To assess serum levels of vitamin D in renal transplant patients, and if the glomerular filtration rate affects them.
MethodsThe study included patients older than 18 years with a kidney transplant for more than 1 year. Demographic, anthropometric, solar exposure, aetiology of chronic pre-transplant renal disease (CKD), bone densitometry, and laboratory variables related to bone and mineral disorders were evaluated.
StudyCross-sectional analytical study with review of medical records. Descriptive statistical methods were used to measure central tendency, dispersion (mean, standard deviation), and absolute and relative frequencies. A lineal regression method was used to determine the correlation between vitamin D levels with each of the laboratory tests included, especially with GFR.
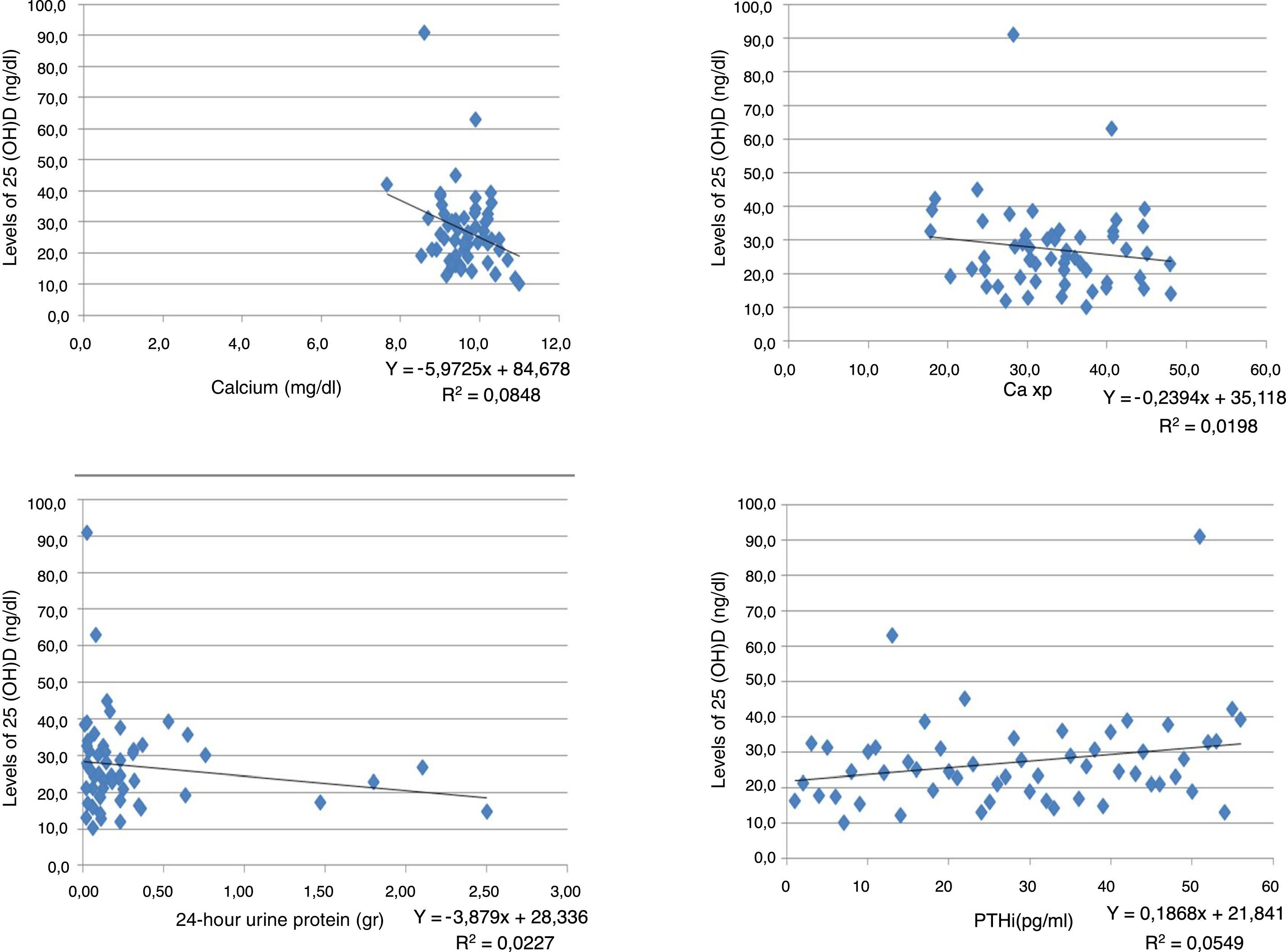
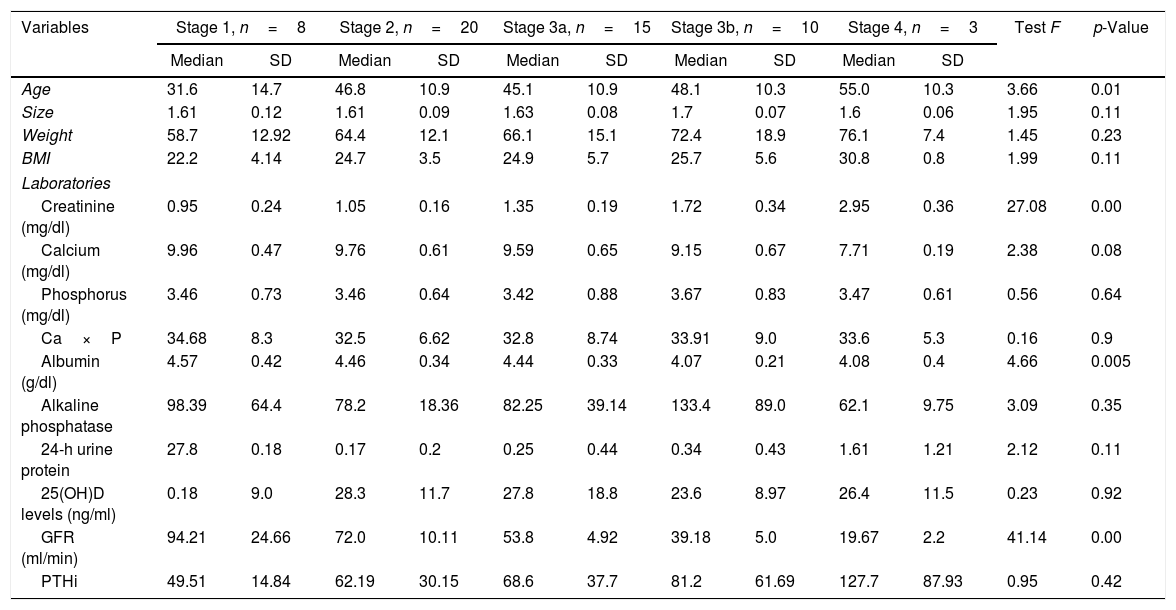
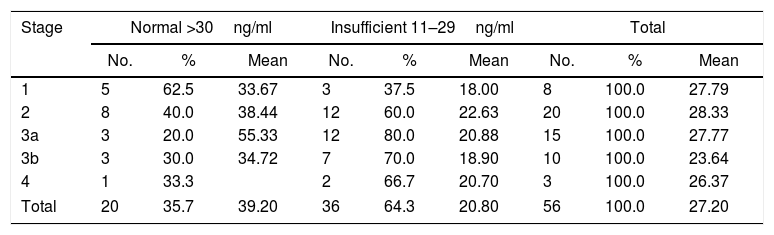
