To assess osteoarthritis in rat models at an early stage (7 days after induction).
Materials and methodsThis is a retrospective study comparing results from previous experiments. The models compared were the following: (a) anterior cruciate ligament transection model (CLX) and an adjuvant-induced arthritis model in two varieties (b) sub-plantar (ASP), and (c) intra-articular (AIA). Seven days after osteoarthritis (OA) induction, an analysis was made of the clinical, histological, radiological, and serological inflammatory markers (IL-1β, IL-4, IL-6, TNF-α, MMP-2, and MMP-9).
ResultsThe AIA model produced significant differences in several parameters, when compared to the control group. However, the levels of IL-1 were higher in all OA models than in the baseline group, being more pronounced in the CXL and AIA groups (p<0.001). Surprisingly, the lowest value for IL-4 was observed in the AIA group (p<0.001 vs. baseline group). Furthermore, the most elevated values of MMP-2 were observed in the ASP model.
ConclusionsAlthough arthritis rat models are used and compared interchangeably as if they were the same, it is shown in this work that at an early stage the models behave quite different in most of the studied variables.
Comparar y caracterizar modelos de osteoartritis inducida en ratas en una fase temprana (7 días luego de la inducción).
Materiales y métodosEste es un estudio retrospectivo que compara resultados de experiencias previas. Se compararon los modelos de: a) corte del ligamento cruzado anterior (CLX), y artritis inducida por adyuvante en sus 2 variedades, b) subplantar (ASP) y c) intraarticular (AIA). Las variables analizadas fueron: estado clínico, histología, radiología y marcadores séricos de inflamación (IL-1β, IL-4, IL-6, TNF-α, MMP-2 y MMP-9).
Resultados El modeloAIA presenta diferencias significativas en diversos parámetros cuando se compara con el grupo control. Además, los niveles de IL-1 fueron elevados en todos los modelos de osteoartritis respecto al basal, siendo más pronunciados en los grupos CLX y AIA (p<0.001). Por otra parte, el valor más bajo de IL-4 fue observado en el grupo AIA (p<0.001 frente al grupo basal). Además, los valores más elevados de MMP-2 fueron observados en el modelo ASP.
ConclusionesSi bien se usan y se comparan de manera indistinta los modelos de artritis en ratas como si fueran similares, en este trabajo demostramos que en un estadio temprano los modelos se comportan bastante diferentes en la mayoría de las variables estudiadas.
Arthritis in its various nosological presentations is a disease that exhibits a progressive course, with some periods of remission, causing pain and usually presenting as sequels. In human medicine, rheumatoid arthritis is of particular importance due to its incidence, whereas in veterinary medicine, the sport horses have arthritis of chronic and progressive course with sporadic exacerbations, usually due to mechanical overload related to the sporting activity. The adequate management of osteoarthritis (OA) would allow reducing the high economic losses caused by the early withdrawal of animals from the sports competition.
For the study of arthritis both in humans and in horses, scientists have been working for a long time with murine models of arthritis, with the purpose of understanding the pathophysiology of the disease and assessing the usefulness of anti-arthritic drugs. However, there are several models of arthritis in rats that keep a relationship with the diseases studied in humans and other species, but they have not been well characterized neither from the point of view of the early response in their clinical, histological and radiological aspects, nor in terms of their serum inflammatory profile. Considering the regulations corresponding to the rational use of laboratory animals,1 there arose the possibility to compare retrospectively the results of different experiments carried out in our laboratory based on the use of some of the models proposed for the study of arthritis.
In this work, we compared 3 murine models, which although they are widely spread in experimental arthritis, it has not been previously clarified as to which is the type of arthritis that each one best represents, and it has not been notified either which are the most relevant complementary methods for the study of each of these arthritis.
That is, on the one hand, these models have been used, indistinctly, to assess both acute and chronic phenomena, and in terms of the determination of molecular markers of inflammation, there are contradictions in the information for each model.
Currently, the role that biomarkers would play in OA is uncertain, and this is largely due to the diversity of arthritis models that have been used. The biological validation and utilization of molecular biomarkers could be very useful for the clinical practice in the diagnosis and monitoring of OA, provided that it is known how the levels of these biomarkers vary according to the different presentations of OA and how their levels are associated with each other.
In this work, we compared 3 models that we have used, these are: transection of cruciate ligaments (CLX) and adjuvant-induced arthritis in 2 modalities, intra-articular (AIA) and sub-plantar (ASP).
The CLX model would suppose a type of chronic arthritis produced by the wear of the joint facets as a consequence of the instability produced in the injured joint; this model could correspond to a good model of the sports wear and tear due to overtraining.
On the other hand, adjuvant-induced arthritis has been a model widely used to induce the pathogenesis of rheumatoid arthritis,2 likewise, this model can be used to resemble other types of reactive arthritis that are usually very frequent in veterinary medicine. Arthritis can be easily induced by subcutaneous inoculation of Mycobacterium tuberculosis H37Ra (Mtb) inactivated by heat and suspended in oily excipient. Moreover, the adjuvant-induced arthritis model has been used as a general model of inflammation which allows evaluating the use of anti-inflammatory agents. It is noteworthy to mention that due to genetic factors3 not all strains of rats are feasible to be induced, however, it works well in Sprague-Dawley4 and Lewis rats.5 Some of the studied factors to which is attributed the susceptibility of these strains to generate the adjuvant-induced arthritis would be the increase in the expression of the articular HSP47 with the increase of type I collagen and the development of anti-HSP47 antibodies,6 as well as the migration of leukocytes to the joint and the role of the MCP-1 chemotactic factor.7 This general model of inflammation has been used to evaluate the anti-arthritic effect of different drugs.8
Certain authors in their works have administered the adjuvant intra-articularly to induce the arthritis in the knee and they evaluated the response at 3 and 5 days post-administration.9
In our work, we decided to compare the experience of the use of these 2 routes of adjuvant, since the different routes of application would seem to generate diverse effects. While the administration of adjuvant produces a model of arthritis that resembles rheumatoid arthritis, this model is also valid for other types of arthritis that occur in veterinary medicine, since this model is characterized by the presence of a good inflammatory component and an important damage of the articular cartilage.10 Conversely, the CLX model has been used to simulate a more chronic evolution of OA.11 Although there are published works with different models,12 the actual characterization and comparison of the model used with respect to others is difficult because the different times, drugs and variables applied are not maintained constant in all the works.
