Tocilizumab (TCZ), an interleukin-6 receptor-α inhibitor, is indicated in patients with moderate to severe rheumatoid arthritis with inadequate response to disease modifying drugs. ACT UP is a multinational project collecting information from several post-marketing TCZ studies.
AimTo determine the proportion of patients in the routine clinical care setting receiving intravenous TCZ after 6 months treatment. Identification of TCZ treatment patterns, efficacy, and safety were also recorded.
MethodThis prospective non-interventional 6-month study, collected real-world information from 169 Central American and Caribbean patients. No interventional procedures or additional visits outside routine clinical care practice were performed. Statistical analysis was essentially descriptive.
ResultsAdherence rate was 74.0%, with 97% of patients receiving TCZ as first biological therapy line and there were no deviations from the local label. Almost 85% of patients started with combination therapy, and the majority remained under this scheme throughout the study. A significant decrease in disease activity assessments and acute phase reactants values were detected during TCZ treatment. The percentage of patients that achieved improvement according to the different levels of the American College of Rheumatology (ACR) increased during the study, and relevant enhancements in quality of life were also accomplished. Adverse events (AEs) occurred in 35 patients, with metabolic and nutritional disorders being the most common. Serious AEs were reported in 3% of patients, and special interest AEs occurred in 6 patients.
ConclusionTreatment adherence was mainly determined by follow-up and compliance with the administration schedule. Efficacy analysis showed better results than those reported in international literature. The incidence of AEs was also lower than in previously published data.
El tocilizumab (TCZ) está indicado en la artritis reumatoide moderada a severa, principalmente en respuestas inadecuadas a fármacos convencionales. ACT UP es un proyecto multinacional que recopila información relacionada con varios estudios de poscomercialización.
ObjetivoDeterminar la proporción de pacientes en la atención clínica de rutina que continúan en tratamiento con TCZ intravenoso después de 6 meses. Se llevó a cabo la identificación de patrones de administración, eficacia y seguridad.
MétodoEste estudio observacional prospectivo recopiló información de la vida real de 169 pacientes de América Central y el Caribe. No se hicieron intervenciones ni visitas adicionales fuera de la práctica clínica habitual. El análisis estadístico fue esencialmente descriptivo.
ResultadosLa tasa de adherencia al tratamiento fue del 74,0%, el 97% de los pacientes recibieron TCZ como primera línea biológica y no existieron desviaciones en las indicaciones de administración según el inserto local. Aproximadamente el 85% de los pacientes inició TCZ como terapia combinada, y la mayoría permaneció bajo este esquema. Se evidenció una disminución en la actividad de la enfermedad y un aumento en el porcentaje de pacientes que lograron respuesta según los diferentes grados del Colegio Americano de Reumatología. En 35 pacientes se presentaron eventos adversos (EA), siendo los relacionados con metabolismo y nutrición los más comunes. Se informaron EA graves en el 3% de los pacientes y de interés especial en 6 casos.
ConclusiónEl seguimiento de los pacientes y el cumplimiento del programa fueron los principales determinantes en la adherencia. El análisis de eficacia mostró mejores resultados que los reportados previamente y la incidencia de EA fue menor que en otros estudios.
