Rheumatoid arthritis is a chronic, systemic inflammatory disease, with a world prevalence of around 1%. The course of the disease may be changed by using synthetic and/or biological disease-modifying antirheumatic drugs alone or in combination. This review assessed whether the use of biological agents in early rheumatoid arthritis can lead to disease remission.
ObjectiveTo evaluate the efficacy of biological therapies in inducing remission in patients with early rheumatoid arthritis.
MethodsType of study: systematic review of the literature. A systematic search of the literature was made in specialized electronic health databases: PubMed, Embase, Cochrane, LILACS, gray literature (doctoral theses, congresses, entity reports, unpublished works) and manual search (Secondary searches of the studies cited in the selected articles). Inclusion criteria: Patients older than 18 years with early rheumatoid arthritis (clinical course less than 12 months) according to criteria of the American College of Rheumatology/European League against Rheumatism (ACR 1987, ACR/EULAR 2010), who received biological therapy Monotherapy or combined with other disease modifying antirheumatic drugs (DMARDs) and were included in randomized controlled trials.
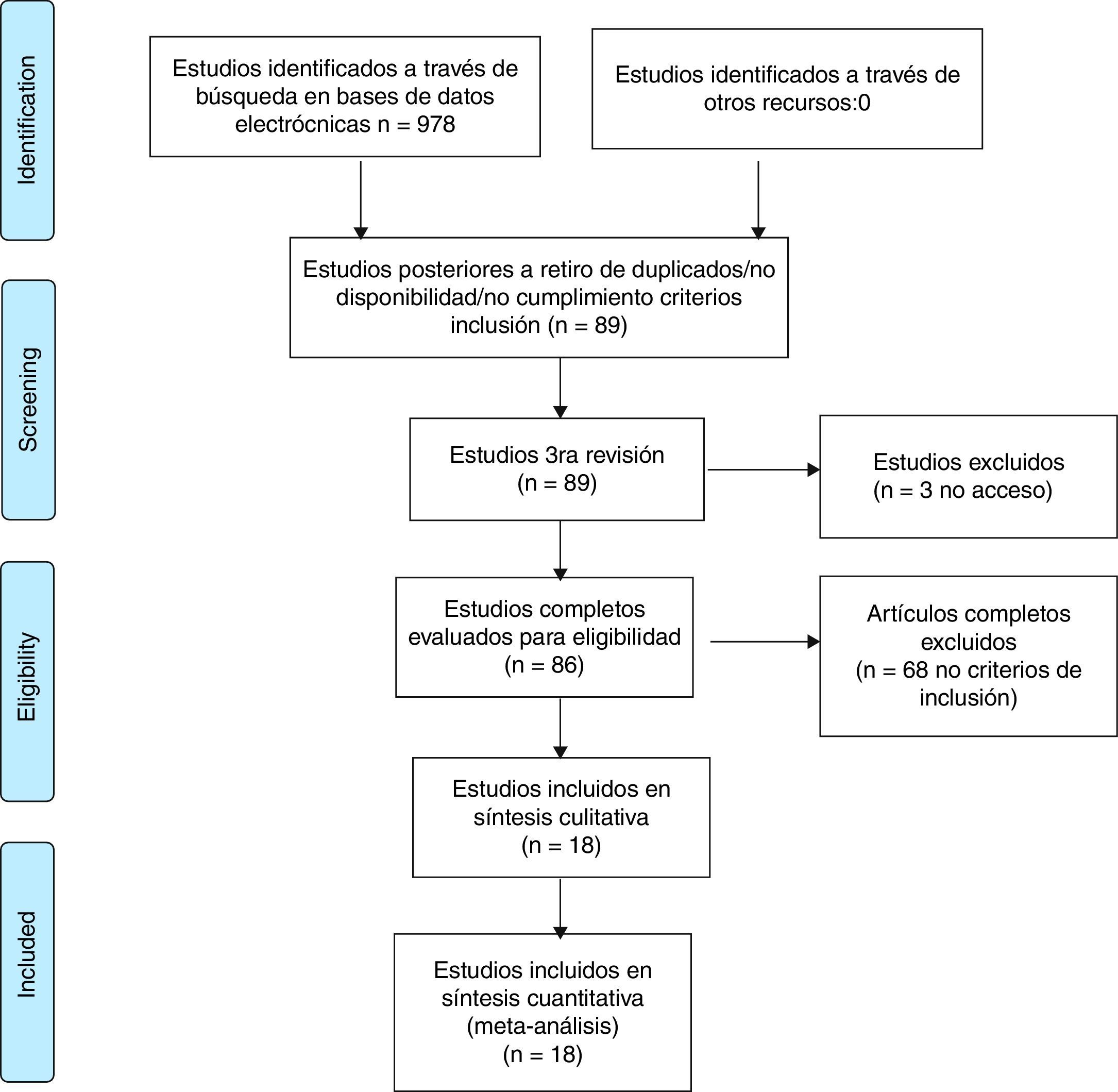
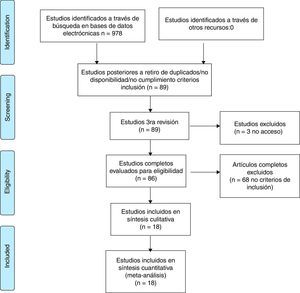
ResultsThe systematic review of the literature identified 978 potentially relevant studies. Of the 86 that were chosen for a thorough evaluation, 68 articles were excluded. A qualitative and quantitative analysis of the remaining 18 studies was performed. A high risk of bias was identified in the studies evaluated and synthesis of the evidence did not show differences in the outcome of remission using biological therapy alone or combined with conventional DMARDs versus synthetic DMARDs (RR 1.08, 95% CI: 0.94–1.23).
