with calcium pyrophosphate related arthritis. Colchicine is usually used in patients with osteoarthritis in whom the presence of arthropathy due to crystals of calcium pyrophosphate is also suspected.12 However, in the clinical practice of many rheumatologists and in some clinical trials it has also been used in patients with primary osteoarthritis (apparently unrelated to calcium pyrophosphate). Despite this, its role in the treatment of primary osteoarthritis is not clear and the international guidelines do not establish recommendations thereon.3 In general, the evidence that supports the use of colchicine in both forms of osteoarthritis has not been systematically reviewed to date.3,12
A systematic review of the literature on the efficacy and safety of the treatment with colchicine in patients with osteoarthritis, both primary and associated with the deposit of calcium pyrophosphate, was conducted in order to establish its usefulness and to allow to update the existing guidelines, to be able to benefit a greater number of patients with this economic and potentially favorable intervention. According to our knowledge this is the first systematic review of the literature that has been conducted on this subject. The PRISMA13 recommendations for the report of systematic reviews evaluating healthcare interventions were followed to carry out this work.
ObjectiveTo evaluate the efficacy and safety of the treatment with colchicine administered orally compared against placebo or active control in adult patients with knee osteoarthritis diagnosed according to the criteria of the American College of Rheumatology,14 with or without suspected association with calcium pyrophosphate crystals disease, for the reduction of pain and improvement of functionality, evaluated at least one month after treatment, as well as its adverse effects (mainly diarrhea) and on the quality of life, through the evaluation of controlled randomized clinical trials of parallel arms.
MethodsProtocol and registrationProtocol registration was not performed to carry out this review.
Eligibility criteriaControlled randomized clinical trials, in full text or in abstract, published or not, using colchicine in any dose and scheme as intervention in adult patients older than 18 years, of either sex, with knee osteoarthritis, primary or related with the deposit of calcium pyrophosphate, which had an active control or placebo control group and which evaluated pain or functionality with a minimum follow-up of one month were included. There was no restriction by language, year, or publication status.
Information sourcesA structured literature search was conducted using the electronic bibliographic databases PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) and LILACS with the keywords “colchicine” and “osteoarthritis” which are Mesh terms, in addition to the keywords “osteoarthrosis”, “osteoartritis”, “osteoartrosis” and “artrosis”. The exact search strategy in PubMed is reported in Annex 1. Additional searches were carried out in Google and Google Scholar. Manual search within the lists of references of the articles obtained was also conducted and some of the authors of the studies found were contacted in order to try to obtain additional references.
Selection of the studiesThe 2 authors performed independently the selection of the articles that met the eligibility criteria established by reading the titles and abstracts of the articles obtained in the different searches. In some occasions it was necessary to review the full text to determine whether or not to include the reference identified. Disagreements between the authors in the selection of the studies were resolved by consensus.
Information extraction processA standardized information extraction format was designed. Both authors extracted the information from the studies finally included. The disagreements between the authors regarding the information obtained were resolved by consensus by reviewing the original sources.
Items of the extracted informationThe information extracted from each study was: first author, country in which the study was conducted, year of publication; the population included was characterized by the total number of participants, average age, distribution by sex, diagnostic criteria, severity of the disease, proportion between patients with probable primary osteoarthrosis and probable osteoarthrosis associated with calcium pyrophosphate, number of patients assigned to each group and final number of participants, in addition to the exclusion criteria; the treatment received by the intervention group and the control group with respect to the dose and the route of administration, as well as the scale used and the effect observed in each group with respect to the outcomes of pain, functionality, quality of life, diarrhea and other adverse effects.
Risk of bias in individual studiesThe 2 authors independently assessed the risk of bias of each of the articles included taking into account the recommendations of the Cochrane collaboration by the use of the RevMan 5.3 tool, considering specifically the use of an adequate method of random allocation, the concealment of the randomization sequence, the blinding of participants, the execution bias, the measurement bias, the reporting bias, the attrition bias and other biases. In addition, an overall assessment of the quality of the body of evidence was conducted using the GRADE approach.15
Summary measuresThe summary measure used was the OR with the Mantel-Haenszel method under a random effects model, to measure the effects observed with the intervention on the outcomes of improvement of at least 30% in the scales of pain and functionality, and on the onset of diarrhea.