In view of our interest in measuring molecular inflammatory mediators such as cytokines and MMP, there are opposed results in the literature probably due to the different models and sampling times. The objective of this study is to characterize and compare patterns related to radiological, histopathological and clinical symptomatology, as well as the study of inflammatory molecular biomarkers in rats that presented different models of arthritis at an early stage (7 days).
Materials and methodsExperimental design and animals. In this work, we compare the results of previous experiences of our group; the day 7 was taken as the reference day, since it was the most representative day for all models. A total of 40 Sprague Dawley rats of 2 months of age (200–250g) bred in the Central Vivarium of the Faculty of Veterinary Sciences of the University of Buenos Aires were used (10 animals per group). All animals were maintained at 22°C with light cycles of 12h, with access to food and water ad libitum. The following were the groups in the study: (i) basal or baseline; animals to which no procedure was performed, (ii) transection of the anterior cruciate ligament (CLX); this group was obtained by a surgical procedure under ketamine/xylazine anesthesia,11 (iii) sub-plantar adjuvant-induced arthritis (ASP); 0.1ml of Freund's complete adjuvant (Sigma, St Louis, USA) was inoculated to this group by sub-plantar route,13 and (iv) intra-articular adjuvant-induced arthritis (AIA); 0.1ml of Freund's complete adjuvant was inoculated to this group by intra-articular route in the femorotibiopatellar joint.14
Of each group of 10 animals, at sacrifice, 5 animals were used for radiological and histopathological studies and the other 5 were used to measure inflammatory biomarkers from articular homogenates. The animals were sacrificed by white bleeding, under general anesthesia with ketamine-xylazine. The procedures were approved by the Institutional Committee for Care and Use of Laboratory Animals (CICUAL) of the Faculty of Veterinary Sciences of the University of Buenos Aires.
Clinical score. It was defined as a score from 0 to 415 according to the degree of severity of the claudication: 0 represents the absence of claudication; 1: barely perceptible claudication; 2: moderate claudication; 3: very marked claudication in which in some occasions the animal subtracts the weight-bearing position of the limb, and 4: occurs with weight-bearing subtraction most of the time. Observation of the symptoms was conducted daily.
Histopathological score. Once the experience was completed, the rats were euthanized and the tibiotarsal and femorotibiopatellar joints were removed for histopathological evaluations. The samples were fixed in 10% formalin and a standard decalcification process for bone samples was conducted. The sections were stained with hematoxylin/eosin. The observed samples were classified from 0 to 6 according to the degree of cellular infiltration and bone resorption of the underlying bone according to the score of Pritzker.12,16
