Psoriasis is treated with biological therapy, which has led to low disease activity or even clinical clearance. The optimization (dose-tapping) process seeks to reduce biological therapy while maintaining the risk–benefit ratio.
ObjectivesTo determine relapse rate in psoriasis optimization strategy (dose tapering) patients.
Material and methodsCohort study (October 2015 to February 2021) of patients with psoriasis in a specialized multicentre health institution in Colombia. Selection criteria included being at least 18 years old with biological therapy and having a sustained response (DLQI=0–5; absolute PASI <3 or BSA <1) for at least 12 months. The optimization strategy was dose reduction or interval application increase. PASI >10 was defined as relapse. Rates were estimated by medication using Kaplan–Meier.
ResultsOf the 467 patients with psoriasis in the cohort, 467 received biologic therapy. 12.2% (n=57) of those who met the inclusion criteria were men, with a median age of 57 years (IQR: 44–66), disease evolution time of 15 years (IQR: 5–30), and time in optimization of 8 months (IQR: 1.54–13.2). Because of the increased application interval, the optimization strategy was 85.8%; 24.5% (n=14) received ustekinumab, 35% (n=20) received adalimumab, 15.8% (n=9) received secukinumab, 14% (n=8) received ixekizumab, 5.2% (n=3) received etanercept, and 5.2% (n=3) received guselkumab. A total of 14% (8 patients) relapsed, the relapse rate was 7.4 cases per 100 person-years (95% CI: 3.4–14).
ConclusionsEighty-six percent of patients in the optimization strategy remain relapse-free after 8 months. The optimization strategy was effective, with a low relapse rate.
La terapia biológica es efectiva en el tratamiento de la psoriasis, su optimización busca reducir la dosis sin afectar la relación riesgo-beneficio.
ObjetivosDeterminar la tasa de recaída en pacientes con optimización.
Materiales y métodosCohorte (octubre 2015-febrero 2021) de pacientes con psoriasis en una institución de salud multicéntrica especializada en Colombia. Se incluyeron pacientes mayores de 18 años con terapia biológica y una respuesta sostenida (DLQI=0-5; PASI absoluto <3 durante 12 meses). La estrategia de optimización fue la reducción de la dosis o el aumento del intervalo de aplicación. El PASI >10 se definió como recaída. Las tasas se estimaron por medicación utilizando Kaplan-Meier.
ResultadosDe 467 pacientes con psoriasis y terapia biológica, se incluyó al 12,2% (n=57). El 65% (n=37) eran varones, con una edad media de 57 años (RIC: 44-66), un tiempo de evolución de la enfermedad de 15 años (RIC: 5-30) y un tiempo de optimización de 8 meses (IQR: 1,54-13,2). En el 85,8% de los casos se aumentó el intervalo de aplicación; el 24,5% (n=14) recibió ustekinumab, el 35% (n=20) adalimumab, el 15,8% (n=9) secukinumab, el 14% (n=8) ixekizumab, el 5,2% (n=3) etanercept y el 5,2% (n=3) guselkumab. El 14% (8 pacientes) recayó, en tanto que la incidencia fue de 7,4 recaídas por cada 100 personas/año (IC 95%: 3,4-14).
ConclusionesEl 86% de los pacientes en la estrategia de optimización permanece sin recaídas a los 8 meses. La estrategia fue efectiva y tuvo una baja tasa de recaída.
Psoriasis affects between 0.5 and 2% of the world's population.1 In some countries, biological therapies such as anti-tumor necrosis factor alpha (TNF), anti-interleukin 17 (IL-17), and anti-interleukin 23 (IL-23) are required for disease control in 25–40% of patients with moderate to severe psoriasis.2,3 These treatments, in a high percentage of cases, achieve complete remission of lesions and symptoms.4
The optimal duration of the biological therapy is unclear beyond the clinical trial data; this justifies the evaluation of new therapeutic strategies in this regard.5 Long-term immunomodulator therapy has safety implications, such as tuberculosis or candidiasis, among others.6,7 Another consideration is the pharmacoeconomic impact of this type of therapy on the health-care system.8
Interventions for therapy optimization include lowering medication doses or increasing the time interval between applications.9 The goal will be to achieve this lower dose while maintaining therapeutic goals in patients based on disease control.10 Previous research has shown that 60% of maintenance is completed with this goal in mind.11 This study aims to show the experience of a population in Colombia in which this treatment strategy has been implemented
MethodsStudy objectivesThe objectives were to: (1) determine the baseline characteristics of the dose-tapering strategy in psoriasis patients. (2) Investigate the dose-tapering frequency in biological therapy. (3) Determine the relapse rate in patients with psoriasis in the optimization strategy (dose tapering), defined as a Psoriasis Area Severity Index (PASI) >10 at the following period.