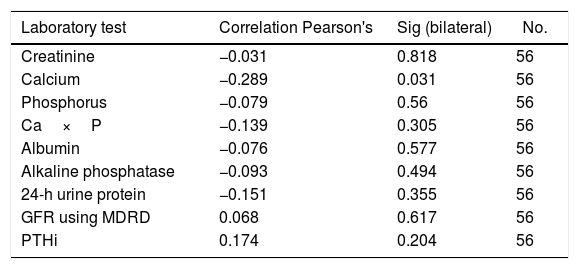
ResultsA total of 56 patients met the inclusion criteria, 29 men and 27 women, mean age 44.8±12.4 years, mostly of mixed race (57.1%), main aetiology of CKD unknown (55.3%). Only 35.7% of the patients had normal levels of vitamin D, and 64.3% had insufficient levels. None of the patients had levels in the deficit range. No significant differences were found between the vitamin D results and the stage of CKD. An inverse relationship was found between vitamin D levels and creatinine, calcium, phosphorus, Ca×P, albumin, and alkaline phosphatase. The ratio was positive for 24-h urine protein, GFR and PTHi, although the ratio is very weak in all of them. The bone density results were within the normal range in 41.07% of patients, with 46.43% osteopenia, and 12.5% osteoporosis. No correlation was found between vitamin D levels and the degree of bone alteration.