ConclusionsThere is no difference in the outcome of remission in patients with early rheumatoid arthritis receiving biological therapy versus patients treated with conventional disease modifying antirheumatic drugs.
Evaluar la eficacia de las terapias biológicas en inducir la remisión de la artritis reumatoide temprana.
MétodosTipo de estudio: revisión sistemática de la literatura. Se realizó una búsqueda sistemática de la literatura en bases de datos electrónicas especializadas en ciencias de la salud: PubMed, Embase, Cochrane, LILACS, literatura gris (tesis doctorales, intervenciones en congresos, informes de entidades, trabajos no publicados) y búsqueda manual (búsquedas secundarias de los estudios citados en los artículos seleccionados). Criterios de inclusión: pacientes mayores de 18 años con artritis reumatoide temprana (curso clínico menor de 12 meses) según criterios del Colegio Americano de Reumatología/Liga Europea contra el Reumatismo (ACR 1987, ACR/EULAR 2010), que recibieron terapia biológica en monoterapia o combinada con otros fármacos antirreumáticos modificadores de la enfermedad (FARME) y fueron incluidos en estudios clínicos controlados aleatorizados.
ResultadosLa revisión sistemática de la literatura identificó 978 estudios potencialmente relevantes; 86 fueron escogidos para la evaluación completa. Se excluyeron 68 artículos por no cumplir los criterios de inclusión, principalmente relacionados con el tiempo de duración de la enfermedad al diagnóstico de la artritis reumatoide temprana y con la metodología del estudio. Se realizó la síntesis cualitativa y cuantitativa de 18 estudios.
Se identificó alto riesgo de sesgos en los estudios evaluados, y la síntesis de la evidencia, a través del metaanálisis, no evidenció diferencias con relación al desenlace remisión de la enfermedad al utilizar terapia biológica en monoterapia o combinada con FARME convencionales versus el uso de FARME (RR: 1,08; IC 95%: 0,94-1,23).
ConclusionesNo hay diferencias en el desenlace de remisión de la enfermedad en pacientes con artritis reumatoide temprana que reciben terapia biológica versus pacientes tratados con FARME convencionales.
Rheumatoid arthritis (RA) is a systemic chronic inflammatory disease, of unknown cause (there are hypothesis concerning the exposure to an external trigger such as cigarette smoking, infection, etc., with consequent autoimmune reactivation, chronic joint swelling and synovial hypertrophy in a genetically predisposed patient), with a world prevalence of around 1%, related to major adverse outcomes such as joint destruction, physical disability and premature mortality. The course of the disease can be modified and structural damage can be slowed and even prevented by means of treatments with synthetic disease-modifying antirheumatic drugs, glucocorticoids and biological agents, in addition to providing the patient a better quality of life when medicines such as non-steroidal anti-inflammatory drugs and physical therapy are used concomitantly. A special scenario is the early RA, in which the early use of disease modifying antirheumatic drugs (DMARDs) and biological agents could generate a symptomatic control and a reduction of the disease activity until its remission. The present article evaluates, through a systematic review of the literature and meta-analysis, whether the use of biological agents in early RA favors the remission of this entity measured by the Disease Activity Score 28 (DAS28).1–6
Materials and methodsType of study: systematic review of the literaturePopulation- •
Patients older than 18 years with early RA according to the criteria of the American College of Rheumatology/European League against Rheumatism (ACR 1987, ACR/EULAR 2010) who received biological therapy in monotherapy or combined with other DMARDs and were included in randomized controlled clinical trials.
- •
Inclusion criteria:
- •
Clinical trials, comparative, controlled, randomized clinical studies that assessed the following interventions: use of anti-TNF (infliximab, adalimumab, certolizumab, etanercept, golimumab) and non-anti-TNF (rituximab, anakinra, tocilizumab, abatacept, ocrelizumab) biological therapy, alone or in combination with conventional DMARDs (methotrexate [MTX], hydroxychloroquine, chloroquine, leflunomide or sulfasalazine) in patients with early RA, defined as the one with a clinical course of less than 12 months. The clinical studies should evaluate as primary or secondary objectives the efficacy of biological therapy in inducing remission of the disease, determined by a score lower than 2.6 in the DAS28 scale or lower than 1.6 in the DAS44 scale.
- •
Articles that include patients with established RA.
- •
Previous use of biological agents.
- •
Articles without access to full text.
- •
Duplicate articles.
- •
Studies that did not evaluate the disease activity using the DAS28 scale or its equivalent.
- •
Pregnant of breast-feeding patients.
- •
Patients under biological therapy for rheumatologic diseases other than RA.
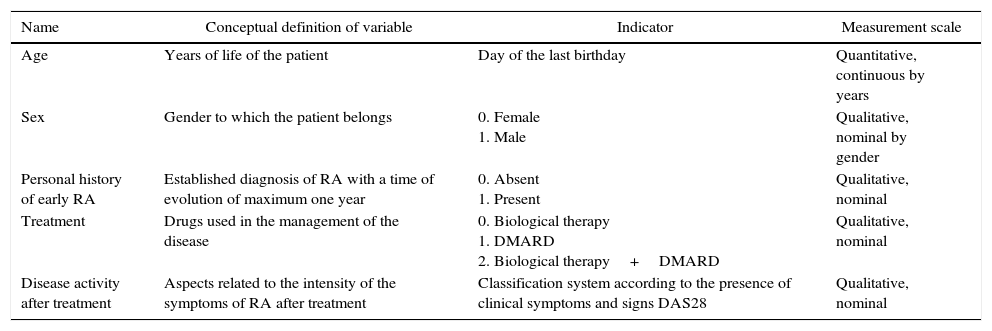
There is a description of the variables corresponding to the study population and those that determine the efficacy of biological therapy in inducing remission of early RA (Table 1).