The epidemiology of Rheumatoid Arthritis (RA) varies according to different regions and is affected by environmental and genetic factors.1 Although data is scarce, it is estimated that Latin America has a prevalence between 0.4 and 1.6%,2,3specifically for Central America and the Caribbean (CAC) Region, there is no precise epidemiological information available. Current treatment for RA considers the initial use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and when an adequate response is not obtained, the addition of other DMARDs or the use of biological agents (bDMARDs) is considered.4 Despite the emergence of biological agents with proven disease modifying activity, that act in several of the individual components and steps of the inflammatory cascade, which have revolutionized RA treatment5; a considerable percentage of patients do not achieve clinical remission with the use of tumor necrosis factor (TNF) α inhibitors.6,7
Between 20 and 55% of patients treated with TNF-α inhibitors are classified as treatment failure associated with either lack of response, resistance or intolerance to the medication.8,9 In these cases, it is necessary to modify the specific agent or use another medication with an alternative mechanism of action. The previous concept regarding the need to modify the initial agent in order to achieve a rapid drug effect is crucial, due to the existence of a relationship between the time to reach remission and the final therapeutic result, therefore agents that show fast response rates are considered the best option to achieve clinical remission.10,11 Tocilizumab (TCZ) is a humanized monoclonal antibody against the α subunit of the interleukin-6 receptor (IL-6R) that prevents the binding of the endogenous ligand with its α subunit.12 Interleukin-6 (IL-6) is a cytokine that acts as a fundamental mediator in the RA inflammatory process.13 Due to its mechanism of action, TCZ prevents the transduction of signals from the inflammatory mediators and is associated with clinical remission in these patients. Currently, this agent is indicated in the treatment of moderate to severe RA, specifically in the absence of a considerable response to csDMARDs.14,15 Clinical studies have demonstrated TCZ's efficacy both in monotherapy and in combination treatment, in addition of being an adequate alternative in case of treatment failure with other bDMARDs.7,16
RA clinical care management in the region is complex and influenced by health systems with limited resources.17 The above in addition to the fact that clinical trials may not represent patients in the usual practice care setting, makes it necessary to generate regional studies that reflect this reality.
MethodologyThis study part of the multinational Actembra Umbrella Project (ACT UP), seeks to describe baseline clinical and demographic characteristics and determine over a 6-month period, patterns of use, efficiency and safety in the usual clinical practice care setting of RA patients who start treatment with intravenous TCZ based on physician‘s criteria, specifically in Central America and the Caribbean Region. The ACT UP research project uses non-interventional, observational, post-marketing, multi-center studies and shares design elements, selection criteria, and basic aspects, which have been extensively described elsewhere.18,19
Treatment dose and duration were determined considering investigator's indication, product prescribing information and local regulations. No additional study visits were scheduled, nor were medications or procedures given outside the routine clinical practice care. The eligibility criteria included patients 18 years of age or older with a diagnosis of moderate to severe RA according to the ACR criteria,20 who had received TCZ within 8 weeks before study enrollment. Patients who received TCZ in a clinical trial, associated to a compassionate use program or who had previously received treatment for more than 8 weeks, were not eligible for inclusion in the study. Additionally, patients who had received any investigational drug within 4 weeks (or 5 half-lives of the experimental agent) prior to treatment onset with TCZ, as well as patients with a history of other autoimmune diseases (e.g., systemic lupus erythematosus, psoriatic arthritis, Sjögren's syndrome) or other inflammatory joint conditions different form RA were not included. There were no restrictions regarding the prescription with concomitant medications corresponding with the investigator's clinical criteria and in accordance with the prescribing drug information for TCZ.