ConclusionsIn renal transplant patients it is common to detect insufficient levels of vitamin D, regardless of the GFR.
Evaluar los niveles séricos de vitamina D en pacientes trasplantados renales, la relación con la tasa de filtración glomerular y la correlación con diversas variables clínicas y de laboratorio que podrían afectarlos.
MétodosPacientes con más de un año de trasplante renal, mayores de 18 años. En ellos se evaluaron variables demográficas, antropométricas, grado de exposición solar, etiología de la enfermedad renal crónica pretrasplante, osteodensitometría y variables de laboratorio relacionadas con desórdenes óseos y minerales.
EstudioAnalítico de corte transversal con revisión de historias clínicas. Se utilizaron métodos estadísticos descriptivos como medidas de tendencia central, de dispersión (media, desviación estándar), frecuencias absolutas y relativas. Para determinar la correlación entre los niveles de vitamina D con cada una de las pruebas de laboratorio incluidas, especialmente con la tasa de filtración glomerular (TFG), se utilizó el método de regresión lineal.
ResultadosCincuenta y seis pacientes cumplieron los criterios de inclusión, 29 hombres y 27 mujeres, edad media de 44,8±12,4 años, la mayoría mestizos (57,1%), principal etiología de enfermedad renal crónica (ERC) desconocida (55,3%). Solo el 35,7% de los pacientes tenían niveles normales de vitamina D y el 64,3%, insuficientes; ninguno de los pacientes tenía niveles en rango de déficit. Al analizar los resultados de la vitamina D y el estadio de ERC, se encontró que no existían diferencias significativas entre ellos. Se encontró relación inversa entre los niveles de vitamina D y las pruebas de creatinina, calcio, fósforo, Ca×P, albúmina, fosfatasa alcalina y proteínas en orina de 24h. Mientras que para la TFG y la hormona paratiroidea intacta la relación fue positiva, aunque en todas las pruebas la relación es muy débil. Los resultados de la osteodensitometría mostraron valores normales para el 41,07% de los pacientes, osteopenia el 46,43% y osteoporosis el 12,5%. No se encontró correlación entre los niveles de vitamina D y el grado de alteración ósea.
ConclusionesEn pacientes trasplantados renales es frecuente detectar niveles insuficientes de vitamina D, independientemente de la TFG que tengan.
Kidney transplant is the best therapeutic option in patients with chronic kidney disease (CKD), because it improves the long term survival, reduces the morbidity and cardiovascular complications, in addition to improving the quality of life and reducing the costs for the healthcare system, as compared with patients undergoing dialysis.1,2 According to the recent recommendation of the KDIGO3 Guidelines, Kidney transplant patients should be included in the group of patients with CKD, in various stages in accordance with their glomerular filtration rate (GFR), including the so called End-Stage Kidney Disease – ESKD – with most of them having a GFR of just 70ml/min in the immediate postoperative period.
Immunosuppressive therapy to prevent rejection of the transplanted organ in these patients has been associated with skin cancer; so the general recommendation for post-transplanted patients is to reduce sun exposure, in order to lower the incidence of skin cancer.4
Ultraviolet light from sun exposure is necessary for the synthesis of active vitamin D (1.25[OH]2D or calcitriol), a process that starts on the skin from 7-dehydrocholesterol, and in the presence of ultraviolet light, produces pre-vitamin D3 that then undergoes thermal isomerization to be converted into vitamin D3. This vitamin D3, under the activity of the CYP2R1 enzyme in the liver, results in 25-hydroxyvitamin D3 (calcidiol [25(OH)D3]), which is filtered in the glomeruli, reabsorbed by the proximal convoluted tubule and exposed to mitochondrial enzyme CYP27B1 (1 alpha hydroxilase), hence generating the active form.5 Active vitamin D is necessary for the optimal intestinal absorption of calcium and phosphorus, for calcium reabsorption in the renal distal tubule, and to inhibit parathyroid hormone (PTH) secretion. Low vitamin D levels result in hypocalcemia which activates the production and release of PTH, increases bone calcium resorption, leading to osteomalacia and osteoporosis.