Study design and populationAn open cohort study of outpatient adults with psoriasis was followed from October 2015 to February 2021 in a specialized multicenter health institution in Colombia with 11 outpatient healthcare facilities. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline was followed.12 Patients eligible for the study were 18 years of age or older, had vulgar psoriasis, and were treated with biological therapy. Patients eligible for optimization therapy (dose tapering) were those with a sustained response (DLQI=0–5; absolute PASI <3) for at least 12 months, and the intervention involved a change, a reduction in dose, or an increase in the interval between dose applications.
Data collection and analysisThe following period's sociodemographic (age, gender), clinical features (PASI, DLQI), treatment optimization, and relapse defined as a PASI >10 were extracted from the structured clinical record software HCM®. The implementation of a psoriasis integral healthcare model, which includes a standardized dermatologist consultation, ensures the evaluation of the clinical scores PASI and the Dermatology Life Quality Index (DLQI). The optimization (dose tapering) protocol was built by the dermatologist, rheumatologist, and knowledge management board with a systematic review of the literature and actual evidence. According to the severity of the disease, all patients were evaluated every 3–6 months.
Statistical analysisBaseline characteristics and demographics were reported using descriptive statistics. The chi-square test was used to evaluate categorical variables if n≥5 and Fisher's exact test otherwise. If the assumption of normality was met or n>30, the Student's t-test was used, otherwise the Wilcoxon rank sum test was used. Relapse rates were estimated by medication using Kaplan–Meier and the density incidence rate. The R statistical software version 4.2.1 was used.
Ethical considerationsThe researchers adhered to the Declaration of Helsinki version 2013. According to resolution 008430/1993 from Colombia's Ministry of Health, this is constituted as a study without risk, so informed consent was not required, as it is considered that this article does not contain personal information that allows to identify the patients.
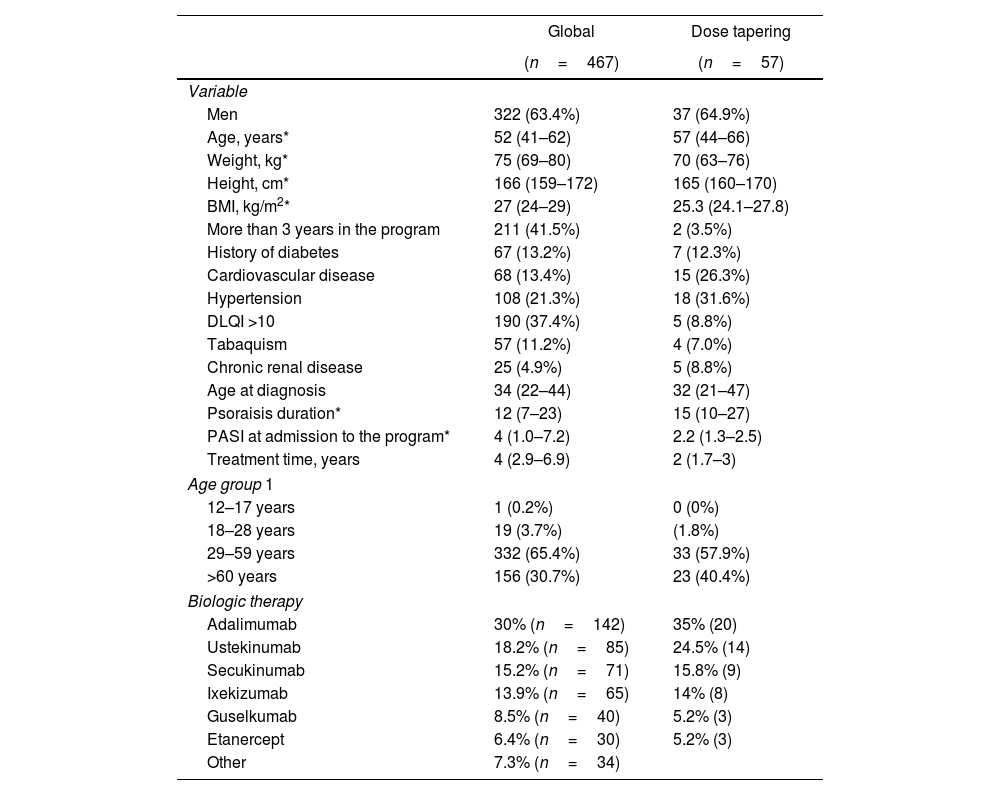
ResultsBaseline demographics and clinical characteristicsFrom a cohort of 467 patients with psoriasis, 12.2% (n=57) achieved the dose tapering inclusion criteria (Fig. 1). The global and dose tapering baseline cohort characteristics are shown in Table 1. Following time represented a total of 108 person-years. In the global cohort, 30% (n=142) received adalimumab, 18.2% (n=85) ustekinumab, 15.2% (n=71) secukinumab, 13.9% (n=65) ixekizumab, 8.5% (n=40) guselkumab, 6.4% (n=30) etanercept, 7.2% (n=34) received other agents. All the clinical data was complete and without missing data. In the dose tapering strategy cohort, 65% (n=37) were men, with a median age of 57 years (IQR: 44–66), disease evolution time of 15 years (IQR 10–27), and time in optimization of 8 months (IQR 1.54–13.2). Patients in the dose-tapering group had lower median BMI (27 vs. 25.3), weight (75 vs. 70), and age at diagnosis (34 vs. 32). Also, lower frequencies of tabaquism (11.2% vs. 7.0%), diabetes (13.2% vs. 12.3%), but a higher frequency of hypertension (21.3% vs. 31.6%), as shown in Table 1.