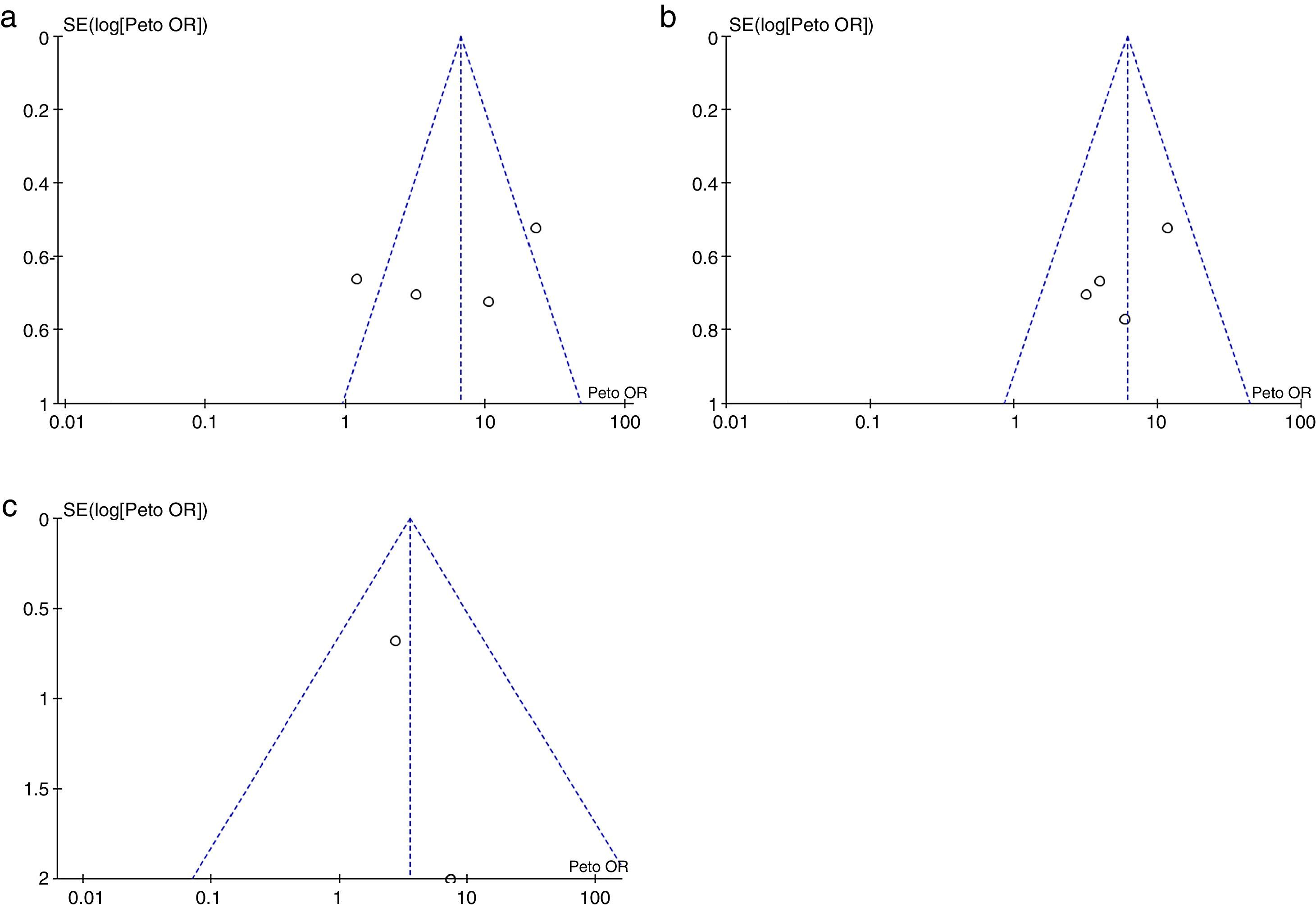
Risk of bias across the studiesThe publication bias was assessed by visual inspection of a funnel plot, which takes into account the size of the effect against the inverse of the variance. We looked for the presence of asymmetry in the funnel plot suggesting the possibility that small studies with negative results would not have been published, taking into account, however, that there are causes of asymmetry other than the publication bias.
Additional analysesWe pre-specified a subgroup analysis to evaluate the intervention in a subgroup of primary knee osteoarthritis and a subgroup of knee osteoarthritis associated with deposition of calcium pyrophosphate crystals.