Recently we were able to establish that in a population of patients with CKD stages 2 to 5, living in Manizales City, Caldas, Colombia, South America, at an altitude of 2200m above sea level, the prevalence of low calcidiol levels affected around 78.80% of the population studied6; moreover, lower GFR values were associated with lower the vitamin D levels.
Kidney transplant patients are considered to have ESKD and low sun exposure, which are predisposing factors for the development of insufficient vitamin D levels. The objective of our study was to determine in a group of kidney transplant patients with various GFRs, the serum vitamin D levels and their correlation with several clinical and laboratory variables that could have an impact.
Materials and methodsKidney transplant patients aged 18 years and older, residents of various cities of the Caldas Department, Colombia, South America, who were under the mandatory health plan and in the post-transplant outpatient clinic of the Internal Medicine-Nephrology Program of Caldas University, Manizales, and the Renal Therapy Service (RTS) Caldas affiliate were included in the trial. The inclusion period and data collection spanned from August 19, 2015 to June 19, 2017. The statistical analysis was conducted over the month of July 2017.
The exclusion criteria were: kidney transplant within less than one year, having received prednisone doses above 10mg per day or IV steroid pulses in the last 12 months; changes in the immunosuppressive therapy over the past six months, parathyroidectomy, primary hyperparathyroidism confirmed through neck ultrasound and sestamibi bone scan, liver disease, hyperphosphaturia, hypocalciuric hypercalcemia, hospitalization in the last 2 months, active infection, having received native vitamin D therapy over the past 12 months, and excessive sun exposure over the last 30 days.
No informed consent was required because at the RTS Caldas affiliate the standard practice adopted since 2013 - and based on the KDIGO Guidelines for bone and mineral disorders of 2009,7 – for transplant patients, included the requirement of once-a-month creatinine test, calcium and phosphorus every three months, alkaline phosphatase every 6 months, and vitamin D, PTH, serum albumin, 24-h urine proteins, and bone densitometry once a year.
The demographic, anthropometric and clinical variables collected were: gender, age, ethnic group, body weight, size, body mass index (BMI), level of sun exposure, pre-transplant CKD aetiology, ESKD stage, and time elapsed since the kidney transplant.
Patients were questioned about the level of sun exposure, and a physical examination of the hands, face, and arms was conducted for confirmation and classification into three levels: level 1 (low) less than 1h per week, level 2 (moderate) between 1 and 3h per week, and level 3(adequate) more than 3h per week.8
The BMI was estimated using the formula: BMI=weight (kg)/height (m2), with the following classification: skinny or low weight <18.49; normal from 18.5 to 24.99; overweight 25.00 to 29.00, and obese >30.00.
The laboratory variables collected were: creatinine, GFR estimated according to the MDRD9 formula, calcium, phosphorus, calcium×phosphorus (Ca×P), albumin, intact PTH (PTHi), alkaline phosphatase, 24-h urine protein, and total native vitamin D (25 [OH]D or calcidiol) levels; the latter were estimated using the electro-chemiluminiscence technique. The result of the bone densitometry recorded within one year of measuring the vitamin D levels was also noted.
The CKD was defined in accordance with the criteria of the KDIGO Guidelines for 20133 and was classified in stages, in accordance with the estimated GFR: 1: >90, 2: 60–90, 3a: 45–59, 3b: 30–44, 4: 15–29, and 5: <15.
The levels of total native vitamin D (25[OH]D) were defined in accordance with the relationship between the serum vitamin D levels, PTH and intestinal calcium transport according to the references by the International Osteoporosis Foundation and the National Osteoporosis Foundation 2005 and 2010,10,11 respectively; the American Geriatrics Society Consensus12 and the National Osteoporosis Society.13 The classification was as follows: normal >30ng/ml, insufficient: between 10 and 30ng/ml, and deficient < 10ng/ml.
