The SARS-CoV-2 virus was first identified in December 2019, the infection was named COVID-19. The initial symptoms and evolution of the disease have been described over the past year. The virus has been shown to increase the risk of thromboembolic events due to the hypercoagulable state triggered by systemic endothelial inflammation. We present the case of a patient with a history of rheumatoid arthritis under prolonged treatment with tofacitinib, who presented COVID-19 and subsequently developed a hypercoagulable state of approximately 6 months’ duration. The possible association between viral infection and the use of tofacitinib is debated.
El virus SARS-CoV-2 se identificó por primera vez en diciembre de 2019; la infección se denominó COVID-19. Los síntomas iniciales y la evolución de la enfermedad se han descrito durante el último año. Se ha demostrado que el virus aumenta el riesgo de eventos tromboembólicos debido al estado de hipercoagulabilidad desencadenado por la inflamación endotelial sistémica. Se presenta el caso de un paciente con antecedente de artritis reumatoide en tratamiento prolongado con tofacitinib, que presentó COVID-19 y posteriormente desarrolló un estado de hipercoagulabilidad de aproximadamente seis meses de duración. Se debate la posible asociación entre la infección viral y el uso de tofacitinib.
The SARS-CoV-2 virus responsible of the COVID-19 pandemic, presents mostly with constitutional symptoms such as fever, myalgias, headaches and respiratory symptoms, however, it has been evidenced that there is a procoagulant pattern with elevated fibrinogen, d-dimer and prothrombin time. The pathophysiology under this mechanism is believed to be due to endothelial damage, which causes microvascular thrombi that impede homogeneous flow and produce a vascular shunt that is reflected in hypoxia and end-organ damage. Tang et al. evidenced that the use of anticoagulation with low molecular weight heparin in patients with COVID-19 and d-dimer >6-fold of upper limit of normal is associated with better prognosis and decreased mortality.1