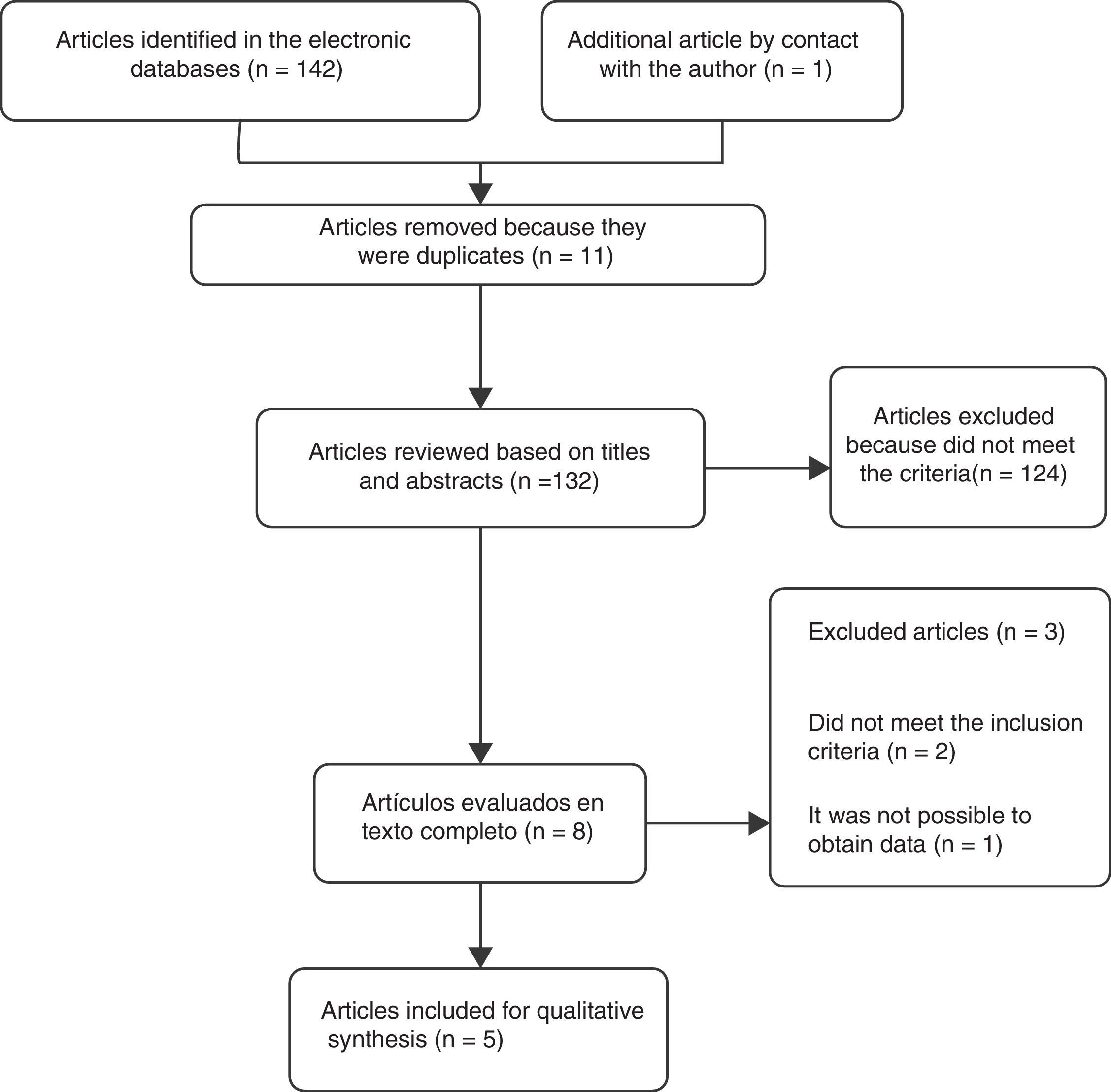
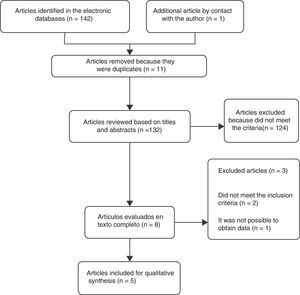
ResultsSelection of the studiesA total of 5 controlled clinical trials were included for the review16–20 (Fig. 1). The search in the electronic databases PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) and LILACS produced a total of 142 citations. 11 duplicate studies were found, and after adjusting for duplicate studies131 remained. Subsequently to the review of titles and abstracts, 124 citations were excluded because they did not clearly meet the inclusion criteria. The remaining 7 citations were reviewed in full text and 3 articles were excluded. Two of these, Marra et al.21 (2012) and Koyuncu et al.22 (2010) did not meet the inclusion criteria. The third article by Ediz et al.23 (2012) was excluded because it was not possible to obtain the translation into English or Spanish and the data required for the review were not found in the abstract of the article. An additional article [Das et al.16 (1999)] was obtained by contacting the main author. After reviewing the full text, the article was included in the review.