Matrix of variables.
| Name | Conceptual definition of variable | Indicator | Measurement scale |
|---|---|---|---|
| Age | Years of life of the patient | Day of the last birthday | Quantitative, continuous by years |
| Sex | Gender to which the patient belongs | 0. Female 1. Male | Qualitative, nominal by gender |
| Personal history of early RA | Established diagnosis of RA with a time of evolution of maximum one year | 0. Absent 1. Present | Qualitative, nominal |
| Treatment | Drugs used in the management of the disease | 0. Biological therapy 1. DMARD 2. Biological therapy+DMARD | Qualitative, nominal |
| Disease activity after treatment | Aspects related to the intensity of the symptoms of RA after treatment | Classification system according to the presence of clinical symptoms and signs DAS28 | Qualitative, nominal |
RA: rheumatoid arthritis; DAS28: Disease Activity Score; DMARDs: disease modifying antirheumatic drugs.
The collection of the information was carried out through the systematic search of the literature in electronic databases specialized in health sciences: PubMed, Embase, Cochrane, LILACS, gray literature (doctoral theses, interventions in congresses, reports of entities, unpublished works) and manual search (secondary searches of the studies cited in the selected articles).
We used the following Mesh terms and free-text terms: («early diagnosis»[ti] AND «arthritis»[ti] AND «rheumatoid»[ti]) AND («antirheumatic agents» OR «biological therapy» OR «biologics») AND («treatment outcome» OR «remission induction»). The following filters were used in the search for information: clinical trials, comparative trials, controlled clinical trials and randomized controlled clinical trials. The search was conducted between the months of April and June of 2014, and it included articles published until June 30, 2014, without language restriction.
Two researchers worked in the evaluation of each title and abstract in order to exclude irrelevant reports; in the case of disagreement about the inclusion of a specific study, it was jointly evaluated, and if an agreement was not reached, a third party (expert in the content-area) was consulted. Independently, the researchers determined whether the full text of each article met the eligibility criteria. When evaluating the relevance of the studies, they had knowledge on the authors, institutions, journal of publication and results.
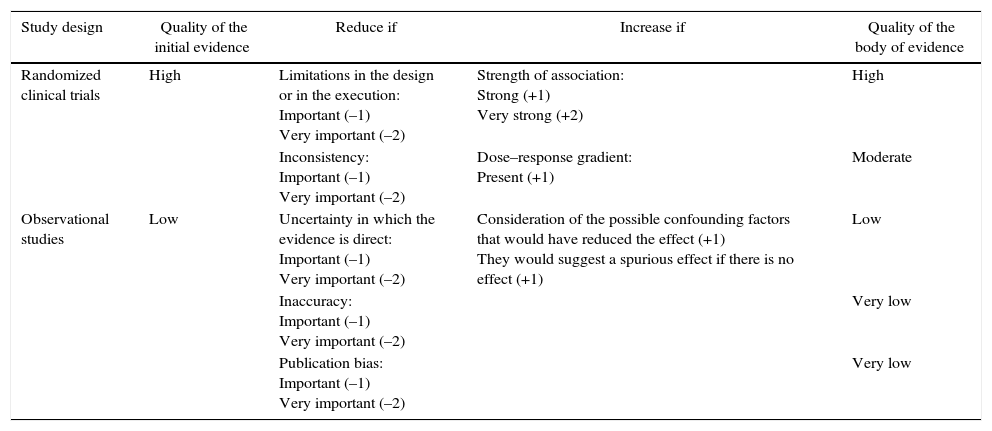
The quality of the studies included in this systematic review was assessed according to the recommendations of the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, in which aspects such as the risk of bias, inaccuracies, inconsistencies and publication bias were taken into account in order to make a more reliable estimation of the efficacy of biological therapy in inducing clinical remission of early RA. By means of this system the studies were classified according to their level of evidence into high, moderate, low or very low6 (Table 2).
Evaluation of the quality of evidence according to the type of design of the studies.
| Study design | Quality of the initial evidence | Reduce if | Increase if | Quality of the body of evidence |
|---|---|---|---|---|
| Randomized clinical trials | High | Limitations in the design or in the execution: Important (–1) Very important (–2) | Strength of association: Strong (+1) Very strong (+2) | High |
| Inconsistency: Important (–1) Very important (–2) | Dose–response gradient: Present (+1) | Moderate | ||
| Observational studies | Low | Uncertainty in which the evidence is direct: Important (–1) Very important (–2) | Consideration of the possible confounding factors that would have reduced the effect (+1) They would suggest a spurious effect if there is no effect (+1) | Low |
| Inaccuracy: Important (–1) Very important (–2) | Very low | |||
| Publication bias: Important (–1) Very important (–2) | Very low |
Source: adapted from Guyatt et al.34
The methodology is reported according to the PRISMA guidelines for the conduction of systematic reviews and meta-analyses. The degree of heterogeneity of the studies was evaluated through the Chi2 and I2 tests.
ResultsThe systematic review of the literature identified 978 potentially relevant studies (Pubmed: 211; Embase: 580; Cochrane: 185; LILACS: 2) of which 86 were chosen for a thorough evaluation in order to evaluate their eligibility.
68 articles were excluded; most of them because the population did not met the criterion established for the time of duration of the disease, corresponding to less than one year in early RA or, in addition, because the methodology of the study did not correspond to clinical trials or studies (comparative, controlled or randomized). A total of 18 studies were included in the evaluation to perform the qualitative and quantitative synthesis (Fig. 1, Table 3). The characteristics of the studies included are fully described in Table 3 of this review.