In this study (NCT01952509) data from 7 sites in Central America and the Caribbean was included, specifically Panama, Guatemala, Costa Rica, and the Dominican Republic, with a total of 169 recruited patients. Gathered information for the clinical and demographic description included: baseline characteristics (evaluated before TCZ administration), medical history, concomitant treatments as well as previous pharmacological therapies prescribed for RA. Drug use pattern was evaluated by means of adherence, regimen modification, changes in concomitant treatments and dosage. Therapy effectiveness evaluations included: clinical disease activity assessments, remission criteria achievement, laboratory determinations and quality of life questionnaires. Aspects related to drug safety comprised laboratory testing and adverse events monitoring. Concomitant diseases and adverse events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) standardized terminology.21,22
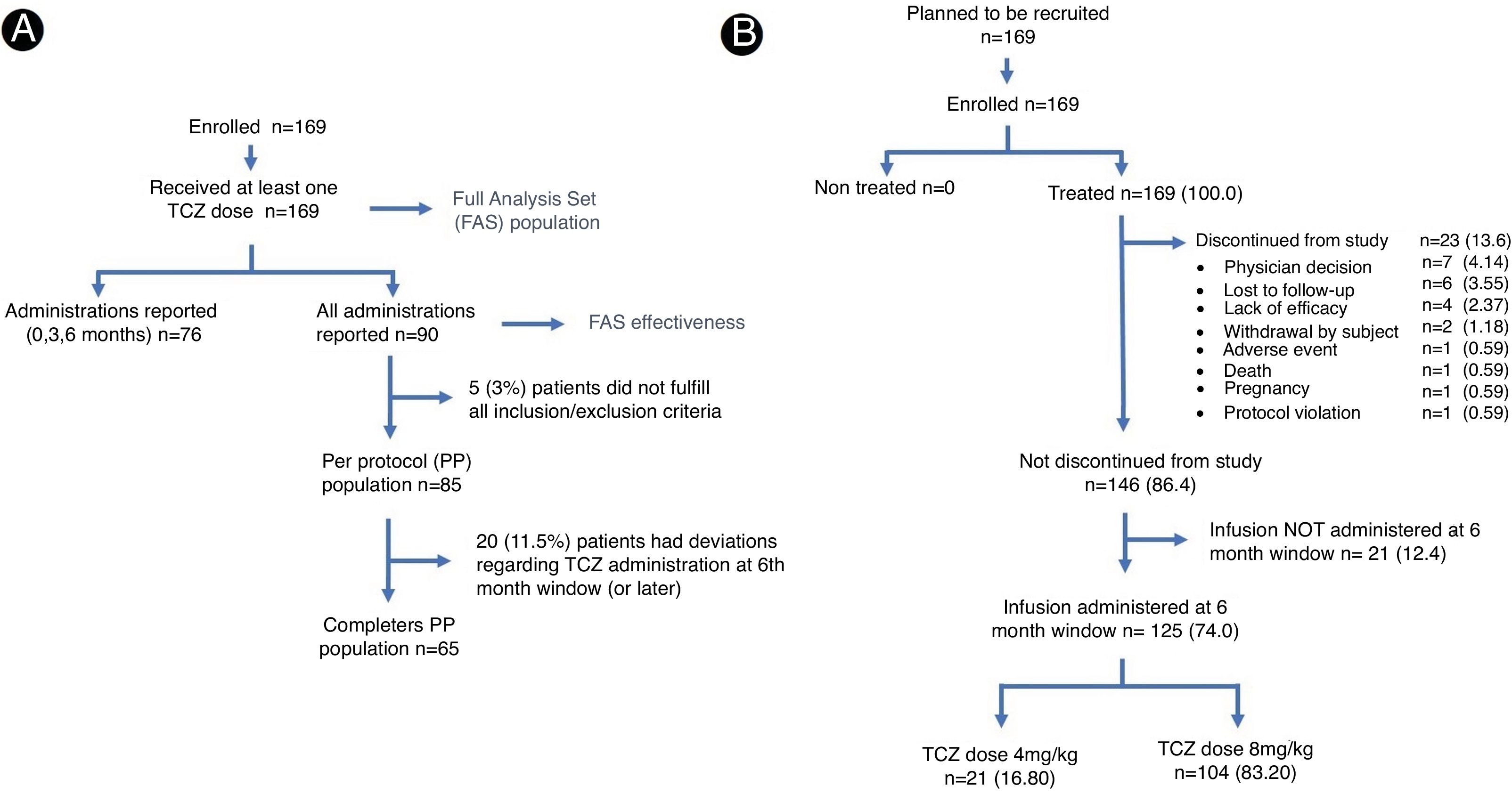
All recruited patients who received at least one dose of TCZ were included in the primary analysis population (FAS, Full Analysis Set), which was used to report safety-related aspects (Fig. 1A). During the follow-up period, two of the participating sites did not report monthly data of TCZ administrations for each patient according to the protocol's specified time frame. Due to the lack of source documentation regarding the exposure of the patient to the study medication on a specific schedule, it was decided that these patients would not be considered in the effectiveness analysis and description of TCZ treatment pattern. As consequence, reference population for the evaluation of efficacy and patterns called FAS effectiveness, included 90 patients (Fig. 1A) and excluded patients from the mentioned centers. For this very reason, Dominican Republic patients were excluded from these analyses.