Radiographic studies. Radiographic studies were carried out with X-ray equipment with digital radiovisiograph (Kodak), which were especially for samples of laboratory animals. The femorotibiopatellar and tibiotarsal joints were radiographed. The plates were analyzed by radiographic scoring as previously described by other authors,17 with slight modifications. In brief, joint space narrowing (0–3) and damage of the articular contour (0–3), with a total range from 0 to 6, were observed.
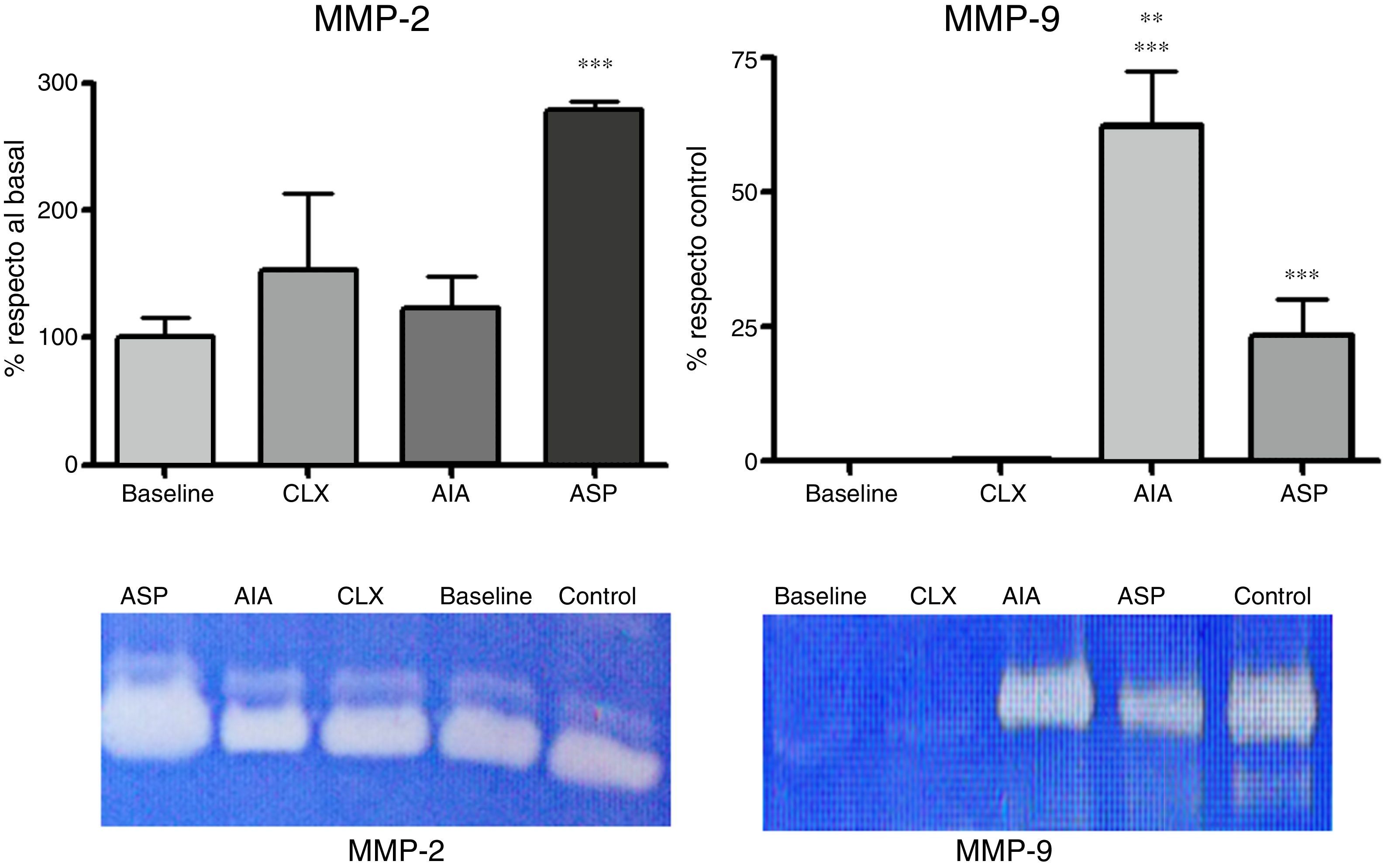
Zymographies. The activity of MMP-2 and MMP-9 was analyzed by zymography on polyacrylamide gels with the aggregate of gelatin. The sample consisted of joint lysates obtained by dissecting the femorotibiopatellar joint for trituration in homogenizer; the samples were recovered in protein extraction buffer (50mM Tris–HCl, 150mM NaCl, 1% Triton X-100), 1ml/g of tissue. The protein concentration of the product obtained was determined using a commercial kit (Wiener Lab.; Argentina). Aliquots of 100μg were mixed with the sample buffer and heated for 3min at 100°C, and then were sowed in the SDS-PAGE 10% with 0.2% of gelatin. Then the gels were washed with 50mM Tris–HCl pH 7.5 with the aggregate of 2.5% of Triton X-100 for 45min, followed by washing with 50mM Tris–HCl, 5mM CaCl, and 1μM ZnCl2 pH 7.5 with the aggregate of 2.5% of Triton X-100 pH 7.5 for 45min. After that, they were incubated with 50mM Tris–HCl, 10mM CaCl2, and 200mM NaCl pH 7.5 at 37°C for 24h. Finally, they were stained with 0.5% Coomassie Brilliant Blue R-250 for 2h and thereafter were discolored with decolorizing solution (25% (v/v) isopropanol and 10% (v/v) acetic acid). The gelatinolytic activity generated clear bands, the gels were digitized and a densitometric study of the bands was carried out using the J Image software. The zymographic activity was expressed as a percentage in relation to the baseline group for MMP-2 and as percentage in relation to the control sample in the case of MMP-9. The explanation for this decision is that because MMP-9 is practically absent in samples of the baseline group, it was necessary to refer it as a percentage of the control sample. The amount of control sample that was loaded was previously titrated according to its activity to produce a percentage of saturation of 50% in the zymography. The data corresponding to different gels were normalized by the use of this control sample, which also served as a point of reference for the densitometric analysis.
ELISA of IL-1β, IL-4, IL-6 and TNF-α. The concentration of cytokines (IL-1β, IL-4, IL-6, and TNF-α) in the supernatants of the articular lysates, prepared as described in the previous item, was determined by ELISA of commercial capture BD OptEIA (BD, Biosciences, San Diego, CA, USA), following the manufacturer's recommendations. The concentrations were determined by a standard curve made with the respective recombinant cytokine supplied in the kit. The samples were read in a Rayto 2100 (China) microplate reader at 450nm.
Statistical analyses. The statistical analyses and graphics were performed using the Instat3 software. ANOVA was applied to establish whether there were statistically significant differences between the different experimental groups (p≤0.05) and then the Tukey's test was applied for multiple comparisons.
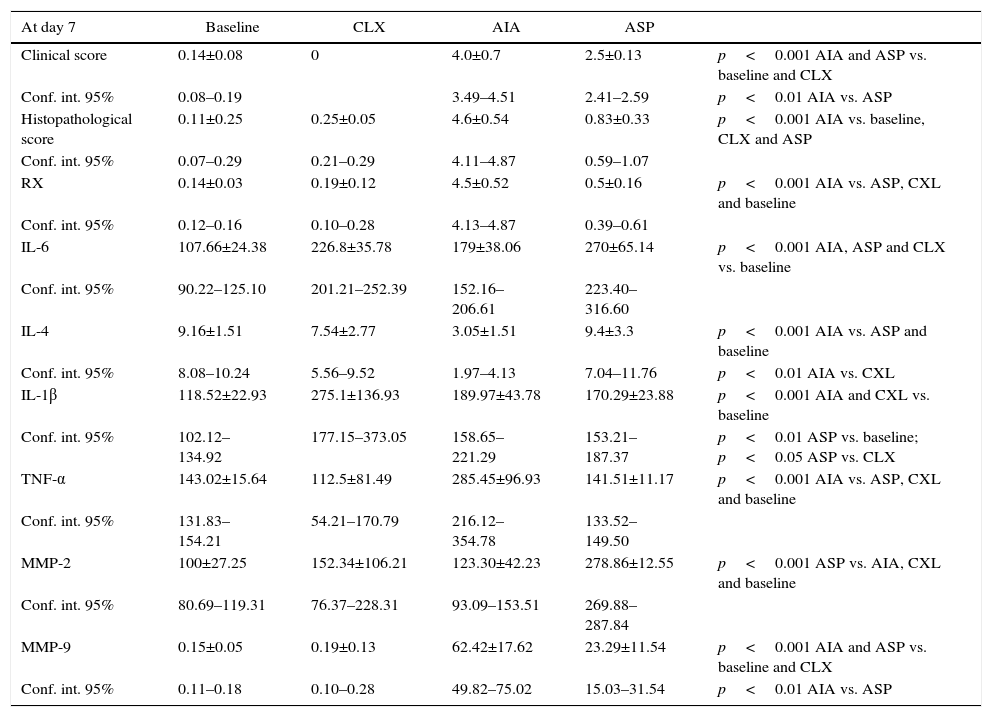
ResultsTable 1 summarizes the values of the results obtained, which will be analyzed in detail below.