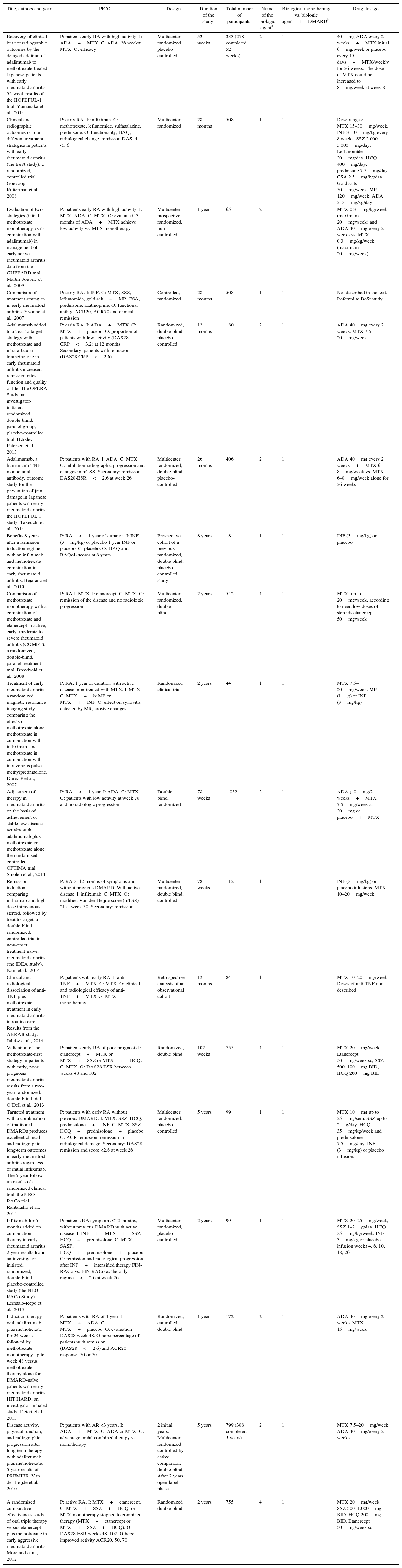
Characteristics of the studies.
| Title, authors and year | PICO | Design | Duration of the study | Total number of participants | Name of the biologic agenta | Biological monotherapy vs. biologic agent+DMARDb | Drug dosage |
|---|---|---|---|---|---|---|---|
| Recovery of clinical but not radiographic outcomes by the delayed addition of adalimumab to methotrexate-treated Japanese patients with early rheumatoid arthritis: 52-week results of the HOPEFUL-1 trial. Yamanaka et al., 2014 | P: patients early RA with high activity. I: ADA+MTX. C: ADA, 26 weeks: MTX. O: efficacy | Multicenter, randomized placebo-controlled | 52 weeks | 333 (278 completed 52 weeks) | 2 | 1 | 40mg ADA every 2 weeks+MTX initial 6mg/week or placebo every 15 days+MTX/weekly for 26 weeks. The dose of MTX could be increased to 8mg/week at week 8 |
| Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Goekoop-Ruiterman et al., 2008 | P: early RA. I: infliximab. C: methotrexate, leflunomide, sulfasalazine, prednisone. O: functionality, HAQ, radiological change, remission DAS44 <1.6 | Multicenter, randomized | 28 months | 508 | 1 | 1 | Dose ranges: MTX 15–30mg/week. INF 3–10mg/kg every 8 weeks, SSZ 2.000–3.000mg/day. Leflunomide 20mg/day. HCQ 400mg/day, prednisone 7.5mg/day. CSA 2.5mg/kg/day. Gold salts 50mg/week. MP 120mg/week. ADA 2–3mg/kg/day |
| Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Martin Soubrie et al., 2009 | P: patients early RA with high activity. I: MTX, ADA. C: MTX. O: evaluate if 3 months of ADA+MTX achieve low activity vs. MTX monotherapy | Multicenter, prospective, randomized, non-controlled | 1 year | 65 | 2 | 1 | MTX 0.3mg/kg/week (maximum 20mg/week) and ADA 40mg every 2 weeks vs. MTX 0.3mg/kg/week (maximum 20mg/week) |
| Comparison of treatment strategies in early rheumatoid arthritis. Yvonne et al., 2007 | P: early RA. I: INF. C: MTX, SSZ, leflunomide, gold salt+MP, CSA, prednisone, azathioprine. O: functional ability, ACR20, ACR70 and clinical remission | Controlled, randomized | 28 months | 508 | 1 | 1 | Not described in the text. Referred to BeSt study |
| Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates function and quality of life. The OPERA Study: an investigator-initiated, randomized, double-blind, parallel-group, placebo-controlled trial. Hørslev-Petersen et al., 2013 | P: early RA. I: ADA+MTX. C: MTX+placebo. O: proportion of patients with low activity (DAS28 CRP<3.2) at 12 months. Secondary: patients with remission (DAS28 CRP<2.6) | Randomized, double blind, placebo-controlled | 12 months | 180 | 2 | 1 | ADA 40mg every 2 weeks. MTX 7.5–20mg/week |
| Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Takeuchi et al., 2014 | P: patients with RA. I: ADA. C: MTX. O: inhibition radiographic progression and changes in mTSS. Secondary: remission DAS28-ESR<2.6 at week 26 | Multicenter, randomized, double blind, placebo-controlled | 26 months | 406 | 2 | 1 | ADA 40mg every 2 weeks+MTX 6–8mg/week vs. MTX 6–8mg/week alone for 26 weeks |
| Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Bejarano et al., 2010 | P: RA<1 year of duration. I: INF (3mg/kg) or placebo 1 year INF or placebo. C: placebo. O: HAQ and RAQoL scores at 8 years | Prospective cohort of a previous randomized, double blind, placebo-controlled study | 8 years | 18 | 1 | 1 | INF (3mg/kg) or placebo |
| Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomized, double-blind, parallel treatment trial. Breedveld et al., 2008 | P: RA I: MTX. I: etanercept. C: MTX. O: remission of the disease and no radiologic progression | Multicenter, randomized, double blind, | 2 years | 542 | 4 | 1 | MTX: up to 20mg/week, according to need low doses of steroids etanercept 50mg/week |
| Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Durez P et al., 2007 | P: RA, 1 year of duration with active disease, non-treated with MTX. I: MTX. C: MTX+iv MP or MTX+INF. O: effect on synovitis detected by MR, erosive changes | Randomized clinical trial | 2 years | 44 | 1 | 1 | MTX 7.5–20mg/week. MP (1g) or INF (3mg/kg) |
| Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomized controlled OPTIMA trial. Smolen et al., 2014 | P: RA<1 year. I: ADA. C: MTX. O: patients with low activity at week 78 and no radiologic progression | Double blind, randomized | 78 weeks | 1.032 | 2 | 1 | ADA (40mg/2 weeks+MTX 7.5mg/week at 20mg or placebo+MTX |
| Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomized, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Nam et al., 2014 | P: RA 3–12 months of symptoms and without previous DMARD. With active disease. I: infliximab. C: MTX. O: modified Van der Heijde score (mTSS) 21 at week 50. Secondary: remission | Multicenter, randomized, double blind, controlled | 78 weeks | 112 | 1 | 1 | INF (3mg/kg) or placebo infusions. MTX 10–20mg/week |
| Clinical and radiological dissociation of anti-TNF plus methotrexate treatment in early rheumatoid arthritis in routine care: Results from the ABRAB study. Juhász et al., 2014 | P: patients with early RA. I: anti-TNF+MTX. C: MTX. O: clinical and radiological efficacy of anti-TNF+MTX vs. MTX monotherapy | Retrospective analysis of an observational cohort | 12 months | 84 | 11 | 1 | MTX 10–20mg/week Doses of anti-TNF non-described |
| Validation of the methotrexate-first strategy in patients with early, poor-prognosis rheumatoid arthritis: results from a two-year randomized, double-blind trial. O’Dell et al., 2013 | P: patients early RA of poor prognosis I: etanercept+MTX or MTX+SSZ or MTX+HCQ. C: MTX. O: DAS28-ESR between weeks 48 and 102 | Randomized, double blind | 102 weeks | 755 | 4 | 1 | MTX 20mg/week. Etanercept 50mg/week sc, SSZ 500–100mg BID, HCQ 200mg BID |
| Targeted treatment with a combination of traditional DMARDs produces excellent clinical and radiographic long-term outcomes in early rheumatoid arthritis regardless of initial infliximab. The 5-year follow-up results of a randomized clinical trial, the NEO-RACo trial. Rantalaiho et al., 2014 | P: patients with early RA without previous DMARD. I: MTX, SSZ, HCQ, prednisolone+INF. C: MTX, SSZ, HCQ+prednisolone+placebo. O: ACR remission, remission in radiological damage. Secondary: DAS28 remission and score <2.6 at week 26 | Multicenter, randomized, placebo-controlled | 5 years | 99 | 1 | 1 | MTX 10mg up to 25mg/sem. SSZ up to 2g/day, HCQ 35mg/kg/week and prednisolone 7.5mg/day. INF (3mg/kg) or placebo infusion. |
| Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomized, double-blind, placebo-controlled study (the NEO-RACo Study). Leirisalo-Repo et al., 2013 | P: patients RA symptoms ≤12 months, without previous DMARD with active disease. I: INF+MTX+SSZ HCQ+prednisolone. C: MTX, SASP, HCQ+prednisolone+placebo. O: remission and radiological progression after INF+intensified therapy FIN-RACo vs. FIN-RACo as the only regime<2.6 at week 26 | Multicenter, randomized, placebo-controlled | 2 years | 99 | 1 | 1 | MTX 20–25mg/week, SSZ 1–2g/day, HCQ 35mg/kg/week, INF 3mg/kg or placebo infusion weeks 4, 6, 10, 18, 26 |
| Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naïve patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Detert et al., 2013 | P: patients with RA of 1 year. I: MTX+ADA. C: MTX+placebo. O: evaluation DAS28 week 48. Others: percentage of patients with remission (DAS28<2.6) and ACR20 response, 50 or 70 | Randomized, controlled, double blind | 1 year | 172 | 2 | 1 | ADA 40mg every 2 weeks. MTX 15mg/week |
| Disease activity, physical function, and radiographic progression after long-term therapy with adalimumab plus methotrexate: 5-year results of PREMIER. Van der Heijde et al., 2010 | P: patients with AR <3 years. I: ADA+MTX. C: ADA or MTX. O: advantage initial combined therapy vs. monotherapy | 2 initial years: Multicenter, randomized controlled by active comparator, double blind After 2 years: open-label phase | 5 years | 799 (388 completed 5 years) | 2 | 1 | MTX 7.5–20mg/week ADA 40mg/every 2 weeks |
| A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Moreland et al., 2012 | P: active RA. I: MTX+etanercept. C: MTX+SSZ+HCQ, or MTX monotherapy stepped to combined therapy (MTX+etanercept or MTX+SSZ+HCQ). O: DAS28-ESR weeks 48–102. Others: improved activity ACR20, 50, 70 | Randomized double blind | 2 years | 755 | 4 | 1 | MTX 20mg/week. SSZ 500–1.000mg BID. HCQ 200mg BID. Etanercept 50mg/week sc |
ACR: American College of Rheumatology; ADA: adalimumab; RA: rheumatoid arthritis; BID: twice a day; CSA: cyclosporine A; DAS: Disease Activity Score; DAS28-CRP: score of disease activity that only includes C-reactive protein), items: TJC28: number of painful joints (0–28); SJC28: number of swollen joints (0–28); CRP: (in mg/l); GH: global health assessment (from 0=best to 100=worse); DAS28-ESR: score of disease activity that only includes ESR (erythrocyte sedimentation rate); DMARDs: disease modifying antirheumatic drugs; FIN-RACo: The Finnish Rheumatoid Arthritis Combination Therapy; HCQ: hydroxychloroquine; INF: infliximab; MP: methylprednisolone; mTSS: modified total Sharp score; MTX: methotrexate; SSZ: sulfasalazine.