Unless otherwise indicated, the values are expressed as absolute quantity and percentage for the qualitative variables, median with quartiles in the case of measurement scales and mean±standard deviation for quantitative variables. Comparisons for discrete variables or values for measurement scales were made at 3 and 6 months using the Wilcoxon range test or paired t-tests in the case of continuous variables. A p value less than 0.05 for bilateral contrasts was considered statistically significant. Patients with missing data were not excluded from the analysis and no imputation was made. The proportion of patients on treatment with TCZ at 6 months was evaluated with descriptive statistical analysis and the confidence intervals were determined using the Clopper–Pearson method. No segregated analysis was performed for monotherapy or combination treatment.
All procedures were carried out in accordance with the Helsinki Declaration and local regulations. In each participating country the research protocol was approved by an ethics committee (Panama: Instituto Conmemorativo Gorgas de Estudios de la Salud (861/CBI/ICGES/14-1070/CBI/ICGES/15), Guatemala: Latins Ethics (ML28747), Costa Rica: Universidad de Ciencias Médicas (CEC/0097/2015-CEC/UCIMED/485/5/2015) and Dominican Republic: Plaza de la Salud (Conabios 023/2013) and prior to study inclusion all patients signed an informed consent form.
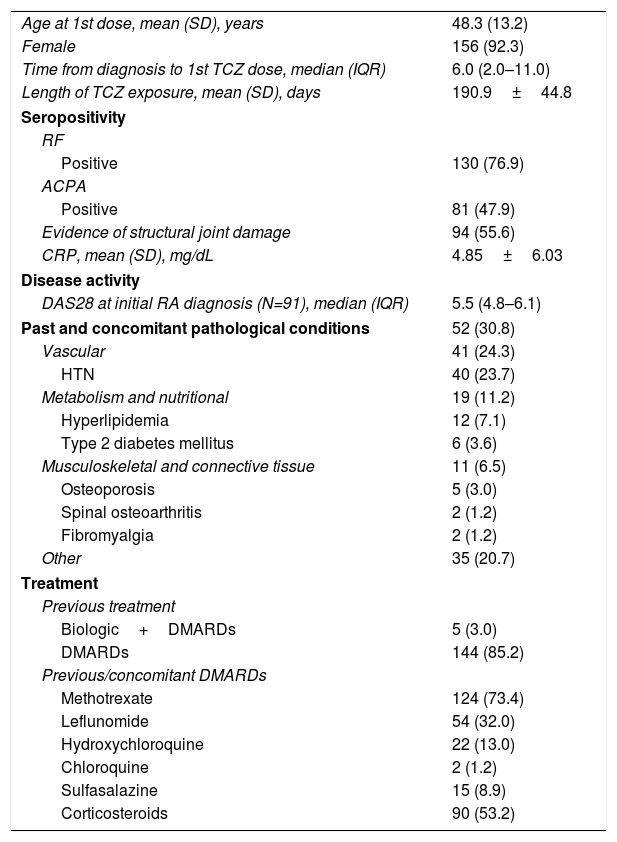
ResultsOne hundred sixty-nine patients were included in the primary analysis and 125 completed study protocol, 23 reported early termination registered as secondary to medical criteria, loss to follow-up and lack of efficacy and 21 cases have no record regarding the 6 month TCZ administration (Fig. 1B). Baseline characteristics demonstrated a population predominantly composed (more than 90%) of women. The average age at study inclusion was 48.3±13.2 (SD) years and the time from diagnosis to TCZ initiation was quite variable, presenting a median of 6.0 years and an interquartile range between 2.0 and 11.0 years. At baseline, the rheumatoid factor was positive in more than 75.0% of the population, while anti/cyclic citrullinated peptide antibodies were confirmed in 88.0% of the patients who underwent the analysis (Table 1).