A PTH above 70pg/ml was considered compatible with secondary hyperparathyroidism.
The bone densitometry report was expressed based on the T-score: normal (−1.0 or higher), osteopenia (between −1.0 and −2.5), and osteoporosis (−2.5 or less), pursuant to the recommendations of the World Health Organization and NBHAWG.14,15
All the medications that the patient was receiving at that time for immunosuppression, prevention, and treatment of osteoporosis were recorded.
The project was approved by the Bioethics Committee of Caldas University, the academic vice-president, and the ethics and RTS research committees for Colombia. The trial was considered risk-free, pursuant to Article 11, Chapter 1 of Resolution No. 008430 of 1993, of the Ministry of Health of the Republic of Colombia, that establishes the scientific, technical, and administrative standards for health research.
Type of studyAnalytical, cross-sectional trial with medical record review.
Statistical analysisThe information was collected on an Excel database which was completed by the investigators and its statistical analysis was done using the SPSS 15.0v statistical software in Spanish, under license for the University of Caldas.
All patients participating in the kidney post-transplantation programme that met the inclusion criteria were included and thus sampling was not considered necessary.
In order to meet the objectives, statistical descriptive methodological approaches were used, such as central tendency measurements, scatter measurements (mean, standard deviation), absolute and relative frequencies; the summary of the information is shown in charts or graphs. In order the establish an association between the vitamin D levels and the laboratory values, the F distribution probability was used, and the criterion to determine statistically significant differences was a test p value<0.05. To determine the correlation, the lineal regression statistical method was used, with its corresponding Pearson correlation coefficient.
ResultsSixty-two patients were evaluated, of which 6 were excluded because they failed to meet the inclusion criteria. Of the remaining 56 patients, 29 (51.8%) were males and 27 (48.2%) were females. The average age was 44.8±12.4 years, and the average body weight and height were 66.1±14.6kg and 1.6±0.1m respectively; the average BMI was 24.9±4.8. With regards to ethnicity, 32 (57.1%) patients were mestizos, 22 (39.2%) were white, and only 2 (3.57%) were black.
The time in years elapsed from the time of transplant was 10.5 years in average, with a standard deviation of 6.1 years.
The level of sun exposure was predominantly “level 1” with 40 (71.4%) patients in this category; there were 15 (26.8%) in “level 2”, and only one (1.79%) patient in “level 3”.
The primary aetiologies resulting in CKD prior to transplantation were: unknown aetiology in 29 (55.3%) patients, lupus nephritis 7 (12.5%), hypertensive nephropathy 5 (8.9%), and diabetic nephropathy 4 (7.1%) patients. Twenty (35.7%) patients had ESKD stage 2 and 15 (26.8%) stage 3a; the rest of the patients were stages 1, 3b and 4 (Table 1).
Demographic, anthropometric, and laboratory characteristics of patients with ESKD.