It is relevant to take into consideration diseases that are based on a chronic inflammatory state such as rheumatoid arthritis, since it can interfere with the management and development of both rheumatoid arthritis and co-infection with SARS-CoV-2. We present a possible association between tofacitinib therapy and increased d-dimer in a patient with rheumatoid arthritis and COVID-19 infection.
Case reportWe present the case of a 32-year-old female with past medical history of rheumatoid arthritis diagnosed approximately 10 years ago, and no other relevant medical condition. No allergies, no surgical and obstetric history. Her treatment included tofacitinib 2.5mg twice a day, leflunomide 20mg daily, folic acid 1mg daily and vitamin D 800UI daily. In the early stages of her disease the patient received etanercept and infliximab with no adequate response. She has been in remission since tofacitinib was initiated approximately 4 years ago. No history of thrombotic events since the initial diagnosis.
On April 2020, the patient reported flu-like symptoms, diarrhea and positive contact exposure to COVID-19. She underwent self-isolation, her symptoms resolved within the next 10 days. She tested positive for COVID-19 RT-PCR via nasopharyngeal swab. Additionally, the household members tested positive for COVID-19 antibodies.
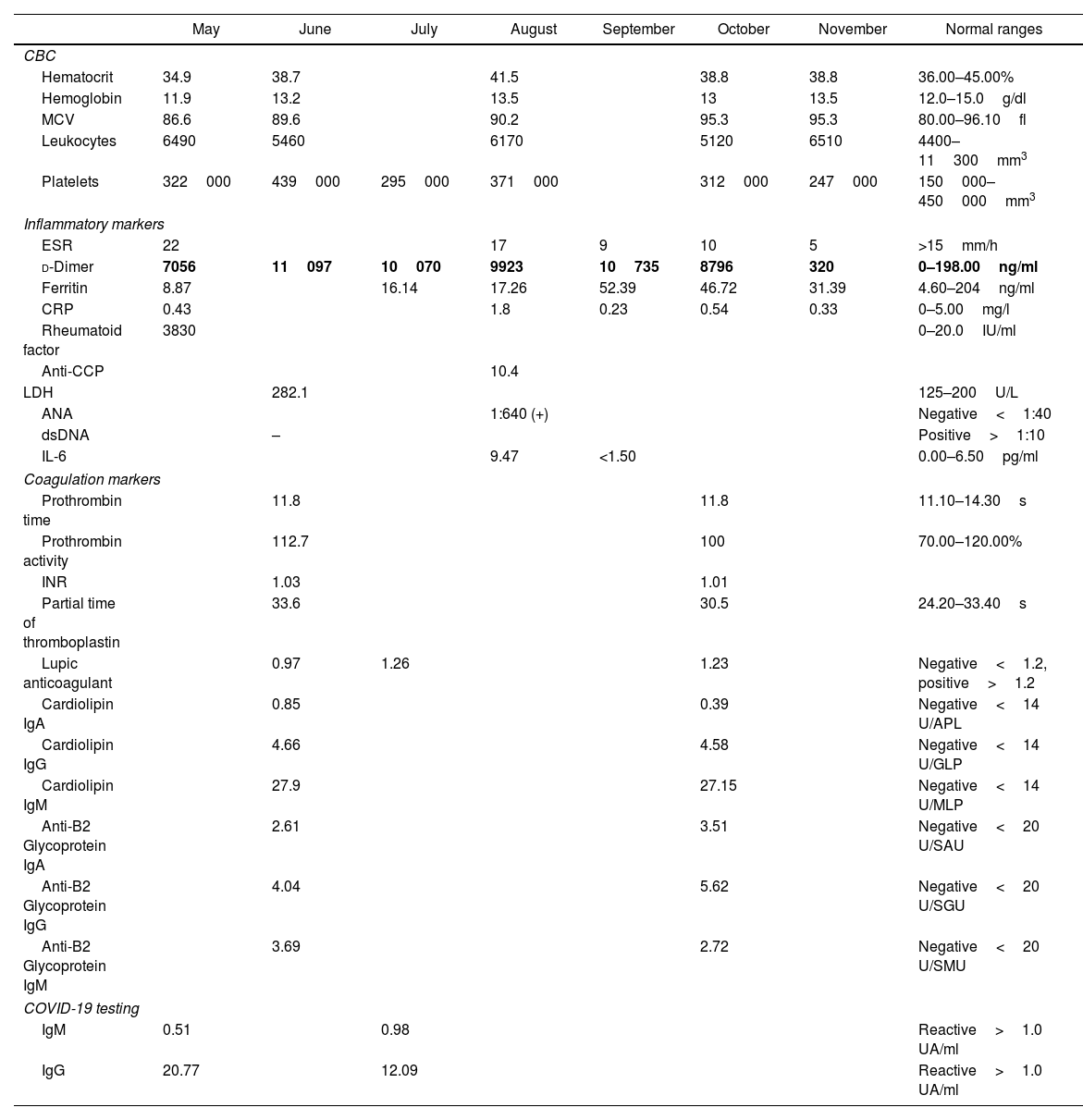
She visited the rheumatology clinic on May 2020 due increased polyarthralgia and fatigue. On physical exam she presented bilateral painful and swollen metacarpophalangeal (MCP) and proximal interphalangeal joints (PIP) with a CDAI of 21 which represented moderate disease activity. Laboratory results are available in Table 1. Elevation of d-dimer was evidenced. COVID-19 antibodies test was IgM 0.51 (−) and IgG 20.77 (+). CXR and chest CT negative for infiltrates or consolidations. Hematology service was consulted in the setting of the hypercoagulable state of COVID-19, recommended rivaroxaban 10mg daily, further modified to rivaroxaban 2.5mg and prednisone 6mg. Further studies included lower extremity doppler negative for deep venous thrombosis, echocardiogram showed an EF of 60% with no structural abnormalities. On July 2020 COVID-19 antibodies test was IgM 0.98 (−) and IGG 12.09 (+).