Values of the results of the different variables studied for each model. ANOVA was applied followed by Tukey's test.
| At day 7 | Baseline | CLX | AIA | ASP | |
|---|---|---|---|---|---|
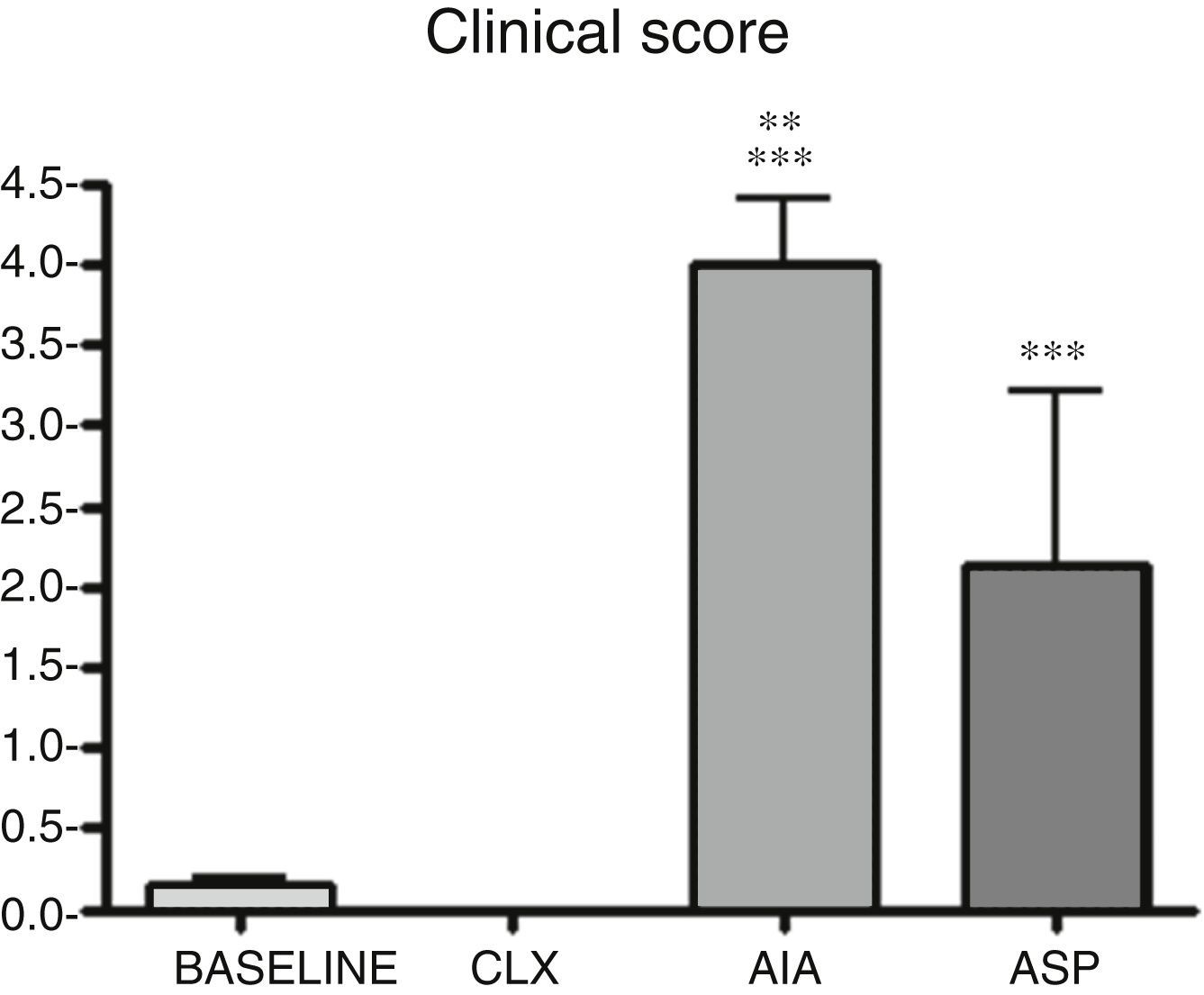
| Clinical score | 0.14±0.08 | 0 | 4.0±0.7 | 2.5±0.13 | p<0.001 AIA and ASP vs. baseline and CLX |
| Conf. int. 95% | 0.08–0.19 | 3.49–4.51 | 2.41–2.59 | p<0.01 AIA vs. ASP | |
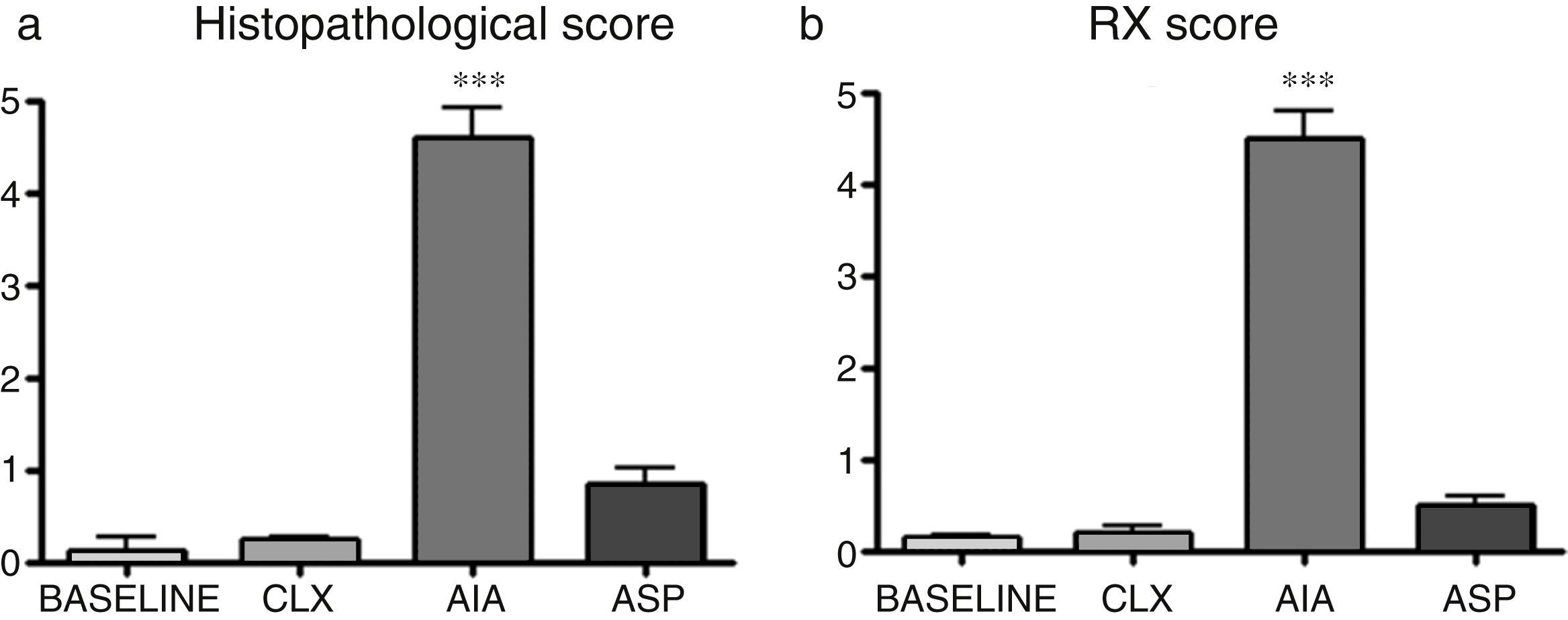
| Histopathological score | 0.11±0.25 | 0.25±0.05 | 4.6±0.54 | 0.83±0.33 | p<0.001 AIA vs. baseline, CLX and ASP |
| Conf. int. 95% | 0.07–0.29 | 0.21–0.29 | 4.11–4.87 | 0.59–1.07 | |
| RX | 0.14±0.03 | 0.19±0.12 | 4.5±0.52 | 0.5±0.16 | p<0.001 AIA vs. ASP, CXL and baseline |
| Conf. int. 95% | 0.12–0.16 | 0.10–0.28 | 4.13–4.87 | 0.39–0.61 | |
| IL-6 | 107.66±24.38 | 226.8±35.78 | 179±38.06 | 270±65.14 | p<0.001 AIA, ASP and CLX vs. baseline |
| Conf. int. 95% | 90.22–125.10 | 201.21–252.39 | 152.16–206.61 | 223.40–316.60 | |
| IL-4 | 9.16±1.51 | 7.54±2.77 | 3.05±1.51 | 9.4±3.3 | p<0.001 AIA vs. ASP and baseline |
| Conf. int. 95% | 8.08–10.24 | 5.56–9.52 | 1.97–4.13 | 7.04–11.76 | p<0.01 AIA vs. CXL |
| IL-1β | 118.52±22.93 | 275.1±136.93 | 189.97±43.78 | 170.29±23.88 | p<0.001 AIA and CXL vs. baseline |
| Conf. int. 95% | 102.12–134.92 | 177.15–373.05 | 158.65–221.29 | 153.21–187.37 | p<0.01 ASP vs. baseline; p<0.05 ASP vs. CLX |
| TNF-α | 143.02±15.64 | 112.5±81.49 | 285.45±96.93 | 141.51±11.17 | p<0.001 AIA vs. ASP, CXL and baseline |
| Conf. int. 95% | 131.83–154.21 | 54.21–170.79 | 216.12–354.78 | 133.52–149.50 | |