| Variables | Stage 1, n=8 | Stage 2, n=20 | Stage 3a, n=15 | Stage 3b, n=10 | Stage 4, n=3 | Test F | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | SD | Median | SD | Median | SD | Median | SD | Median | SD | |||
| Age | 31.6 | 14.7 | 46.8 | 10.9 | 45.1 | 10.9 | 48.1 | 10.3 | 55.0 | 10.3 | 3.66 | 0.01 |
| Size | 1.61 | 0.12 | 1.61 | 0.09 | 1.63 | 0.08 | 1.7 | 0.07 | 1.6 | 0.06 | 1.95 | 0.11 |
| Weight | 58.7 | 12.92 | 64.4 | 12.1 | 66.1 | 15.1 | 72.4 | 18.9 | 76.1 | 7.4 | 1.45 | 0.23 |
| BMI | 22.2 | 4.14 | 24.7 | 3.5 | 24.9 | 5.7 | 25.7 | 5.6 | 30.8 | 0.8 | 1.99 | 0.11 |
| Laboratories | ||||||||||||
| Creatinine (mg/dl) | 0.95 | 0.24 | 1.05 | 0.16 | 1.35 | 0.19 | 1.72 | 0.34 | 2.95 | 0.36 | 27.08 | 0.00 |
| Calcium (mg/dl) | 9.96 | 0.47 | 9.76 | 0.61 | 9.59 | 0.65 | 9.15 | 0.67 | 7.71 | 0.19 | 2.38 | 0.08 |
| Phosphorus (mg/dl) | 3.46 | 0.73 | 3.46 | 0.64 | 3.42 | 0.88 | 3.67 | 0.83 | 3.47 | 0.61 | 0.56 | 0.64 |
| Ca×P | 34.68 | 8.3 | 32.5 | 6.62 | 32.8 | 8.74 | 33.91 | 9.0 | 33.6 | 5.3 | 0.16 | 0.9 |
| Albumin (g/dl) | 4.57 | 0.42 | 4.46 | 0.34 | 4.44 | 0.33 | 4.07 | 0.21 | 4.08 | 0.4 | 4.66 | 0.005 |
| Alkaline phosphatase | 98.39 | 64.4 | 78.2 | 18.36 | 82.25 | 39.14 | 133.4 | 89.0 | 62.1 | 9.75 | 3.09 | 0.35 |
| 24-h urine protein | 27.8 | 0.18 | 0.17 | 0.2 | 0.25 | 0.44 | 0.34 | 0.43 | 1.61 | 1.21 | 2.12 | 0.11 |
| 25(OH)D levels (ng/ml) | 0.18 | 9.0 | 28.3 | 11.7 | 27.8 | 18.8 | 23.6 | 8.97 | 26.4 | 11.5 | 0.23 | 0.92 |
| GFR (ml/min) | 94.21 | 24.66 | 72.0 | 10.11 | 53.8 | 4.92 | 39.18 | 5.0 | 19.67 | 2.2 | 41.14 | 0.00 |
| PTHi | 49.51 | 14.84 | 62.19 | 30.15 | 68.6 | 37.7 | 81.2 | 61.69 | 127.7 | 87.93 | 0.95 | 0.42 |
| No. | % | No. | % | No. | % | No. | % | No. | % | x2 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnicity | ||||||||||||
| Mestizo | 5 | 62.5 | 9 | 45.0 | 10 | 66.7 | 5 | 50.0 | 3 | 100.0 | 4.31 | 0.36 |
| White | 2 | 25.0 | 11 | 55.0 | 5 | 33.3 | 4 | 40.0 | 0 | 0.0 | 2.82 | 0.42 |
| Black | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 | 0 | 0.0 | ||
The evaluation of the medicines used indicated that 100% of the patients received a low dose of steroid (prednisone or deflazacort), so a statistical analysis was not feasible. With regards to the other medications, 12 (21.4%) of the patients received azathioprine, 22 (39.3%) received mycophelonate mofetil, and 20 (35.7%) sodium mycophenolate. With regards to mTOR, only 3 (5.3%) patients received sirolimus and 7 (12.5%) everolimus. In terms of calcineurin inhibitors, 16 (28.6%) cyclosporine and 26 (46.4%) tacrolimus. No association was found between the type of immunosuppressant that the patients received and the serum vitamin D levels.
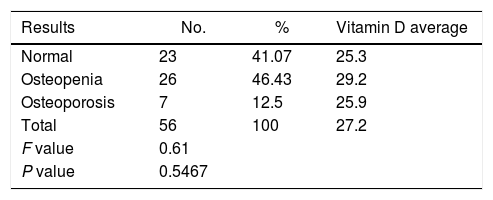
The results of vitamin D values showed that of the total number of patients analyzed, 20 (35.7%) had normal vitamin D levels, 36 (64.3%) had insufficient levels and none had a deficit of vitamin D.
Only among patients in ESKD stage 1, the percentage of patients with normal levels exceeded the percentage of patients with insufficient levels (Table 2), although when considering the association of the vitamin D levels and ESKD, no statistically significant differences were found, since the test result was 0.9207. An association was identified however with regards to creatinine, GFR, and albumin, with statistically significant differences for the various stages (Table 3).