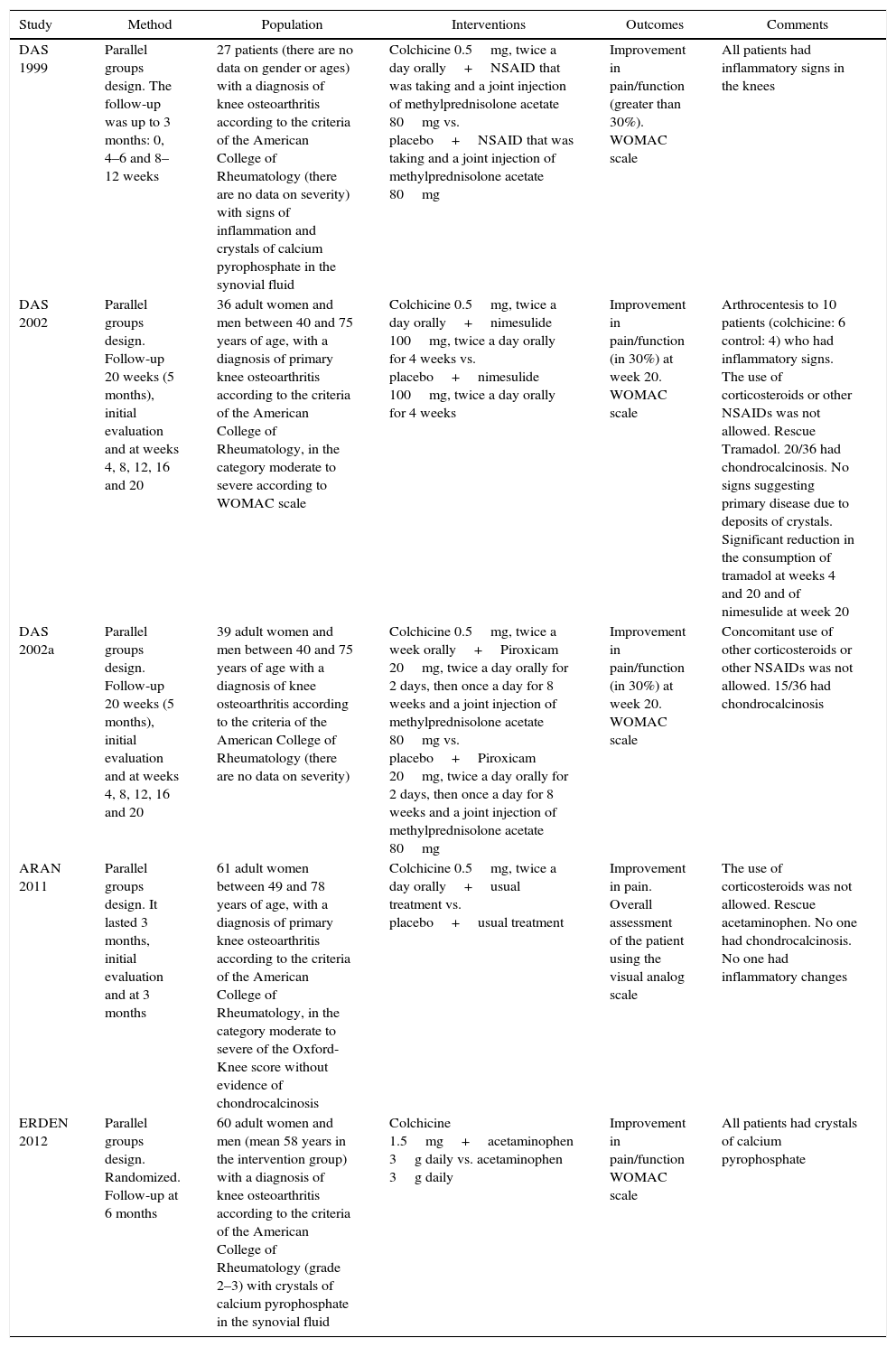
Characteristics of the studiesThe characteristics of the studies included in the review are summarized in Table 1. All 5 articles included are in English language and were published between the years 1999 and 2012. Three of them are by the same author [Das et al.16 (1999), Das et al.17 (2012) and Das et al.18 (2012a)] carried out in India. Another was conducted in the United States of America [Aran et al.19 (2011)] and the fifth was carried out in Turkey [Erden et al.20 (2012)]. Only one study (Das, 1999) expresses the scenario from where the patients were recruited, stating that it was conducted in a Rheumatology clinic. The scenario is not mentioned in the other articles.
Characteristics of the studies included in the review.