Summary of baseline demographics, disease, and clinical variables.
| Global | Dose tapering | |
|---|---|---|
| (n=467) | (n=57) | |
| Variable | ||
| Men | 322 (63.4%) | 37 (64.9%) |
| Age, years* | 52 (41–62) | 57 (44–66) |
| Weight, kg* | 75 (69–80) | 70 (63–76) |
| Height, cm* | 166 (159–172) | 165 (160–170) |
| BMI, kg/m2* | 27 (24–29) | 25.3 (24.1–27.8) |
| More than 3 years in the program | 211 (41.5%) | 2 (3.5%) |
| History of diabetes | 67 (13.2%) | 7 (12.3%) |
| Cardiovascular disease | 68 (13.4%) | 15 (26.3%) |
| Hypertension | 108 (21.3%) | 18 (31.6%) |
| DLQI >10 | 190 (37.4%) | 5 (8.8%) |
| Tabaquism | 57 (11.2%) | 4 (7.0%) |
| Chronic renal disease | 25 (4.9%) | 5 (8.8%) |
| Age at diagnosis | 34 (22–44) | 32 (21–47) |
| Psoraisis duration* | 12 (7–23) | 15 (10–27) |
| PASI at admission to the program* | 4 (1.0–7.2) | 2.2 (1.3–2.5) |
| Treatment time, years | 4 (2.9–6.9) | 2 (1.7–3) |
| Age group 1 | ||
| 12–17 years | 1 (0.2%) | 0 (0%) |
| 18–28 years | 19 (3.7%) | (1.8%) |
| 29–59 years | 332 (65.4%) | 33 (57.9%) |
| >60 years | 156 (30.7%) | 23 (40.4%) |
| Biologic therapy | ||
| Adalimumab | 30% (n=142) | 35% (20) |
| Ustekinumab | 18.2% (n=85) | 24.5% (14) |
| Secukinumab | 15.2% (n=71) | 15.8% (9) |
| Ixekizumab | 13.9% (n=65) | 14% (8) |
| Guselkumab | 8.5% (n=40) | 5.2% (3) |
| Etanercept | 6.4% (n=30) | 5.2% (3) |
| Other | 7.3% (n=34) | |
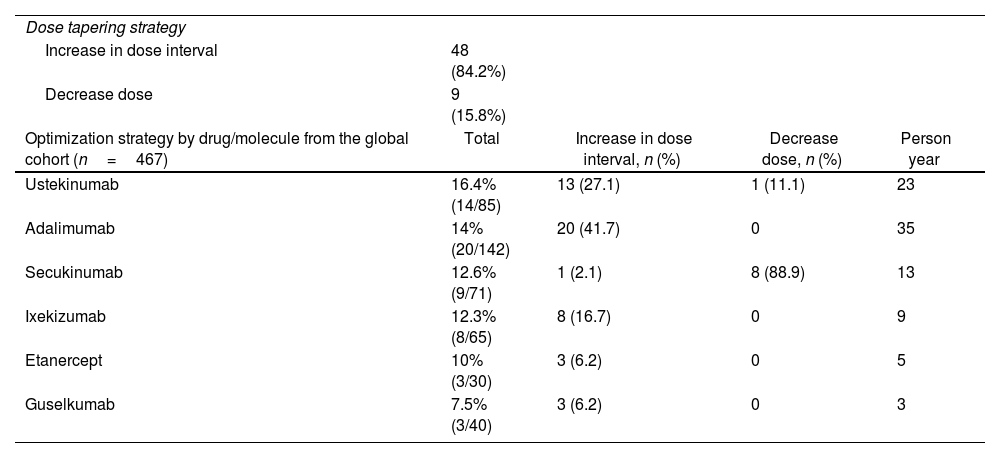
The optimization strategy was 85.8% due to an increase in the application interval and 14.2% due to a dose decrease; all the biological therapies had a similar frequency of optimization, although Guselkumab was the least optimized (Table 2). Although 12% (7 patients) relapsed, the incidence rate was 7.4 for 100 person-years (95% CI 3.44–14). Three patients and five patients, at a median time of 14 and 8 months, relapsed with adalimumab and ustekinumab, respectively. Those patients restart the previous regimen after achieving clearance (Fig. 2).