Patient baseline characteristics (FAS population).
| Age at 1st dose, mean (SD), years | 48.3 (13.2) |
| Female | 156 (92.3) |
| Time from diagnosis to 1st TCZ dose, median (IQR) | 6.0 (2.0–11.0) |
| Length of TCZ exposure, mean (SD), days | 190.9±44.8 |
| Seropositivity | |
| RF | |
| Positive | 130 (76.9) |
| ACPA | |
| Positive | 81 (47.9) |
| Evidence of structural joint damage | 94 (55.6) |
| CRP, mean (SD), mg/dL | 4.85±6.03 |
| Disease activity | |
| DAS28 at initial RA diagnosis (N=91), median (IQR) | 5.5 (4.8–6.1) |
| Past and concomitant pathological conditions | 52 (30.8) |
| Vascular | 41 (24.3) |
| HTN | 40 (23.7) |
| Metabolism and nutritional | 19 (11.2) |
| Hyperlipidemia | 12 (7.1) |
| Type 2 diabetes mellitus | 6 (3.6) |
| Musculoskeletal and connective tissue | 11 (6.5) |
| Osteoporosis | 5 (3.0) |
| Spinal osteoarthritis | 2 (1.2) |
| Fibromyalgia | 2 (1.2) |
| Other | 35 (20.7) |
| Treatment | |
| Previous treatment | |
| Biologic+DMARDs | 5 (3.0) |
| DMARDs | 144 (85.2) |
| Previous/concomitant DMARDs | |
| Methotrexate | 124 (73.4) |
| Leflunomide | 54 (32.0) |
| Hydroxychloroquine | 22 (13.0) |
| Chloroquine | 2 (1.2) |
| Sulfasalazine | 15 (8.9) |
| Corticosteroids | 90 (53.2) |
Values expressed as total quantity and percentage unless otherwise mentioned. SD: standard deviation, IQR: interquartile range, TCZ: tocilizumab, DAS 28: Disease Activity Score 28, DMARDs: disease modifying antirheumatic drugs.
Initially, most of the patients showed high disease activity measured by DAS-28 and more than half presented articular damage on the physical examination; patient's previous medical history was also considered for articular damage classification. Vascular and metabolic alterations were the main previous and concomitant conditions registered, with a frequency greater than 10.0% (Table 1).
Prior the administration of TCZ, five patients had concomitantly used biological agents (TNF-α inhibitors, monoclonal antibodies or immunomodulatory agents) and csDMARDs in combination therapy. Only one patient had been treated with 2 biological drugs and 80% of treatment interruption in these cases was due to therapeutic failure.
The vast majority of patients (85.2%) had previously been treated with csDMARDs (mainly methotrexate (73.4%)) (Table 1) and generally, the use of such DMARDs continued during the administration of TCZ. Methotrexate and antimalarials were the most frequently suspended drugs before the first dose of TCZ. The percentage of patients treated with csDMARDs and the proportion of each remained virtually unchanged during the study. Prior to the use of TCZ, more than half of the participants reported corticosteroids use, with prednisone being the most employed at a daily average dose of 7.7mg±2.6 (SD). Likewise, approximately 40% reported previous or concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics. At the end of the study, the number of patients treated with these medications remained constant. Four patients receiving concomitant treatment with corticosteroids modified the dose, also dose was adjusted in 3 patients using NSAIDs and in one patient treated with cyclooxygenase-2 inhibitors.