| MMP-2 | 100±27.25 | 152.34±106.21 | 123.30±42.23 | 278.86±12.55 | p<0.001 ASP vs. AIA, CXL and baseline |
| Conf. int. 95% | 80.69–119.31 | 76.37–228.31 | 93.09–153.51 | 269.88–287.84 | |
| MMP-9 | 0.15±0.05 | 0.19±0.13 | 62.42±17.62 | 23.29±11.54 | p<0.001 AIA and ASP vs. baseline and CLX |
| Conf. int. 95% | 0.11–0.18 | 0.10–0.28 | 49.82–75.02 | 15.03–31.54 | p<0.01 AIA vs. ASP |
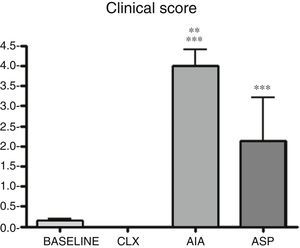
Clinical score. It can be observed that AIA and ASP are the arthritis models that produce significant differences in the clinical score in relation to the baseline group (p<0.001). In addition, the AIA demonstrated to be clinically more aggressive than the ASP (p<0.01). These results would indicate that, to carry out studies evaluating clinical symptoms and responses to a drug, the ASP and AIA models require less time than the CLX model. In addition to the observed claudication, these 2 models showed deformation and edema of the limb in many cases (Fig. 1).
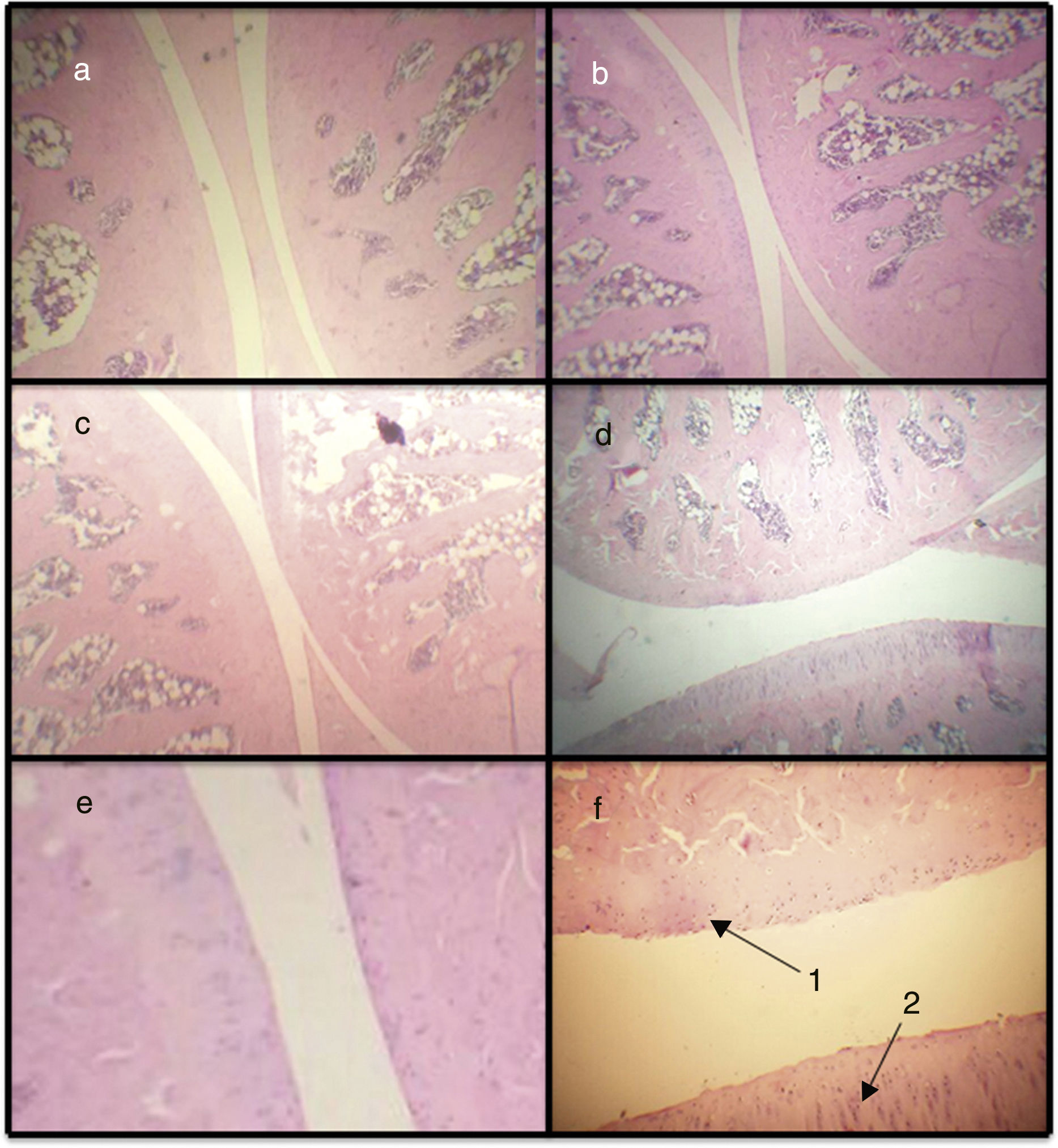
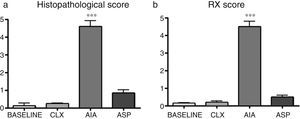
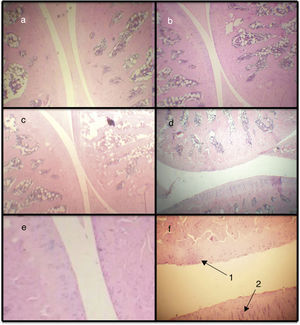
Histopathological score. By means of the histopathological score, it can be observed that the 3 models show significant differences with respect to the baseline model, with the AIA model being the one that presents more difference (Figs. 2a and 3). In this way, the AIA generates an intense inflammatory reaction at day 7, whereas the other 2 models, ASP and CLX, develop lesions in a less intense manner. The CLX and ASP models exhibited mainly occasional areas of superficial fibrillation of the structure of the cartilage. On the other hand, the AIA presented fissures in the articular cartilage with loss of the cartilage matrix and hypercellularity (Fig. 3).