Laboratory results.
| May | June | July | August | September | October | November | Normal ranges | |
|---|---|---|---|---|---|---|---|---|
| CBC | ||||||||
| Hematocrit | 34.9 | 38.7 | 41.5 | 38.8 | 38.8 | 36.00–45.00% | ||
| Hemoglobin | 11.9 | 13.2 | 13.5 | 13 | 13.5 | 12.0–15.0g/dl | ||
| MCV | 86.6 | 89.6 | 90.2 | 95.3 | 95.3 | 80.00–96.10fl | ||
| Leukocytes | 6490 | 5460 | 6170 | 5120 | 6510 | 4400–11300mm3 | ||
| Platelets | 322000 | 439000 | 295000 | 371000 | 312000 | 247000 | 150000–450000mm3 | |
| Inflammatory markers | ||||||||
| ESR | 22 | 17 | 9 | 10 | 5 | >15mm/h | ||
| d-Dimer | 7056 | 11097 | 10070 | 9923 | 10735 | 8796 | 320 | 0–198.00ng/ml |
| Ferritin | 8.87 | 16.14 | 17.26 | 52.39 | 46.72 | 31.39 | 4.60–204ng/ml | |
| CRP | 0.43 | 1.8 | 0.23 | 0.54 | 0.33 | 0–5.00mg/l | ||
| Rheumatoid factor | 3830 | 0–20.0IU/ml | ||||||
| Anti-CCP | 10.4 | |||||||
| LDH | 282.1 | 125–200U/L | ||||||
| ANA | 1:640 (+) | Negative<1:40 | ||||||
| dsDNA | – | Positive>1:10 | ||||||
| IL-6 | 9.47 | <1.50 | 0.00–6.50pg/ml | |||||
| Coagulation markers | ||||||||
| Prothrombin time | 11.8 | 11.8 | 11.10–14.30s | |||||
| Prothrombin activity | 112.7 | 100 | 70.00–120.00% | |||||
| INR | 1.03 | 1.01 | ||||||
| Partial time of thromboplastin | 33.6 | 30.5 | 24.20–33.40s | |||||
| Lupic anticoagulant | 0.97 | 1.26 | 1.23 | Negative<1.2, positive>1.2 | ||||
| Cardiolipin IgA | 0.85 | 0.39 | Negative<14 U/APL | |||||
| Cardiolipin IgG | 4.66 | 4.58 | Negative<14 U/GLP | |||||
| Cardiolipin IgM | 27.9 | 27.15 | Negative<14 U/MLP | |||||
| Anti-B2 Glycoprotein IgA | 2.61 | 3.51 | Negative<20 U/SAU | |||||
| Anti-B2 Glycoprotein IgG | 4.04 | 5.62 | Negative<20 U/SGU | |||||
| Anti-B2 Glycoprotein IgM | 3.69 | 2.72 | Negative<20 U/SMU | |||||
| COVID-19 testing | ||||||||
| IgM | 0.51 | 0.98 | Reactive>1.0 UA/ml | |||||
| IgG | 20.77 | 12.09 | Reactive>1.0 UA/ml | |||||
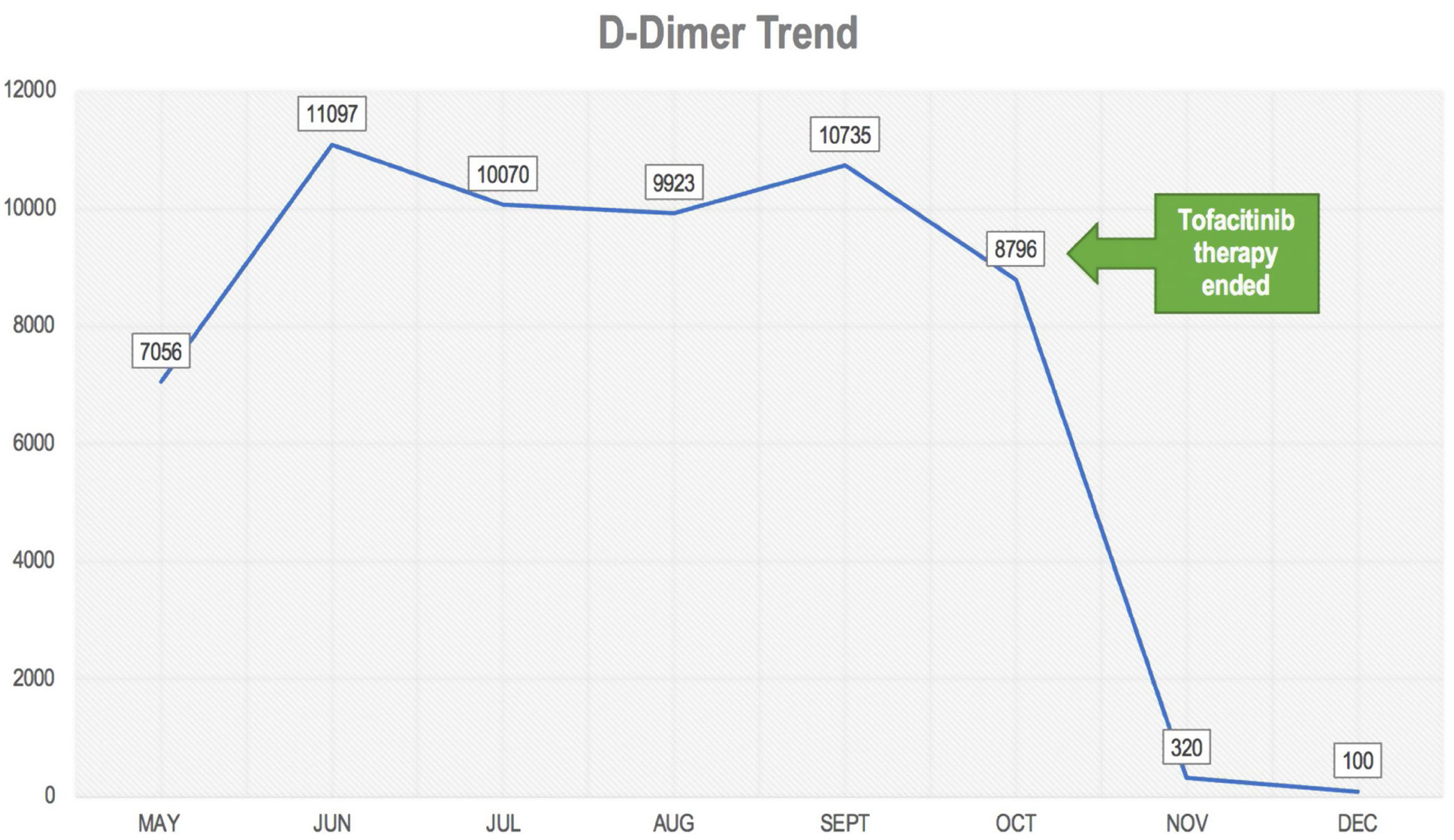
Bold text indicates the D-Dimer trend.