Twenty-six participants (15.4%) received TCZ as monotherapy. Of these, 20 were drug naive patients and 6 had interrupted all disease modifying treatment. At six months, 18 patients remained with the same regimen, 2 had early treatment suspension, and in 6 cases a valid evaluation was not obtained within the established time. Of the 143 patients who started TCZ in combination therapy, 1 patient switched to monotherapy, 106 reported no modification in their treatment plan (3 presented changes in TCZ dose), in 21 cases TCZ administration was applied outside of the time period established and 15 patients were registered as early termination.
Treatment adherence rate was 74.0% (95% CI 66.7–80.4), more than 95% (n=86) of patients included in the FAS effectiveness subgroup received 5 doses of TCZ, and in 77 (85.6%) of the cases 6 doses were administered. The time between infusions was every 28 to 33 days.
At the end of the study, 83.2% of patients in the FAS population reported the use of 8mg/kg of TCZ (Fig. 1A), this dose was used in about 63% of the FAS effectiveness population. Dose modifications in FAS effectiveness subgroup correspond to increases related to low efficacy, which were reported in 5 (5.6%) cases. Throughout the study, no dose reductions, incomplete infusions, interruptions or deviations from local label recommendations were reported. In Costa Rica, most patients received the 4mg/kg dose.
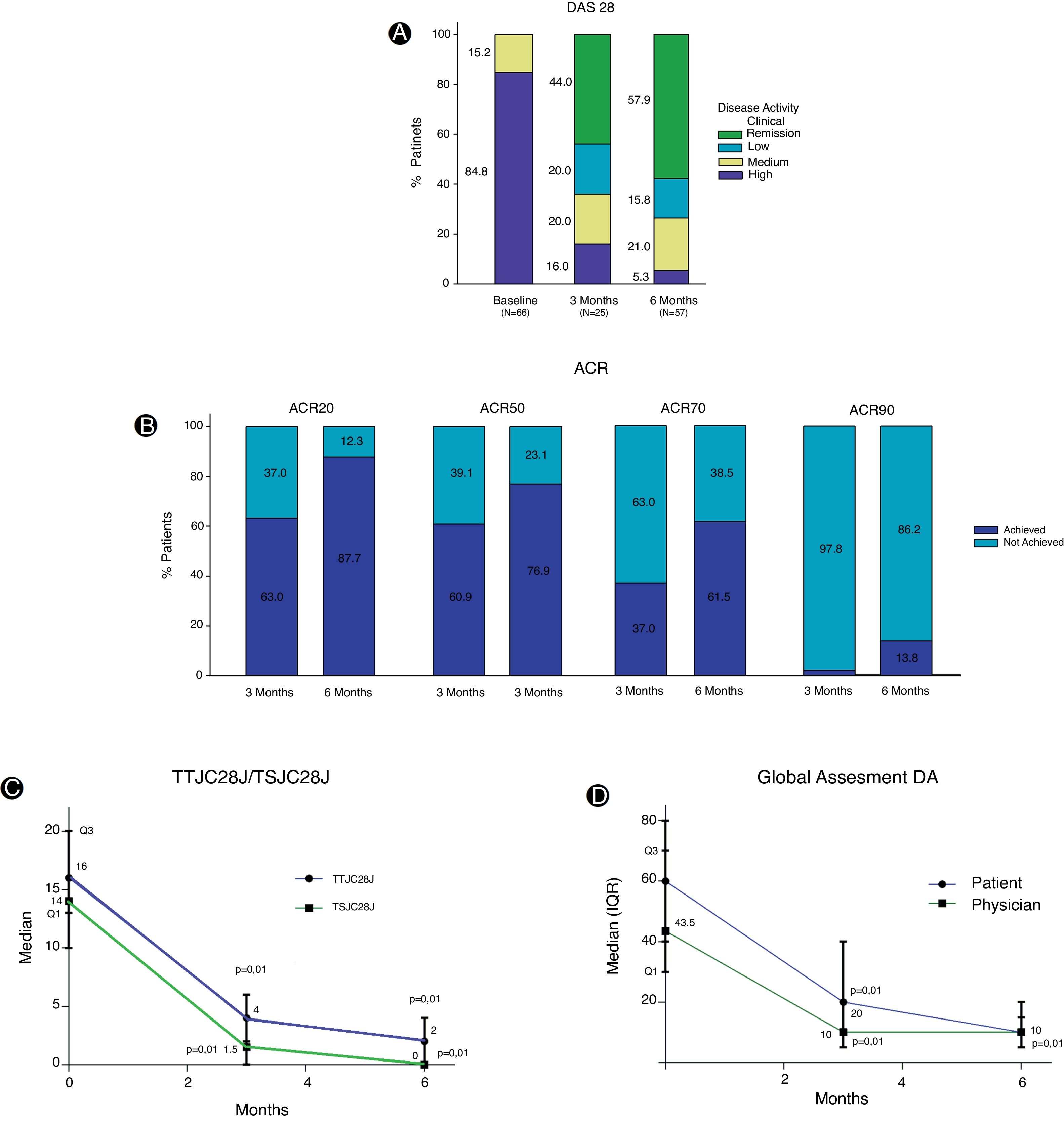
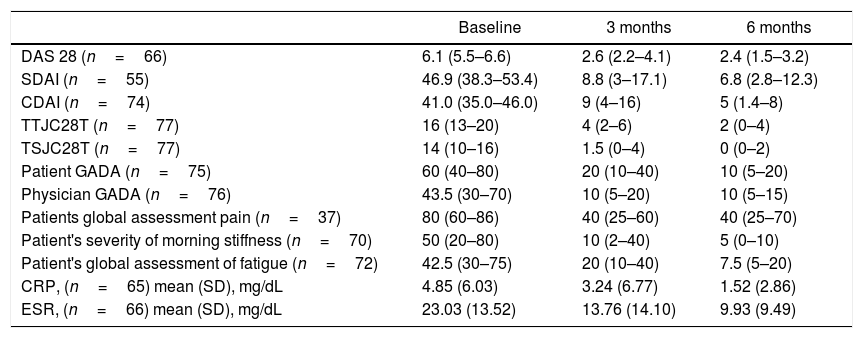