The results related to the outcome of clinical remission were reported in the majority of studies according to the score in the DAS28 scale and in a lower proportion using the DAS44 scale, considering as remission a score lower than 2.6 and 1.6, respectively.
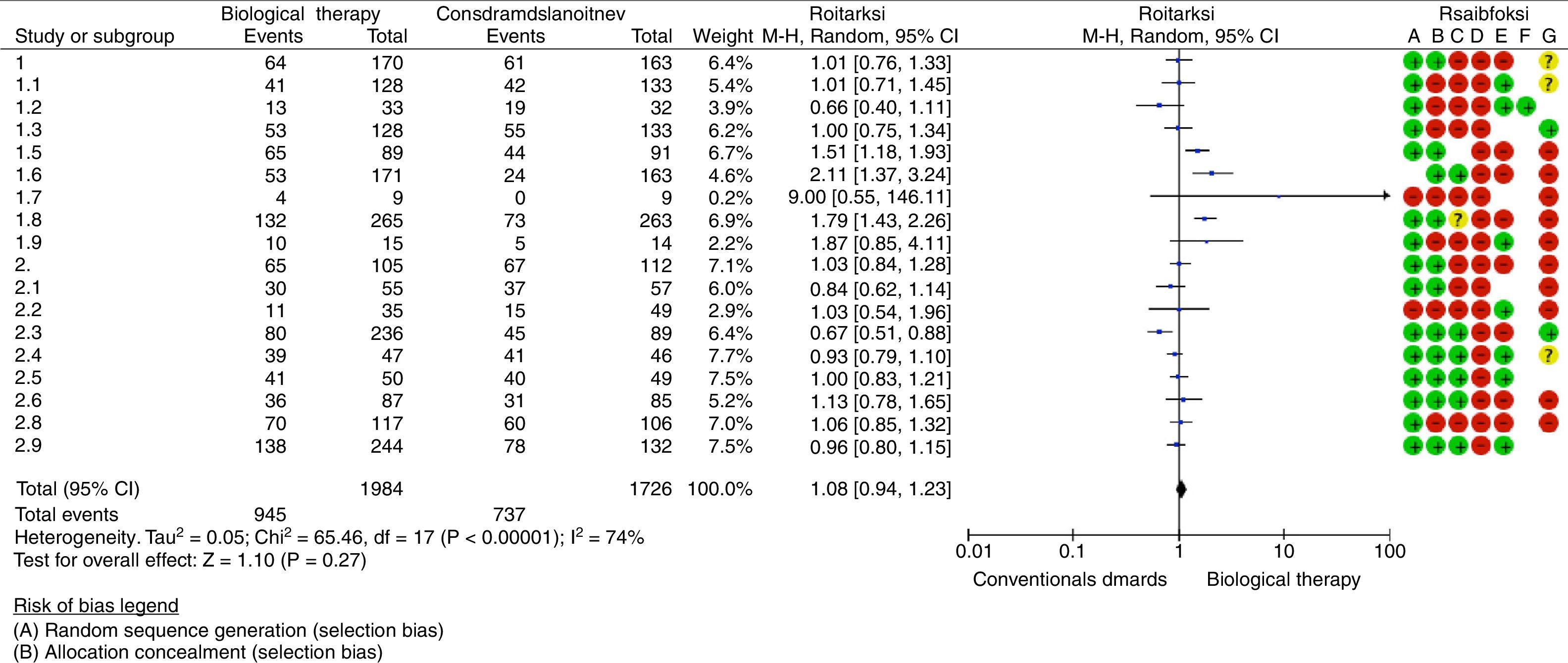
Nine studies reported as an outcome the remission of the disease in a follow-up period equal to or less than one year; 3 of these studies were able to demonstrate statistically significant differences when comparing biological therapy versus conventional antirheumatic drugs. Hørslev-Petersen et al. found a higher proportion of patients with DAS28<2.6 at 12 months in the group of adalimumab/MTX/intra-articular corticosteroid compared with MTX/intra-articular corticosteroid, being of 74 and 49%, respectively, with a RR=1.51 (95% CI: 1.18–1.93; p=0.001). In the group studied by Takeuchi et al., it was evidenced that the patients treated with adalimumab plus MTX were more likely to achieve clinical remission (31%) compared with the group assigned to MTX in monotherapy (14.5%) at week 26 of treatment, with a RR=2.11 (95% CI: 1.37–3.24; p=0.007). In addition, the study conducted by Emery et al. showed a greater number of patients with clinical remission at 52 weeks in the group treated with etanercept/MTX (50%) in relation to patients treated with MTX in monotherapy (28%), with a RR=1.79 (95% CI: 1.43–2.26; p=0.00001).