(a) Differences in the histopathological analyses between the different groups quantified by a histological score. ANOVA was applied followed by the Tukey's test. The AIA group shows significant differences (***p<0.001) vs. the 3 remaining groups. (b) Results of the radiographic score in which is seen that the model which presents the greatest lesions is the AIA (***p<0.01 vs. the rest of the models).
Microphotographs of histological sections corresponding to the femorotibiopatellar joint. Baseline (a), CLX (b), ASP (c) and AIA (d) (100×). Sections (e) and (f) correspond to magnification of healthy and damaged sections, respectively (400×). (d) Damage in the articular surface and is magnified. In (f) is observed articular fibrillation (1) and hypercellularity (2) at the level of the articular cartilage.
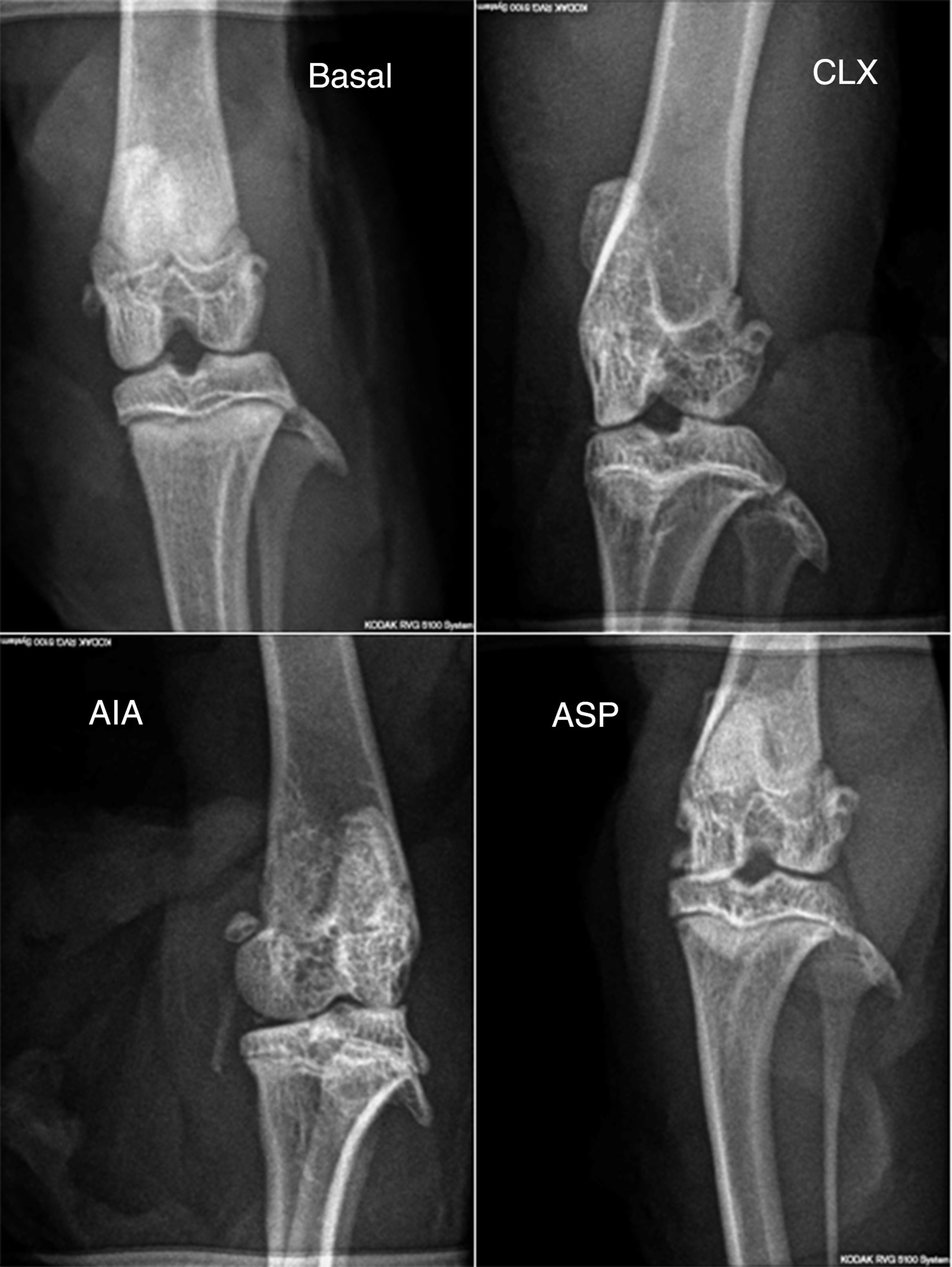
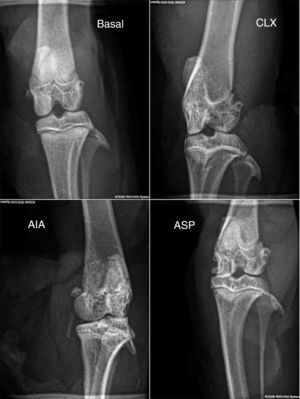
Radiological score. In the radiological score were obtained results similar to those observed with the models in histopathology. The AIA model showed significant differences (p<0.01) with respect to the rest of the models (Fig. 2b and 4), with the AIA model being the one that presented the greatest radiological damage, showing severe loss of the articular contour and narrowing of the joint space. On the other hand, the ASP and CLX models evidenced mild degrees of deformation and narrowing of the joint space (Fig. 4).
