Belimumab, a monoclonal type Ig G1 antibody that binds and inhibits the soluble form of the Blys (B lymphocyte stimulator) has shown to be effective in the management of systemic lupus erythematosus (SLE). However, its effectiveness is unknown in an ethnically variable population, such as in Colombia.
MethodsA prospective observational study was conducted between February 2015 and February 2016 on patients with active SLE disease despite being on standard treatment and who were treated with Belimumab.
ResultsBelimumab showed a significant improvement in joint, cutaneous and hematological involvement, with an increase in complement levels, a decrease in lupus crises and hospital admissions. After 3 months there was lower activity, calculated by SLEDAI, with stability for 9 months.
ConclusionsIn a real-life patient setting, it was observed that belimumab was useful in Colombian patients with SLE and refractory to standard therapy, especially in the joint, hematological, and cutaneous manifestations.
El belimumab es un anticuerpo monoclonal tipo IgG1 que se une e inhibe la forma soluble de Blys (estimulador de linfocitos B) y ha mostrado efectividad en el manejo del lupus eritematoso sistémico (LES). Sin embargo, se desconoce su efectividad en una población tan variable étnicamente como la colombiana.
MétodosSe realizó un estudio prospectivo observacional entre febrero de 2015 y febrero de 2016, en pacientes con LES, con enfermedad activa a pesar del tratamiento estándar, quienes fueron tratados con belimumab.
ResultadosEl uso de belimumab se relacionó con una mejoría significativa en los compromisos articular, cutáneo y hematológico, con aumento de los niveles de complemento, disminución de las exacerbaciones por LES y de las hospitalizaciones, además de una menor actividad calculada por SLEDAI después de 3 meses de utilización y con una estabilidad mantenida hasta los 9 meses.
ConclusionesSe observó que el belimumab es útil en pacientes colombianos con LES que son refractarios a la terapia estándar, especialmente en manifestaciones articulares, hematológicas y cutáneas, en un entorno de pacientes de la vida real.
Systemic lupus erythematosus (SLE) is a multisystemic disease, of autoimmune origin and unknown etiology. The main pathogenic mechanisms include the production of autoantibodies, activation of complement, production of cytokines and costimulatory molecules, activation of B cells and deposition of immune complexes.
The drug belimumab is a monoclonal type IgG1 antibody that binds and inhibits the soluble form of the Blys (B lymphocyte stimulator, also known as BAFF), which has shown effectiveness in the management of SLE.1,2 The safety and usefulness of belimumab was demonstrated in phase II and III studies (BLISS-52 and BLISS-76).3,4 These randomized trials demonstrated several benefits in the group treated with belimumab, such as less use of steroids, a lower disease activity (in several evaluation scores including PGA, SF-36 and Selena-SLEDAI), and a greater response in patients with higher disease activity (positive double-stranded anti-DNA antibodies and those with SELENA-SLEDAI >8). These findings were maintained in the follow-up study (BLISS-76) during 7 years in 1746 patients worldwide.4 Taking into account the short time for which belimumab has been used in patients with SLE and the few studies conducted in the real clinical setting in different ethnic populations, we describe the effect of belimumab in Colombian patients treated during 9 months.
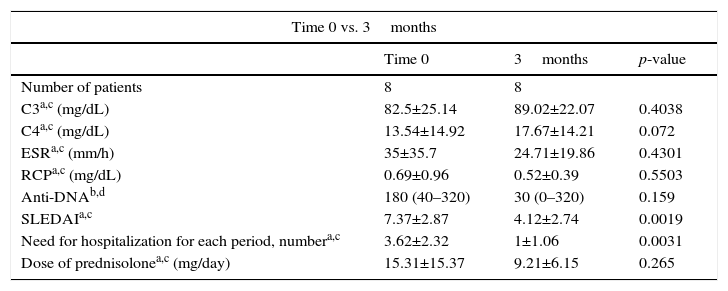
MethodsA prospective observational study was conducted between February 2015 and February 2016 in SLE patients with active disease despite standard treatment, who were treated with belimumab (10mg/kg/dose, 0-2-4 and then every 4 weeks). All patients met the classification criteria for SLE5 and were assessed in the Reference Center of Autoimmune Diseases of the Foundation Valle del Lili, Cali; the study was authorized by the institutional ethics committee and the patients who were included signed informed consent. Clinical and immunological information was collected at the beginning and every 3 months. The main variables included doses of prednisolone or its equivalent, presence of exacerbations, need for hospitalization, levels of acute phase reactants, levels of complement, anti-double stranded DNA titers, and levels of disease activity determined by the Systemic Lupus Erythematosus Disease Activity Score (SLEDAI). The results at baseline and those obtained every 3 months were compared using the Wilcoxon test for unpaired data and the T test for paired data according to the distribution thereof (Table 1).