| Study | Method | Population | Interventions | Outcomes | Comments |
|---|---|---|---|---|---|
| DAS 1999 | Parallel groups design. The follow-up was up to 3 months: 0, 4–6 and 8–12 weeks | 27 patients (there are no data on gender or ages) with a diagnosis of knee osteoarthritis according to the criteria of the American College of Rheumatology (there are no data on severity) with signs of inflammation and crystals of calcium pyrophosphate in the synovial fluid | Colchicine 0.5mg, twice a day orally+NSAID that was taking and a joint injection of methylprednisolone acetate 80mg vs. placebo+NSAID that was taking and a joint injection of methylprednisolone acetate 80mg | Improvement in pain/function (greater than 30%). WOMAC scale | All patients had inflammatory signs in the knees |
| DAS 2002 | Parallel groups design. Follow-up 20 weeks (5 months), initial evaluation and at weeks 4, 8, 12, 16 and 20 | 36 adult women and men between 40 and 75 years of age, with a diagnosis of primary knee osteoarthritis according to the criteria of the American College of Rheumatology, in the category moderate to severe according to WOMAC scale | Colchicine 0.5mg, twice a day orally+nimesulide 100mg, twice a day orally for 4 weeks vs. placebo+nimesulide 100mg, twice a day orally for 4 weeks | Improvement in pain/function (in 30%) at week 20. WOMAC scale | Arthrocentesis to 10 patients (colchicine: 6 control: 4) who had inflammatory signs. The use of corticosteroids or other NSAIDs was not allowed. Rescue Tramadol. 20/36 had chondrocalcinosis. No signs suggesting primary disease due to deposits of crystals. Significant reduction in the consumption of tramadol at weeks 4 and 20 and of nimesulide at week 20 |
| DAS 2002a | Parallel groups design. Follow-up 20 weeks (5 months), initial evaluation and at weeks 4, 8, 12, 16 and 20 | 39 adult women and men between 40 and 75 years of age with a diagnosis of knee osteoarthritis according to the criteria of the American College of Rheumatology (there are no data on severity) | Colchicine 0.5mg, twice a week orally+Piroxicam 20mg, twice a day orally for 2 days, then once a day for 8 weeks and a joint injection of methylprednisolone acetate 80mg vs. placebo+Piroxicam 20mg, twice a day orally for 2 days, then once a day for 8 weeks and a joint injection of methylprednisolone acetate 80mg | Improvement in pain/function (in 30%) at week 20. WOMAC scale | Concomitant use of other corticosteroids or other NSAIDs was not allowed. 15/36 had chondrocalcinosis |
| ARAN 2011 | Parallel groups design. It lasted 3 months, initial evaluation and at 3 months | 61 adult women between 49 and 78 years of age, with a diagnosis of primary knee osteoarthritis according to the criteria of the American College of Rheumatology, in the category moderate to severe of the Oxford-Knee score without evidence of chondrocalcinosis | Colchicine 0.5mg, twice a day orally+usual treatment vs. placebo+usual treatment | Improvement in pain. Overall assessment of the patient using the visual analog scale | The use of corticosteroids was not allowed. Rescue acetaminophen. No one had chondrocalcinosis. No one had inflammatory changes |