Radiographs in anteroposterior position of the members of each group (baseline, CLX, AIA, and ASP). The greatest lesions were presented by the ASP group, where is observed some degree of deformation, bone erosion, periostitis, narrowing of the joint space, osteopenia and loss of the bone contour.
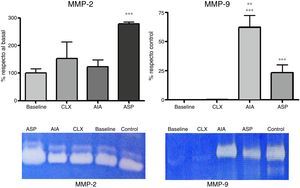
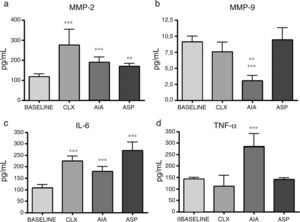
Zymographies. The zymographic results in SDS-PAGE with the aggregate of gelatin showed 2 bands corresponding to MMP-2 and MMP-9, respectively. According to the densitometric studies referred with the standard samples, we could observe that the levels of MMP-2 were very high in the ASP model, with it being the only one that presented significant differences with respect to the baseline model (p<0.01) (Fig. 5). Likewise, when analyzing the MMP-9, we observed that it was increased in the 2 adjuvant-induced arthritis models (ASP and AIA), being that MMP-9 increases at the joint level exclusively in pathological processes. It is highlighted that the CLX model does not present high levels of MMP-9 at day 7 (Fig. 5).
Expression of MMP-2 and MMP-9 from articular homogenates for each of the models, the control corresponds to a sample of MMP-2 and MMP-9, respectively, which is used to locate the bands and refer the densitometry of different gels. MMP-2 was expressed as the percentage of activity in relation to the baseline group. MMP-9 was expressed as the percentage of activity in relation to the control sample. ANOVA was applied followed by Tukey's test. For MMP-2: ***p<0.001 ASP vs. AIA, CXL and baseline. For MMP-9: ***p<0.001 AIA and ASP vs. baseline and CLX, **p<0.01 AIA vs. ASP.
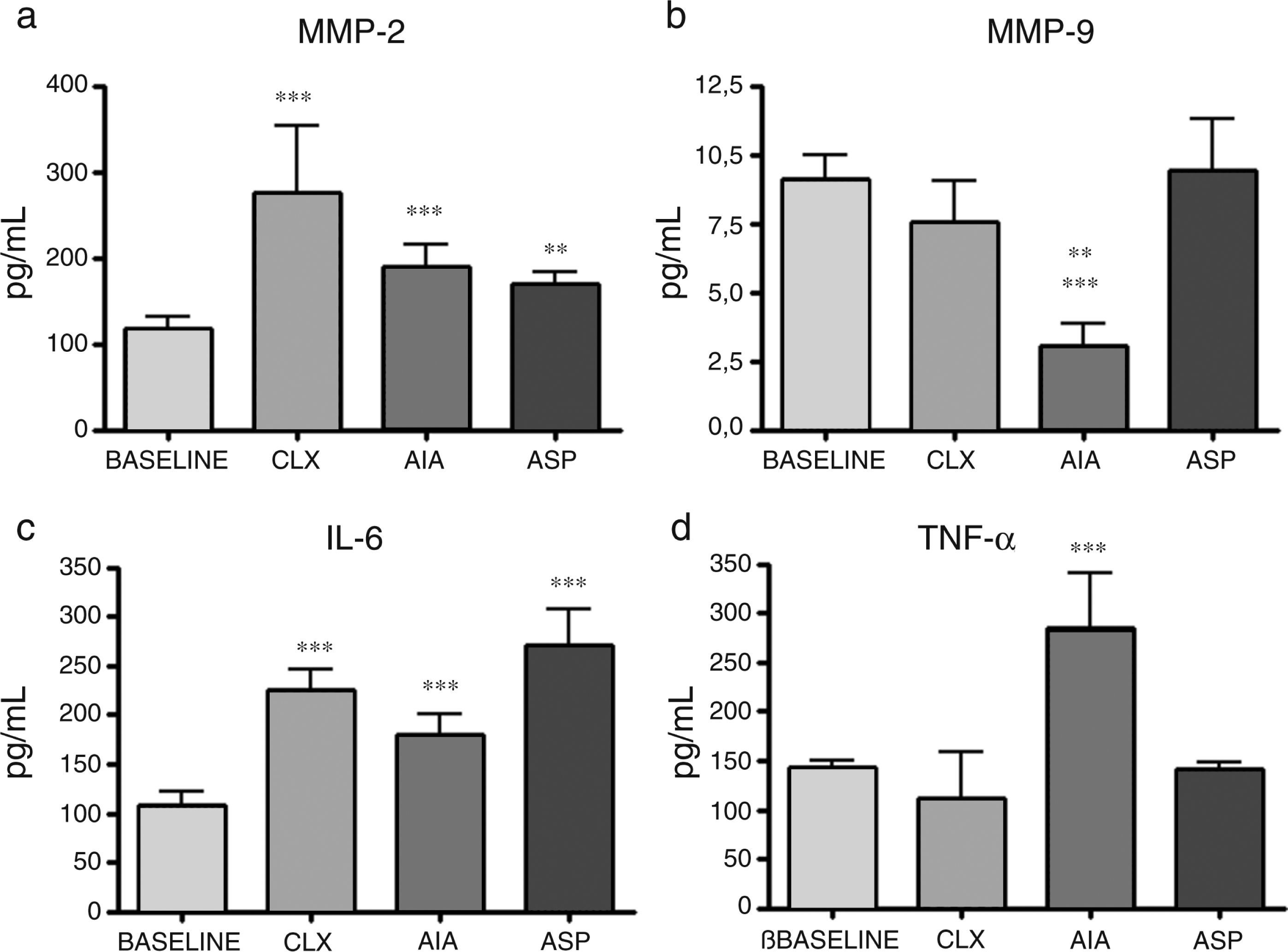
ELISA of cytokines. The cytokines presented variable results between them and according to the model in question. Regarding IL-1β, while all models had increases with respect to the baseline model, the CLX and AIA models showed the differences with the highest level of significance (p<0.001), although the ASP model also presented significant difference with respect to the baseline model (p<0.01). On the other hand, IL-4, which is a cytokine related with the profile of TH2 and anti-inflammatory response, showed in the AIA model a significant drop in relation to the baseline group and the ASP group (p<0.001); the AIA model also showed significant difference compared with the CLX model, although with less significance (p<0.01). In addition, IL-6 showed increases in the 3 models, being most notable in the ASP and CLX models (p<0.001) vs. the baseline model. Finally, TNF-α showed a significant increase only in the AIA model vs. the other 3 models (p<0.001) (Fig. 6).