Classification of vitamin D levels according to stage.
| Stage | Normal >30ng/ml | Insufficient 11–29ng/ml | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | No. | % | Mean | No. | % | Mean | |
| 1 | 5 | 62.5 | 33.67 | 3 | 37.5 | 18.00 | 8 | 100.0 | 27.79 |
| 2 | 8 | 40.0 | 38.44 | 12 | 60.0 | 22.63 | 20 | 100.0 | 28.33 |
| 3a | 3 | 20.0 | 55.33 | 12 | 80.0 | 20.88 | 15 | 100.0 | 27.77 |
| 3b | 3 | 30.0 | 34.72 | 7 | 70.0 | 18.90 | 10 | 100.0 | 23.64 |
| 4 | 1 | 33.3 | 2 | 66.7 | 20.70 | 3 | 100.0 | 26.37 | |
| Total | 20 | 35.7 | 39.20 | 36 | 64.3 | 20.80 | 56 | 100.0 | 27.20 |
Deficit: zero cases.
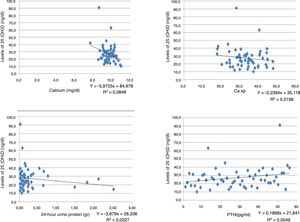
Correlation between the levels of vitamin D and the laboratory tests of individuals with chronic kidney disease.
| Laboratory test | Correlation Pearson's | Sig (bilateral) | No. |
|---|---|---|---|
| Creatinine | −0.031 | 0.818 | 56 |
| Calcium | −0.289 | 0.031 | 56 |
| Phosphorus | −0.079 | 0.56 | 56 |
| Ca×P | −0.139 | 0.305 | 56 |
| Albumin | −0.076 | 0.577 | 56 |
| Alkaline phosphatase | −0.093 | 0.494 | 56 |
| 24-h urine protein | −0.151 | 0.355 | 56 |
| GFR using MDRD | 0.068 | 0.617 | 56 |
| PTHi | 0.174 | 0.204 | 56 |
An inverse correlation was found between the levels of vitamin D and the creatinine, calcium, phosphorus, Ca×P, albumin, alkaline phosphatase and 24-h urine protein tests. In contrast, the correlation was positive for the GFR and PTHi, although in all tests the correlation was very weak because the coefficients were very low (Table 3) (Fig. 1).
With regards to sun exposure, no significant differences were found between the average vitamin D levels and the degree of exposure, keeping in mind that most patients were in level 1 (71.4%). A similar situation occurred with the bone densitometry results. No correlation was found between the level of bone disorder and the levels of vitamin D, since the results were > 5% (Table 4).
In only 5 (8.9%) patients a specific osteoporosis therapy was identified, and the agent used was alendronate in all cases, stating just a few months earlier. With regards to prophylaxis for osteoporosis, 21 (37.5%) patients were not being treated, 5 (8.9%) received calcium carbonate, 12 (21.4%) received calcitriol, and 18 (32.1%) received the combination of calcium carbonate+calcitriol.
DiscussionHaving normal 25-hydroxivitamin D (25[OH]D) o calcidiol levels is very important for kidney transplant patients, in order to achieve an adequate production of the active form of vitamin D; i.e., calcitriol.5 Normal serum levels are required to inhibit the secretion of PTH and these range between 30 and 40ng/ml.10,11 Low levels of vitamin D are a risk factor for osteoporosis, high blood pressure, cardiovascular disease, diabetes mellitus, infections, autoimmune diseases, rickets, some types of cancer and higher mortality among the population in general.16–21
In has been found that low vitamin D levels in kidney transplant patients represent a risk factor for various complications, including most notably: (1) delayed graft function and increased risk of acute rejection with accelerated kidney function loss, interstitial fibrosis, tubular atrophy and low GFR one year after transplantation.22–25 Vitamin D may affect the adaptive and innate immune response, modulating the allogeneic response,26 and possibly promoting the tolerance for inducing regulatory T-cells.27–29 (2) Stronger tendency towards the development of opportunistic viral infections such as polyomavirus type BK,30,31 and bacterial infections (mainly those that involve the urinary tract),32 but non-fungal.33 A further contribution could be the stimulating effect of vitamin D in macrophage antimicrobial activity.34–36 (3) Major presence of depressive symptoms and fatigue.37,38 (4) Persistence of secondary hyperparathyroidism, with increased risk of mineral bone loss and fractures.39,40 (5) Post-transplantation diabetes mellitus due to a decreased insulin secretion by beta cells which express vitamin D receptors.41 (6) Risk of cancer.42,43