| ERDEN 2012 | Parallel groups design. Randomized. Follow-up at 6 months | 60 adult women and men (mean 58 years in the intervention group) with a diagnosis of knee osteoarthritis according to the criteria of the American College of Rheumatology (grade 2–3) with crystals of calcium pyrophosphate in the synovial fluid | Colchicine 1.5mg+acetaminophen 3g daily vs. acetaminophen 3g daily | Improvement in pain/function WOMAC scale | All patients had crystals of calcium pyrophosphate |
The characteristics of the population were diverse among the studies, as women were included in some of them and men and women were included in others. The spectrum of severity was variable, in some studies including moderate-severe arthritis and in others the severity was not taken into account. In some studies the population had signs of joint inflammation, in others not, and in others was mixed. Some authors included in their population the presence of chondrocalcinosis as a sign of joint disease due to calcium pyrophosphate and others excluded this population. The shortest follow-up time was 3 months.
In the intervention group all patients received colchicine, 4 of them (except Erden 2012) at a dose of e 0.5mg orally, twice a day. However, additional co-interventions varied from one study to another from NSAIDs and acetaminophen to intra-articular corticosteroids. In the comparison group the treatment also varied from study to study.
In most studies (except Aran, 2011) the WOMAC scale (or its validated modification) was used to measure the main outcome. The number of participants included in the studies is low, ranging between 27 and 61 participants, including both the intervention group and the control group.
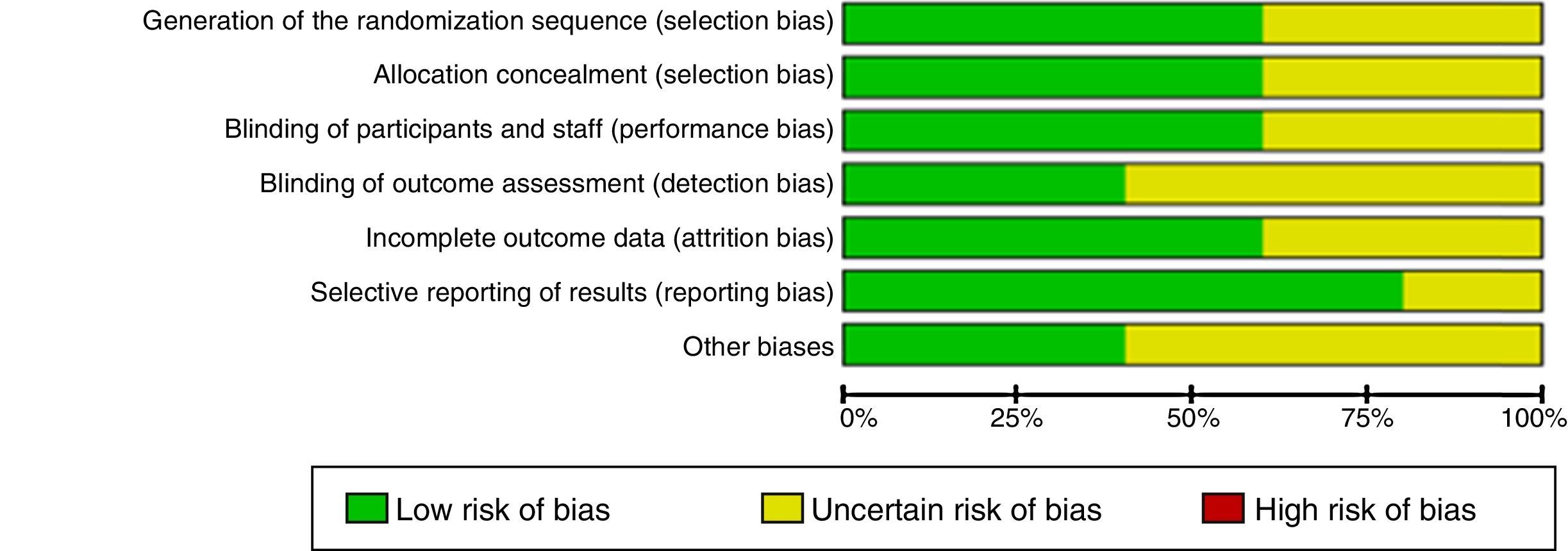
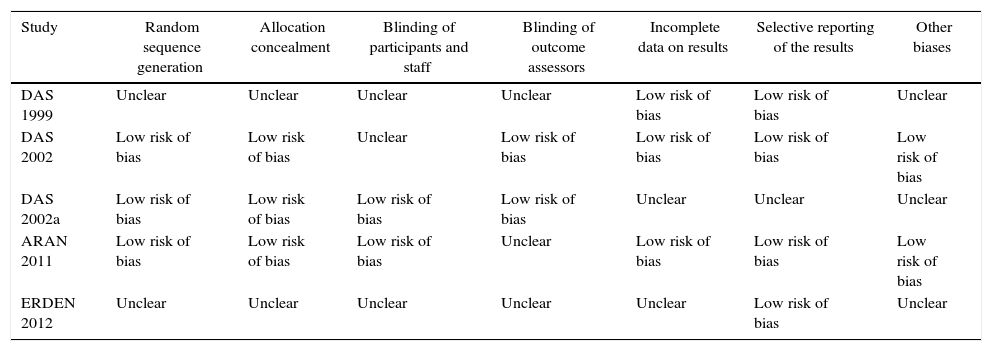
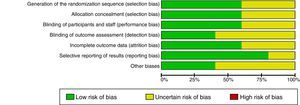
Risk of bias in the studiesTable 2 and Fig. 2 show the summary of the risk of bias for each of the studies and the risk of bias by domains, respectively, according to the Cochrane Collaboration's tool for assessing the risk of bias. Fundamental domains that affect the quality are committed in three of the included studies (Das, 1999, Das, 2002 and Erden, 2012).
Risk of bias in each study.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and staff | Blinding of outcome assessors | Incomplete data on results | Selective reporting of the results | Other biases |
|---|---|---|---|---|---|---|---|
| DAS 1999 | Unclear | Unclear | Unclear | Unclear | Low risk of bias | Low risk of bias | Unclear |
| DAS 2002 | Low risk of bias | Low risk of bias | Unclear | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias |
| DAS 2002a | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Unclear | Unclear | Unclear |
| ARAN 2011 | Low risk of bias | Low risk of bias | Low risk of bias | Unclear | Low risk of bias | Low risk of bias | Low risk of bias |
| ERDEN 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Low risk of bias | Unclear |
The quality of the evidence as a whole, using the GRADE approach, was “very low”. Although only clinical trials were included, factors such as limitations in design, inaccuracies in the results and high probability of publication bias, diminish the level of quality of the evidence.