Patient's follow-up.
| Time 0 vs. 3months | |||
|---|---|---|---|
| Time 0 | 3months | p-value | |
| Number of patients | 8 | 8 | |
| C3a,c (mg/dL) | 82.5±25.14 | 89.02±22.07 | 0.4038 |
| C4a,c (mg/dL) | 13.54±14.92 | 17.67±14.21 | 0.072 |
| ESRa,c (mm/h) | 35±35.7 | 24.71±19.86 | 0.4301 |
| RCPa,c (mg/dL) | 0.69±0.96 | 0.52±0.39 | 0.5503 |
| Anti-DNAb,d | 180 (40–320) | 30 (0–320) | 0.159 |
| SLEDAIa,c | 7.37±2.87 | 4.12±2.74 | 0.0019 |
| Need for hospitalization for each period, numbera,c | 3.62±2.32 | 1±1.06 | 0.0031 |
| Dose of prednisolonea,c (mg/day) | 15.31±15.37 | 9.21±6.15 | 0.265 |
| Time 0 vs. 6months | |||
|---|---|---|---|
| Time 0 | 6months | p-value | |
| Number of patients | 8 | 7 | |
| C3b,d (mg/dL) | 79.5 (67.8–85) | 90 (84–127) | 0.625 |
| C4a,c (mg/dL) | 15.19±15.31 | 23.67±17.54 | 0.0199 |
| ESRa,c (mm/h) | 48.6±34.28 | 22.2±10.89 | 0.114 |
| RCPb,d (mg/dL) | 0.58 (0.11–1.06) | 1.1 (0.029–1.4) | 0.1763 |
| Anti-DNAa,c | 250±140 | 125±148.21 | 0.1912 |
| SLEDAIa,c | 7.85±2.73 | 2.71±1.70 | 0.002 |
| Need for hospitalization for each period, numbera,c | 3.57±2.50 | 0.71±0.75 | 0.0117 |
| Dose of prednisoloneb,d (mg/day) | 10 (7.5–25) | 10 (5–12.5) | 0.1677 |
| Time 0 vs. 9months | |||
|---|---|---|---|
| Time 0 | 9months | p-value | |
| Number of patients | 8 | 5 | |
| C3a,c (mg/dL) | 72.52±13.17 | 94.99±32.2 | 0.164 |
| C4b,d (mg/dL) | 10.3 (30.1.8–12) | 12.1 (8.7–14) | 0.568 |
| ESRa,c (mm/h) | 33.8±10.18 | 17.2±10.18 | 0.3293 |
| RCPb,d (mg/dL) | 0.11 (00.01–00.58) | 0.24 (0.024–0.45) | 0.6858 |
| Anti-DNAb,d | 320 (40–320) | 160 (20–320) | 0.5862 |
| SLEDAIa,c | 8.2±3.19 | 2.2±1.78 | 0.0414 |
| Need for hospitalization for each period, numbera,c | 4±2.91 | 0.6±0.54 | 0.0388 |
| Dose of prednisoloneb,d (mg/day) | 18.5±19.49 | 18.5±19.49 | 0.2511 |
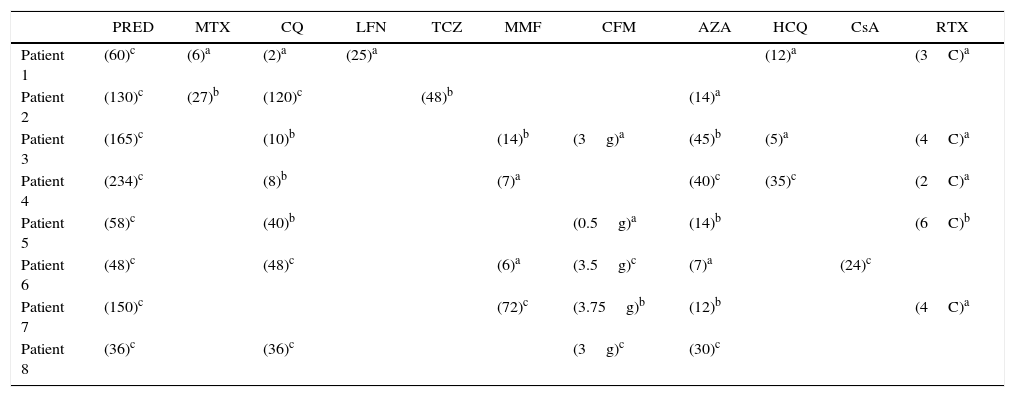
After an average number of 7 cycles, positive results were evidenced. Eight patients were included in the baseline, all of them women. The mean age of inclusion was 32.2±3.2 years and the mean duration of the disease was 11.25 years. Two patients had an association with antiphospholipid syndrome. The mean basal dose of prednisolone was 15.31mg/day and the initial SLEDAI was high (7.37). The treatment with belimumab was indicated by the presence of a refractory involvement of the joints (n=8), cutaneous (n=7) and hematological (n=3) given by hemolytic anemia (one patient) and leukocytes <4000/μL (2 patients), despite standard therapy which included steroids, antimalarial drugs, methotrexate, mycophenolate mofetil, azathioprine or rituximab. Table 2 shows the immunosuppressive agents previously received and the time of treatment with these drugs. No infusion-related adverse events were demonstrated during the treatment with belimumab. Two patients developed infectious complications (one case of urinary tract infection and one case of sinusitis). Three patients discontinued the belimumab, one case due to adverse events (patient with headache during the 12 weeks of treatment without response to conventional management of headache and with complete improvement when the belimumab was discontinued) and other 2 cases due to lack of effectiveness (one patient at 24 weeks of treatment due to the presence of lupus nephritis and renal failure, and another patient at 21 weeks due to cutaneous involvement that did not improve with the drug).