Native vitamin D serum levels have been evaluated in various kidney transplant population groups. These levels of vitamin D have been reported low in 97% of patients in Germany, Spain, England, and Denmark,44–47 based on studies in the Northern hemisphere, where sun exposure is low; therefore, generalizing these findings to tropical regions would be inappropriate. In Brazil – tropical region – 65% of the renal transplant patients exhibited hypovitaminosis D (53% insufficient and 12% with deficit), with a higher impact on obese women.48 In another trial in that same country, the rate was 80.7% among 83 patients studied.49 In Israel, inadequate vitamin D levels were identified in 75% of 103 kidney transplant patients, 52.4% in the insufficiency range and 23% in the deficit range.50 In Korea, in 25 kidney transplant patients, the native vitamin D levels were low in 40% of the patients.51 In Iran no seasonal changes of vitamin D were described in 96 kidney transplant patients, but during the summer the patients avoided a high sun exposure and used sun screens.52 In India, in 51 kidney transplant patients, only 8% had adequate calcidiol levels, 33% were insufficient, 51% had deficit, and 8% had severe deficit.53
Currently, the most common factors involved in the identification of low levels of vitamin D in kidney transplant patients are: low sun exposure, low intake of foods rich in vitamin D, and the type of immunosuppressive therapy administered. Low sun exposure is the consequence of the general recommendation given to these patents to avoid the risk of skin neoplasms, particularly when receiving cytostatic therapy such as azathioprine and mycophenolate.4,54–56
The reason why there is a low intake of foods rich in vitamin D is that most transplanted patients have CKD and a low protein intake is recommended.
The type of immunosuppression may be important for metabolizing vitamin D. Steroids, due to their ability to increase the 24-hydroxilase activity, may result in low levels of vitamin D.57 Steroids have a direct toxic effect on the osteoblasts and osteocytes by inducing apoptosis and increasing the osteoclastic activity through the elevation of RANK-L; furthermore, steroids reduce intestinal and renal tubular calcium absorption.58–60 Mycophenolate and azathioprine have a neutral effect on bone metabolism and vitamin D. Calcineurin inhibitors are controversial,61–64 though they may cause suppression of the renal vitamin D receptor, hence increasing the urine calcium losses.63 mTOR inhibitors have not been shown to affect the serum vitamin D levels.63,65
In our study, the prevalence of insufficient levels of calcidiol were high, except in patients in ESKF stage 1. This is probably associated with shorter post-transplant times and higher pre-transplant sun exposure, since the effects of a low sun exposure and the side effects of the immunosuppressive medication are evidenced in the long term, because of the prolonged half-life of calcidiol.
The progressive elevation of PTH as the GFR drops, and the reduction of serum albumin may be the result of nutritional factors, low renal excretion of phosphates, hypocalcemia, and insufficient vitamin D levels. The lack of correlation between the levels of vitamin D and the bone scan findings is striking, reflecting the difficult interpretation in patients with CKD since in these patients, a high PTH hinders the conclusion of whether the findings are the result of renal osteodystrophy and its variations, or of genuine osteoporosis.
The strength of this study is that this is the first report in our country describing the above findings. The limitations include the small number of patients and the lack of long-term follow-up.
ConclusionsA high percentage of kidney transplant patients exhibit low levels of vitamin D, at all stages of their ESKD. This could be a significant factor in the development of osteoporosis and secondary hyperparathyroidism. It is then recommended that the levels of 25(OH)D be measured in al kidney transplant patients, regardless of their GFR.
Conflict of interestThe authors have no conflict of interest to disclose.
Please cite this article as: Restrepo Valencia CA, Aguirre Arangob JV, Escobar DC. Determinación de niveles de vitamina D (25[OH]D) en pacientes trasplantados renales y su importancia de acuerdo con la tasa de filtración glomerular. Rev Colomb Reumatol. 2018;25:161–168.