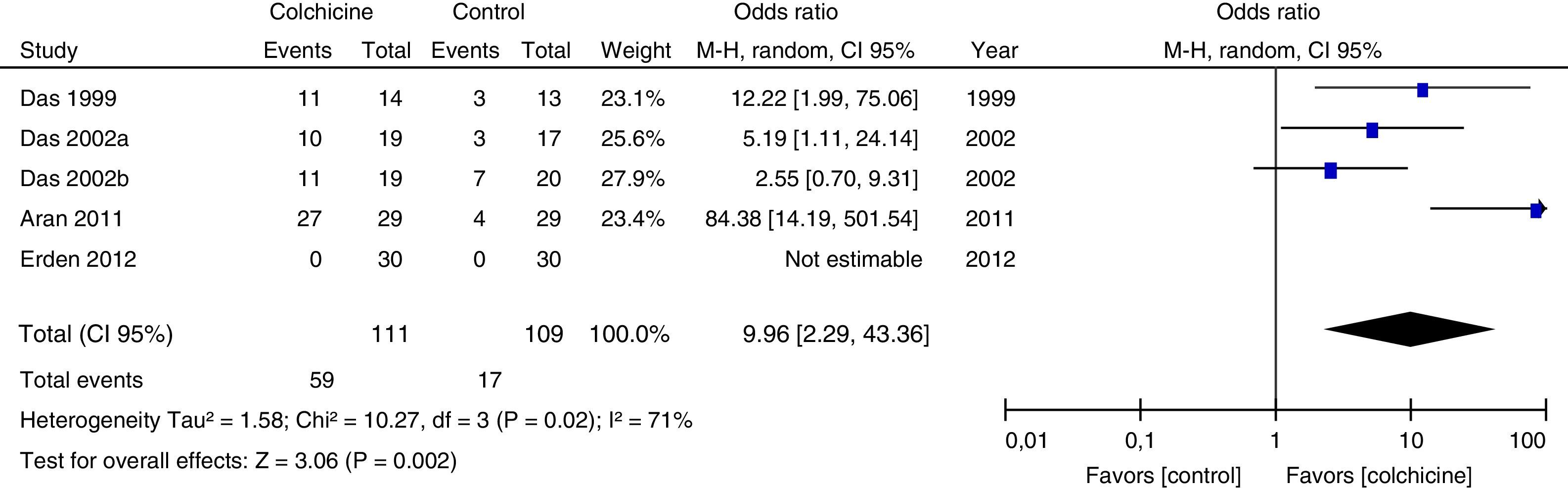
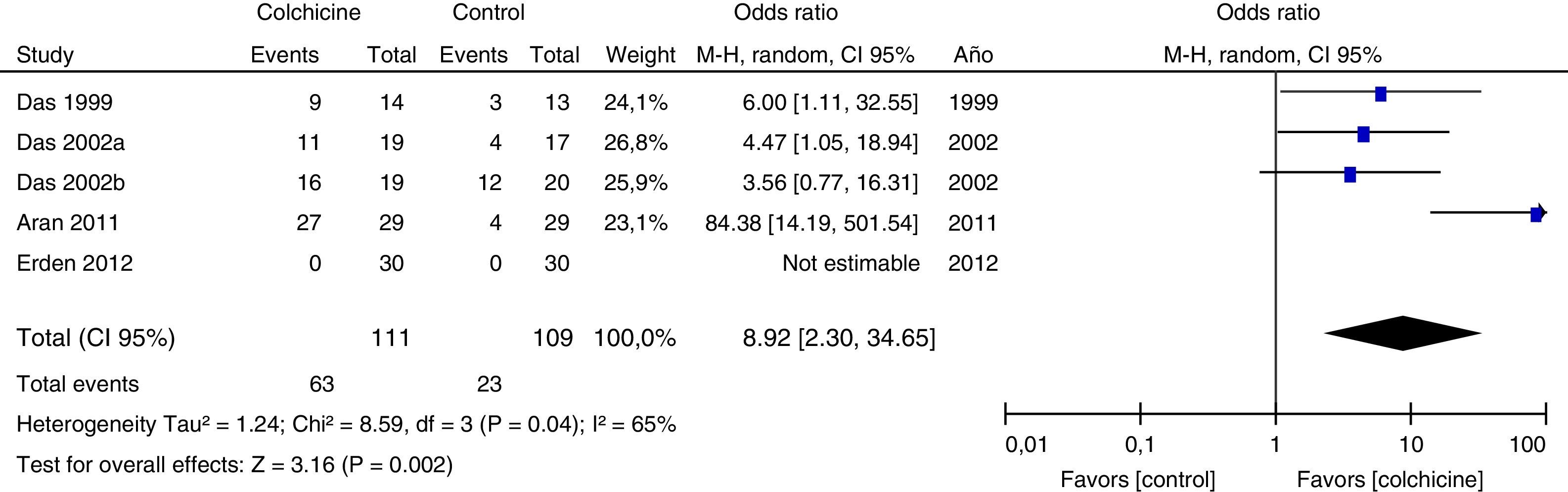
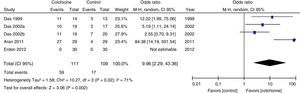
Results of individual studiesFigs. 3–5 show the summary of the data, the estimation of the effect and the confidence intervals for each study included in the review, regarding the outcomes of pain reduction (Fig. 3), improvement in functionality (Fig. 4) and gastrointestinal adverse effects (Fig. 5).
Synthesis of resultsGiven that the populations, their clinical characteristics and the interventions vary markedly, as well as the methodological quality of the studies, the review was focused on describing the studies, their results, applicability and limitations in a qualitative synthesis. The additional meta-analysis confirmed a high heterogeneity among studies, especially for the efficacy outcomes (Figs. 3 and 4).
All articles included in the review assessed the improvement in pain. In the forest plot (Fig. 3) is observed a common trend in all the point estimates of the articles, which tend to favor the use of colchicine for the improvement in pain, when added to conventional treatments, compared with conventional treatment alone. A similar trend is observed with the outcome of improvement in functionality (Fig. 4), which was also evaluated in all articles included.