Characteristics of optimization strategy.
| Dose tapering strategy | ||||
| Increase in dose interval | 48 (84.2%) | |||
| Decrease dose | 9 (15.8%) | |||
| Optimization strategy by drug/molecule from the global cohort (n=467) | Total | Increase in dose interval, n (%) | Decrease dose, n (%) | Person year |
| Ustekinumab | 16.4% (14/85) | 13 (27.1) | 1 (11.1) | 23 |
| Adalimumab | 14% (20/142) | 20 (41.7) | 0 | 35 |
| Secukinumab | 12.6% (9/71) | 1 (2.1) | 8 (88.9) | 13 |
| Ixekizumab | 12.3% (8/65) | 8 (16.7) | 0 | 9 |
| Etanercept | 10% (3/30) | 3 (6.2) | 0 | 5 |
| Guselkumab | 7.5% (3/40) | 3 (6.2) | 0 | 3 |
All major pivotal studies focus on clearance and safety and do not involve dose reduction or discontinuation of biological therapy.4 Although, there is an ongoing randomized non-inferiority clinical trial, the current data comes from registries that reported reduced doses without decreasing efficacy.13 The most frequent inclusion criteria for dose tapering in non-clinical trial psoriasis registries were therapeutic clearance at PASI and maintaining a PASI value, which are similar to the present study definitions. In this regard, the incidence of 7.4 cases per 100 person-years and 14% of relapse is lower compared with other studies that report 40% relapses in patients with previous clearances.11 It is also low compared with the 67.7% with 300mg and 52.4% with 150mg of the secukinumab retreatment-as-needed strategy relapse rate.5,14,15 Unfortunately, the following time was short and do not bring up long-term results data. Another limitation of the current data is its focus on anti-TNF, ustekinumab, and secukinumab.9,16 This study extended the focus and also included ixekizumab and guselkumab.
At the moment, no dose tapering clinical trials in dermatology is available. A rheumatology consensus recommended reducing the dose or interval between 20 and 50% of the regular dose depending on the drug and patient characteristics.17,18 20–50% of patients in psoriasis registries under biologic treatment were receiving a reduced dose,9 but in the present study, it was lower (14%). The patients who started the dose-tapering protocol usually begin after 5 years of treatment19; however, in our data only 3% of the patient had more than 3 years in the program. The tendency was to start early the dose tapering strategy and it included anti-IL17 and anti-IL23 therapy.
Obesity, rapid attainment of PASI 100,11,18 and specific treatment gene polymorphism20 are prognostic factors associated with psoriasis relapse; this is consistent with our findings and other studies that reported better outcomes in patients with a lower BMI and lower weight when treated with dose-tapering. In our cohort, patients with dose tapering had a lower BMI compared to the non-tapered group (Table 1). The patients who relapsed went back to the previous therapy regimen without any adverse events, which is consistent with other studies.5,9,16
Many aspects of the dose-tapping strategy are still unknown. The optimal duration of the dose tapering strategy is unclear with the current data, however, a second step in reducing the dose has been reported.16 Prognostic models for the prediction of relapse based on unbiased factors and cost-utility studies that determine the impact of dose-tapering strategies in the health system are needed to close the gap between clinical practice and public health in psoriasis. The generalizability of this dose-tapering program is low because it reflects our clinical practice and demographics; further studies are needed to confirm and build a stronger approach.20
ConclusionsMost patients in the optimization strategy remain in sustained clinical response after 8 months. The low relapse rates reported in this study support the optimization strategy as effective and without adverse events. Further studies are needed to improve the dose-tapering strategy to reduce drug exposure, adverse events, and costs to the health system and increase effectiveness, quality of life, and patient compliance.
FundingMedicarte.
Conflict of interestNone declared.