Further follow up showed an increase d-dimer trend for approximate 6 consecutive months (Fig. 1). In the mist of this situation, it was decided to discontinue tofacitinib in late October and start tocilizumab 8mg/kg. Anticoagulation treatment was discontinued after 6 months and d-dimer reduction was seen. During her last visit in December 2020, the patient denied polyarthralgia, further CDAI was 0 and d-dimer levels were back to normal.
DiscussionWe present the case of a patient with rheumatoid arthritis under treatment with tofacitinib of approximately 4 years duration without major complications, who in the presence of SARS-CoV-2 infection developed a hypercoagulable state with persistent elevation of d-dimer of approximately 6 months. The patient did not present any thromboembolic event, however, due to a history of rheumatoid arthritis, it was decided to start anticoagulant therapy. A normalization of d-dimer level was not evident until tofacitinib was discontinued, suggesting a possible association between the increase in d-dimer and background medication, which is potentiated with SARS-CoV-2 infection.
Miesbach et al. reported an incidence of thromboembolic events in 40% of critical patients infected with SARS-CoV-2,2 Zhang et al. reported an incidence of stroke in 5.7% of critically ill patients with the infection.3 Due to the presence of a hypercoagulable state, it is considered to start anticoagulation with LMWH in patients with elevated d-dimer in 3- to 4-fold increase.4
Mehta et al. highlighted in a letter to the editor the possible association between increased risk of thromboembolic events and the use of janus kinase inhibitors (JAKi).5 Tofacitinib is a JAK 1/3 inhibitor used in the treatment of rheumatoid arthritis and ulcerative colitis, and has an FDA box warning for venous or arterial thrombotic events. The discussion is whether tofacitinib is a causal agent of thrombotic events,6 or if there is a background factor that contributes to the hypercoagulable state, as is the case of RA, where the risk of DVT is 0.3–0.7 per 100 patients/year compared to the general population where the risk is 0.1–0.4 per 100 patients/year.7
The FORWARD study has a registry of approximately 530 patients with rheumatic diseases who were asked if they made any changes to their therapeutic regimen during these months of the COVID-19 pandemic, 14% reported having made changes on their own and 11% Based on medical advice, this demonstrated a perception of an increased risk of complications from COVID-19 secondary to the immunosuppressants indicated for their underlying disease.8 However, it has been shown that patients with rheumatic diseases have a similar incidence of contagion and evolution of the disease compared to the general population.9,10
Because SARS-CoV-2 is a procoagulant risk factor and rheumatoid arthritis can be a causative agent as well, it is important to evaluate these patients, and if necessary, consider anticoagulant therapy.
ConclusionThe presence of rheumatic diseases such as rheumatoid arthritis is a risk factor for venous and/or arterial embolic events, however, the possible association with COVID-19 is uncertain. In addition, the added factor of the use of tofacitinib should be studied. In this case, the levels of d-dimer decreased to normal after discontinuing tofacinitib, which makes a possible association between the drug and an increase in the hypercoagulable state in COVID-19 more evident. More studies are needed to confirm this possible relationship.
Data availabilityThe authors confirm that the data supporting the findings of this study are available within the article.
ConsentThe patient signed an inform consent for the release of the information mentioned in the case report.
Ethical approvalThe study was approved by the Ethics and Teaching Committee of the Centro de Reumatología y Rehabilitación (CERER) (Registration number: 006/2021; Folio 01: Book of Acts No. 1).
FundingThis work was not financially supported by any organization. Authors are part of the Rheumatology Department of the Universidad Espiritu Santo and Internal Medicine Department of Loyola MacNeal Hospital.
Conflicts of interestThe authors declare that they have no conflicts of interest.