Prior and concomitant drugs along with belimumab therapy.
| PRED | MTX | CQ | LFN | TCZ | MMF | CFM | AZA | HCQ | CsA | RTX | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | (60)c | (6)a | (2)a | (25)a | (12)a | (3C)a | |||||
| Patient 2 | (130)c | (27)b | (120)c | (48)b | (14)a | ||||||
| Patient 3 | (165)c | (10)b | (14)b | (3g)a | (45)b | (5)a | (4C)a | ||||
| Patient 4 | (234)c | (8)b | (7)a | (40)c | (35)c | (2C)a | |||||
| Patient 5 | (58)c | (40)b | (0.5g)a | (14)b | (6C)b | ||||||
| Patient 6 | (48)c | (48)c | (6)a | (3.5g)c | (7)a | (24)c | |||||
| Patient 7 | (150)c | (72)c | (3.75g)b | (12)b | (4C)a | ||||||
| Patient 8 | (36)c | (36)c | (3g)c | (30)c |
AZA: azathioprine; CFM: cyclophosphamide; CQ: chloroquine; CsA: cyclosporine; HCQ: hydroxychloroquine; LFN: leflunomide; MMF: mycophenolate; MTX: methotrexate; PRED: prednisolone; RTX: rituximab; TCZ: tocilizumab.
The patient received the treatment before belimumab, the number indicates the months during which he received it.
RTX: In rituximab the value inside parentheses indicates the number of cycles (C).
CFM: In cyclophosphamide the value inside parenthesis indicates the cumulative dose of the drug in grams (g).
In the 5 remaining cases, belimumab showed a significant improvement in the joint (this improvement was assessed clinically and with the SLEDAI score), cutaneous and hematological involvements, with increase in the levels of complement, reduction in lupus exacerbations and hospitalizations, as well as a lower activity calculated by SLEDAI (Table 1) after 3 months and stable after 9 months. Anti-DNA titers remained stable during the treatment with belimumab.
DiscussionThis study shows the effects of belimumab in an ethnically variable group such as the Colombian population. The results are consistent with those reported in other series of real-life patients. A Brazilian study of a cohort of 48 patients with SLE, who were managed with belimumab, evidenced an improvement in the SLEDAI from 12±3 to 2.5±2.5 with a decrease in the dose of steroids from 30±12.5 to 7.5±5mg/day, with significant p-values.6 Our study showed a decrease in the SLEDAI from 8.2±3.19 to 2.2±1.78 with a p<0.05 at 9 months of follow-up, however, the decrease in the dose of steroids was not statistically significant in our cohort, this probably due to the small number of patients, since, as shown in Table 1, there was a tendency to the reduction of steroids especially between time-zero and the evaluation at 3 months. In 2014 was published an Italian cohort with 18 patients who were treated with belimumab for 9 months and, like in the series previously reported, the results were consistent with our findings. These patients presented a reduction in SLEDAI from 9 to 6 with a p<0.05 and a reduction in the use of steroids from 66.3 to 46.9mg/week with a p<0.05.7
Our study did not show a significant increase in complement of decrease in ESR, CRP or anti-DNA. This is consistent with what was reported in both series, Italian and Brazilian, where no significant changes were found in the acute-phase reactants. Our study evidenced, in addition, a statistically significant decrease in the number of hospitalizations, between time-zero compared with the 9 months, from 4±2.91 to 0.6±0.54, being an interesting result and not reported in other series, which could have a significant impact from the viewpoint of utilization of healthcare resources. An additional point of discussion is related to the time to achieve significant changes. Previous studies mention that the improvement in SLEDAI parameters and the reduction in the doses of steroids became notorious after 6 months of treatment. However, in our series, these findings were present since the first control carried out at 3 months and remained until 9 months.
Regarding the lack of effectiveness, it was necessary to discontinue the drug in 2 of our patients, who represent 25% of the patients, but given that the sample is small, we cannot conclude that this will occur in larger samples.
ConclusionsIt was observed that belimumab is useful in Colombian patients with SLE who are refractory to standard therapy, especially in joint, hematological and cutaneous manifestations, in a setting of real-life patients. These results cannot be widely generalizable since the sample of patients is small and that is the major limitation of our study, but they are valuable results, given that they show the behavior of the drug in a real context, mainly regarding the number of hospitalizations, and they are similar to those observed in the other published series. A larger study is required to be able to assess the effect of the drug in the long term.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Echeverri A, Posso-Osorio I, Figueroa C, Suso J-P, Hormaza A, Bonilla-Abadía F, et al. Efectos del belimumab en pacientes colombianos con lupus eritematoso sistémico, un estudio prospectivo observacional. Rev Colomb Reumatol. 2017;24:159–163.