Six studies evaluated the remission of early RA in patients who were followed-up during 13 and 24 months after the start of treatment, showing significant differences the study conducted by O’Dell et al., which supports the use of MTX in monotherapy as initial management of patients with early RA with poor prognostic factors, achieving remission in 50.6% of the patients treated with MTX compared with 34.2% of the patients who received progressive combination therapy (MTX+etanercept or MTX+sulfasalazine+hydroxychloroquine) (RR=0.67 [95% CI: 0.51–0.88]; p=0.004).
The outcome of remission of the disease for a period longer than 2 years was described in 3 studies without finding differences when comparing biological therapy with DMARD.
An assessment of the risk of bias was carried out taking into account the following aspects: presence of selection bias measured by sequence generation and allocation concealment, presence of performance bias evaluating the blinding of participants and staff, existence of detection bias if there was not blinding of outcome assessors and attrition bias related to incomplete outcome data (loss of data/patients greater than 10% of those included at the beginning of the study). In general, it was found a high risk of bias (Fig. 2).
The synthesis of the evidence through meta-analysis did not evidence differences concerning the outcome of remission of the disease, when using biological therapy in monotherapy or combined with conventional DMARDs versus the use of DMARDs (RR=1.08; 95% CI: 0.94–1.23). Data analysis was performed using the RevMan 5.3 software provided by Cochrane (http://tech.cochrane.org/revman/download).
DiscussionRA is a chronic, destructive and persistent disease, predominantly articular, whose cause is not clearly known so far, that affects multiple systems although its main target organ is the synovial membrane; it is characterized by autoimmune inflammatory symmetric polyarthritis (synovitis), in the context of positive serological tests for self-reactivity; although there is no pathognomonic laboratory test for the diagnosis of RA, the presence of rheumatoid factor and anti-citruline antibodies is very suggestive of this condition. In the extra-articular disease, cardiovascular, cutaneous, renal, ocular, gastrointestinal, neurological and vascular commitment may exist, and untreated patients can progress to marked joint deformity, severe joint destruction, functional limitation and increased mortality related to accelerated cardiovascular disease.1,2
RA is the most frequent and serious inflammatory chronic joint disease in the world, with an annual incidence of approximately 3 cases per 10,000 inhabitants, and a prevalence close to 1%, which increases with age and has its highest peak at the ages of 35–50 years. This entity affects all populations, although its prevalence is higher in some groups. In Latin America, between 0.4% and 1% of the population may suffer from it, being more frequent in female patients, with a ratio of 6–8:1 in this region.1–5,7
As in other diseases, RA requires a comprehensive approach that should include non-pharmacological interventions, such as lifestyle modifications, with dynamic exercises, occupational therapy, hydrotherapy and smoking cessation; classically, in the management of RA have been used conventional DMARDs, which include MTX, antimalarials (hydroxychloroquine, chloroquine), leflunomide, sulfasalazine and particularly in the last decades have begun to be used, more and more frequently, new groups of drugs that like biological therapy or biological DMARDs (anti-TNF and not anti-TNF) have started to demonstrate an important role as modifiers of the clinical course and the remission of RA. The strategies for the management of RA have changed significantly in the last years in order to reduce the symptomatic burden of the disease, the progression of joint damage and the functional disability. There is evidence that early initiation of pharmacological measures results in better outcomes, based on the «window of opportunity» concept, which indicates that in the initial phase of the disease there is a greater response to treatment, being able to alter its natural evolution and in some cases, to return to normality.8
Biological DMARDs have been studied in different scenarios, the inhibitors of TNF (etanercept, infliximab, adalimumab) and IL-1 (anakinra) have demonstrated their efficacy and safety in long-standing RA, but the studies in early TA are scarce.
The ATTRACT (AntiTNF Therapy in RA with Concomitant Therapy) study shows how the combination therapy of infliximab and MTX increases the clinical and radiographic benefit in patients with active RA despite having received therapy with MTX (study not addressed to patients with early RA although about 20% had an evolution shorter than 3 years); more than 400 patients were included and in the analyses at 52 and 102 weeks was evidenced that the patients treated with either of the regimens of infliximab (3 or 10mg/kg every 4 or 8 weeks) plus MTX had a significant improvement in the radiographic indexes, erosion indexes and impingement of the joint space versus the group of MTX alone; in the sub-analysis of the group of patients with early RA it was documented that those who received infliximab showed greater inhibition of the progression of structural damage in a treatment period of 2 years, and for this reason, it was concluded that the early use of anti-TNF agents could provide benefits in the long term regarding the progression of the disease and the preservation of the joint integrity.9,10 Subsequent studies such as the ASPIRE (Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset) with more than 1000 patients evaluated showed similar results in terms of clinical response rates when using combinations of doses of infliximab with MTX. In the study conducted by Quinn et al., which evaluated 20 patients with RA of less than one year of evolution, with poor prognostic factors, the patients were randomized to receive MTX plus infliximab 3mg/kg or MTX plus placebo, it was observed that all radiological indexes evaluated by magnetic resonance were significantly better, without new erosions and with improvement of functional ability in the group of infliximab plus MTX; as a relevant finding, one year after the suspension of the induction therapy, the response was sustained in 70% of the patients in the group of infliximab plus MTX, with a mean DAS28 of 2.05 (remission range).11,12