As for the outcome of adverse effects, it was evaluated in 4 studies and in one did not (Erden, 2012). Three of them (Das, 1999, Das, 2002 and Aran, 2011) reported one episode of diarrhea in each one, in the colchicine group. In the Das 2002a study, diarrhea was not reported but rather 8 cases of dyspeptic symptoms in the colchicine group vs. 4 cases in the control group. Only Das 2002a reported adverse effects other than gastrointestinal; among them, 5 cases of infection of the upper respiratory tract and 8 cases of increase in musculoskeletal pain in the colchicine group vs. one case of infection of the upper respiratory tract and 28 cases of increase in musculoskeletal pain in the control group. All studies included in the review are small, with inaccurate confidence intervals. None of the studies assessed the quality of life.
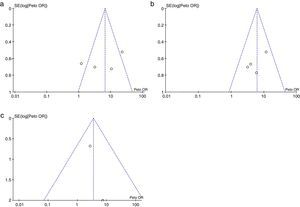
Risk of biases across the studiesIn the visual inspection of the funnel plot for each of the outcomes evaluated, it is not possible to rule out the possibility of publication bias (Fig. 6).
Additional analysesBecause of the small number of studies found, it was not possible to perform subgroup analyses.
DiscussionSummary of the evidenceA systematic review of the literature was conducted to evaluate the efficacy and safety of the intervention with colchicine added to the conventional management of adult patients with knee osteoarthritis. Due to the marked variability between the populations, their clinical characteristics and the interventions, as well as in the methodological quality, the review was focused on performing a qualitative synthesis instead of a meta-analysis. In total only 5 articles met the eligibility criteria.
The most representative finding of the review is the trend in favor of the use of colchicine for the improvement in pain and functionality, compared with the control group. In addition, as for the adverse effects, the forest plot shows a trend to produce more gastrointestinal adverse effects with the use of colchicine, however, the difference is not statistically significant in the individual studies. It is important to highlight that the dose of colchicine used in the majority of the studies was 0.5mg, twice a day, which coincides with the doses currently recommended for the treatment of the calcium pyrophosphate crystals deposition disease12 and also for the treatment of gout; for the latter it has been demonstrated that high doses do not increase the effectiveness but they do increase the adverse effects.24
There was not a clear statement of the scenario of the studies, which might hinder the generalizability. The strength of the evidence is poor, having few studies of small size, imprecise and of middle methodological quality. None of the studies took into account the assessment of the quality of life of the participants.
Only the study conducted by Aran et al.19 (2011) was exhaustive in including only patients with primary knee osteoarthritis, including radiological evaluation of the chondrocalcinosis and excluding those with inflammatory changes. However, this does not rule out completely the possibility of association of osteoarthritis with deposit of crystals of calcium pyrophosphate. The result of this study was also in favor of colchicine.
LimitationsAmong the limitations of the review we found that the quality of the evidence varies and none of the articles included meets all quality standard criteria. The studies are small and with low precision. It was not possible to rule out publication bias for the included comparisons.
ConclusionsColchicine appears to be an effective and safe alternative for the treatment of adult patients with knee osteoarthritis, either primary or related with deposit of crystals of calcium pyrophosphate. Its use reduces pain and improves functionality, although it can produce gastrointestinal symptoms in some patients. The great clinical and methodological heterogeneity of the studies included in the review makes it inappropriate to obtain a measure of the summary effect of all these studies. It would be ideal to carry out controlled clinical trials of better quality, with a greater number of patients, clearly discriminating between those with primary osteoarthritis or associated with the deposit of calcium pyrophosphate and that also include outcomes such as quality of life. It is important to note that a recently completed clinical trial evaluating the use of colchicine in knee arthritis appears currently registered. Until the last search conducted in November 2016 we did not found published results.25
Conflict of interestThe authors declare they do not have any conflict of interest.
- 1.
Search: “osteoarthritis”[MeSH Terms]
- 2.
Search: “osteoarthritis, knee”[MeSH Terms]
- 3.
Search: “osteoarthritis”[Text Word]
- 4.
Search: “osteoarthrosis”[Text Word]
- 5.
Search: “arthrosis”[Text Word]
- 6.
Search: “osteoartrosis”[Text Word]
- 7.
Search: “artrosis”[Text Word]
- 8.
Search: #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
- 9.
Search: “colchicine”[MeSH Terms]
- 10.
Search: “colchicine”[Text Word]
- 11.
Search: “colchicina”[Text Word]
- 12.
Search: #9 OR #10 OR #11
- 13.
Search: (((randomized controlled trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR random allocat* [tw] OR randomly allocat* [tw] OR double-blind method[mh] OR single-blind method [mh] OR double blind* [tw] OR single blind* [tw] OR triple blind* [tw] OR clinical trial [pt] OR clinical trials [mh]) NOT (animal [mh] NOT human [mh])))
- 14.
Search: #8 AND #12 AND #13
Please cite this article as: Restrepo-Escobar M, Carmona-Franceschi MdJ, Donado Gómez JH. Revisión sistemática de la literatura sobre el tratamiento con colchicina en pacientes adultos con osteoartritis de rodilla. Rev Colomb Reumatol. 2017;24:102–111.