Concentration of cytokines in the baseline rats and in those belonging to the different models of arthritis studied. ANOVA was applied followed by the Tukey's test. (a) IL-1β: CLX and AIA models (***p<0.001) vs. baseline, ASP vs. baseline (**p<0.01). (b) IL-4: AIA vs. baseline and ASP (***p<0.001), AIA vs. CLX (**p<0.01). (c) IL-6: ASP, AIA and CLX, (***p<0.001) vs. baseline. (d) TNF-α: AIA (***p<0.001) vs. the baseline group and the other 2 models (CLX and ASP).
In this work, when comparing the results in different models of induced arthritis, we can observe that although in the literature the models are used indistinctly to test different drugs or to evaluate the response to them, the difference in the clinical manifestation induced by them should be considered. In our work, we focused on the early phase of OA, which would correspond to an acute phase model. As we can see in Table 1, only the adjuvant-induced arthritis models show a clinical symptomatology at day 7. Many authors use the intradermal route at the base of the tail in Lewis inbred rats and describe the development of edema and arthritis after 2 weeks.18 Certain differences could be due to the susceptibility of the strains of rats to generate systemic arthritis; in our work, we used rats of the Sprague Dawley strain, which according to the bibliography are among the strains capable of producing systemic arthritis.4,19 Other authors who also used sub-plantar routes observed edema and inflammation in the paw, which reached the peak at days 5–7, and then entered into a more chronic stage by the day 20.20
The CLX model has been previously obtained in different animal species such as canines, rabbits, guinea pigs and rats.12 The CLX model is indicated to generate instability in the joint and to trigger arthritis in the medium or long term; however, some works have observed histopathological and radiological lesions within 2 weeks.21 Like in other models not studied in this work, such as collagen-induced arthritis and antigen-induced arthritis, histopathological lesions are observed in the AIA and ASP models at day 7. These lesions are characterized by synovial inflammation and early erosion of the articular cartilage.
IL-1β is found increased with respect to the control in the 3 arthritis models tested, which could indicate that this cytokine would be a possible general target for the control of arthritis of different types. The use of the IL-1β receptor antagonist showed variable results in different inflammatory models22 and in patients with OA.23 On the other hand, some works showed protective effects in relation with the degradation of the articular cartilage and the progression of bone erosion.24 As for the cytokines, the AIA model is the one that has produced the highest increase in TNF-α. The increase in TNF-α has been associated with an increase in leukocyte infiltrates that leads to joint inflammation, pain and damage. The increase in TNF-α has been associated in human medicine with certain types of arthritis such as rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Increases in IL-1β and TNFα have been related to the induction of MMP, which would be linked to the destruction of the extracellular matrix and to the joint damage itself.25 Likewise, in models of injection of IL-1β into the joint space, it was observed that in the arthritis that was triggered there was a loss of proteoglycans of the joint cartilage.26 There was also an increase in IL-1β in other models, as is the case of collagen-induced arthritis.27 Other works demonstrated that TNF-α would be elevated in early stages of joint disease, whereas IL-1β would be elevated for a longer period of time.28 The suppression of TNF-α has demonstrated beneficial effects in different models of autoimmune arthritis.29 In addition to the importance of these cytokines by their role in the inflammatory process, it has been described that IL-1β, IL-6 and TNF-α participate directly by mediating pain in the articular nociceptive terminations.30 It has also been demonstrated that IL-6 plays an important role in the destruction of the articular cartilage in induced arthritis. In the same way, in our work, IL-4 was found to have the lowest levels in the AIA model. IL-4 is related to an effect in the arthritis models, which would be to reduce inflammation, proinflammatory cytokines, vascularization and bone destruction. The MMP-9 in the CLX models exhibits minimal values, similar to those of the baseline animals, while this protease is extremely elevated in the AIA model, in coincidence with the greater joint damage.
The model that showed significant variation with respect to the control group in the greatest number of variables was the AIA model. But IL-1, at day 7, showed a significant difference in all models with respect to the baseline, being more marked in the CXL, and for IL-4, the lowest value was observed in the AIA group. This work allowed us to evaluate and compare the behavior of the different variables in each model, in an early but acute phase of the joint disease, which could be useful at the time of choosing to work in an acute model of OA. On the other hand, the ASP model proved to be very useful as a model of clinical inflammation at 7 days and was the one that presented the highest elevation in MMP-2. Although the arthritis models in rats are used and compared indistinctly as if they were similar, in this work, we demonstrated that in an early stage the models behave in a different manner in most of the studied variables.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingProject UBACYT 20020120100092BA.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: De Simone E, Lastra Y, Caggiano N, Díaz J, Rubatino F, Ferretto A, et al. Estudio comparativo de la fase temprana de artritis experimental en 3 modelos de ratas. Rev Colomb Reumatol. 2017;24:92–101.