The ERA (early RA) study evaluated the efficacy and safety of etanercept in the management of RA compared with MTX; in this study, more than 600 patients with RA of less than 3 year of duration, not previously treated with MTX, were randomized and assigned to 3 groups of treatment: oral MTX (mean, 19mg/week); etanercept, 10mg/twice a week, and etanercept, 25mg/twice a week. Compared with MTX, the group of etanercept-25mg had a more rapid improvement at 3, 6, 9 and 12 months. In this study is also evident that etanercept and MTX in monotherapy demonstrated to be effective in the treatment of RA of less than 3 years of evolution, although the combination of anti-TNF and MTX is superior to both in monotherapy, both for clinical and radiological outcomes; additional information documented by the TEMPO (Trial of Etanercept and MTX with Radiographic Patient Outcomes) study, not exclusive of patients with early RA, shows that such combination is better in the reduction of the disease activity, improvement of functional capacity and delay of radiographic progression with statistical significance.13–15
Another anti-TNF agent, adalimumab, has demonstrated efficacy in the treatment of RA established in the ARMADA (The Anti-Tumor Necrosis Factor Research Study Program of the Monoclonal Antibody Adalimumab [D2E7] in Rheumatoid Arthritis) study, and the results of a sub-analysis of the DEO19 study indicate that early treatment with adalimumab can be more effective that its late use in the course of RA. The recent PREMIER study compared the safety and efficacy of adalimumab (40mg/every 15 days) plus MTX (in rapid escalation at 20mg weekly) with MTX or adalimumab in monotherapy in 799 patients with RA of less than 3 years of evolution and who were not previously treated with MTX. The ACR50 response at week 52 was significantly greater in the combination arm than with MTX or adalimumab in monotherapy (62% versus 46% and 42%, respectively) (p<0.001); this difference was maintained during the 2 years of duration of the study. In 50% of the patients in the combination arm it was induced a sustained remission measured by the DAS28 index <2.6 and a greater clinical response was also observed. With the combination treatment, it was obtained also an inhibition of the radiographic progression significantly greater than with either of the treatments in monotherapy. According to what is observed in Fig. 2, Forest Plot, the drugs that have more statistically significant likelihood to induce response and remission would be adalimumab and etanercept, although the studies that support them have methodological limitations.16–18
The final outcome expected when instituting the medical management in patients with RA is to achieve complete remission, although attaining a low disease activity may be an acceptable goal in certain cases. To reach this goal, several strategies have been suggested: in 2013 the EULAR proposed the recommendations which describe an intervention by phases; phase I includes patients with clinical diagnosis of RA in whom, according to the severity of the condition, management with MTX or combination of therapy with conventional DMARD plus steroid at low doses should be started and, in case of contraindication for MTX, DMARD therapy in monotherapy or in combination should be used excluding this drug; phase II includes patients who have had failure in phase I or presence of toxicity, in them, the presence of unfavorable prognostic factors (high levels of rheumatoid factor or anti-citruline antibodies, very high disease activity, early joint damage) should be evaluated; in the absence of risk factors, is indicated a change to a second DMARD agent, alone or combined, and the presence of risk factors for poor prognosis is the scenario where the addition of therapy with biological agents would apply; phase III is constituted by patients in whom phase II has failed, in this group of patients it is indicated to change to a second biological agent in combination with conventional DMARD therapy. According with these recommendations, biological agents would be reserved for patients in whom the management with conventional therapy has failed, however, more and more studies (among which stands out the BeSt study with more than 500 patients) begin to show a new space in which the biological DMARDs would have a place in earlier stages of the disease to achieve higher rates of remission of RA. A strategy that is suggested to define which patients can benefit more from the early initiation of the use of biological agents is the determination of poor prognostic factors, the identification of patients with early RA who will have persistent disease and the prediction of the response to biological agents; in addition, the cost and the possible adverse effects (severe infections, malignancy) derived from the use of these drugs, should be taken into consideration. The present study evaluates the ability of biological agents to achieve such remission of the disease, particularly in patients with early RA, without finding differences in the remission of the disease between the use of biological therapy versus conventional DMARDs.4,19–31
LimitationsIt stands out the heterogeneity of the studies analyzed, mainly for clinical differences and epidemiological methods. In the present study was demonstrated that there is a significant heterogeneity (I2=74%) among the studies meta-analyzed, as well as a high risk of bias demonstrated through the GRADE system. This heterogeneity lied in differences in the design of the analyzed studies, highlighting those in the comparator (for example, in some studies it was MTX monotherapy, while in others it was combined therapies); in the same way in the time of outcome measurement, which was variable among all studies (proportion in remission at 6, 12, 24 or 60 months), among others.
There are limitations when the remission of the disease is assessed by the DAS28 because an important group of patients, who are classified in remission by means of this tool, exhibit inflammatory signs such as edema or pain corresponding to a low level of activity of RA. With the above would be included a greater proportion of patients in remission by DAS28 compared with the mACR, SDAI and CDAI criteria.32,33 Given that the effectiveness of biological therapy in inducing ACR20, ACR50 and ACR70 responses was not defined as inclusion or outcome criteria in the different studies, this variable was not included, which limits its analysis.
All these limitations invite the reader to analyze carefully the results of the study at the time of putting them into clinical practice.
ConclusionsWhen evaluating this systematic review, no statistically significant differences are found regarding the outcome of remission of the disease between patients with early RA who receive biological therapy versus conventional DMARDs.
It is necessary to carry out clinical studies of better quality in order to be able to determine the efficacy of biological therapy in inducing remission of the disease in patients with early RA.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Camargo Barrios CB, Ibargüen JER, Quintana-López G. Terapia biológica en la artritis reumatoide temprana: eficacia en la remisión de la enfermedad. Rev Colomb Reumatol. 2017;24:164–176.