Baseline evaluation for disease activity in the FAS effectiveness population exhibited great affectation (Table 2). The DAS28 index shows at the beginning of the study that most of the patients (84.8%) reported high disease activity, while, at 3 months of treatment, despite the low number of patients evaluated, a decrease in disease activity was reported. At 6 months, with a greater number of evaluations registered, it was determined that 57.9% showed clinical remission and in 15.8% the disease activity was reported as low (Fig. 2A). Variations in DAS28 scores were similar at 3 and 6 months of treatment and show a statistically significant difference (p=0.01) when compared to baseline values (Table 2).
Clinical determinations (FAS effectiveness).
| Baseline | 3 months | 6 months | |
|---|---|---|---|
| DAS 28 (n=66) | 6.1 (5.5–6.6) | 2.6 (2.2–4.1) | 2.4 (1.5–3.2) |
| SDAI (n=55) | 46.9 (38.3–53.4) | 8.8 (3–17.1) | 6.8 (2.8–12.3) |
| CDAI (n=74) | 41.0 (35.0–46.0) | 9 (4–16) | 5 (1.4–8) |
| TTJC28T (n=77) | 16 (13–20) | 4 (2–6) | 2 (0–4) |
| TSJC28T (n=77) | 14 (10–16) | 1.5 (0–4) | 0 (0–2) |
| Patient GADA (n=75) | 60 (40–80) | 20 (10–40) | 10 (5–20) |
| Physician GADA (n=76) | 43.5 (30–70) | 10 (5–20) | 10 (5–15) |
| Patients global assessment pain (n=37) | 80 (60–86) | 40 (25–60) | 40 (25–70) |
| Patient's severity of morning stiffness (n=70) | 50 (20–80) | 10 (2–40) | 5 (0–10) |
| Patient's global assessment of fatigue (n=72) | 42.5 (30–75) | 20 (10–40) | 7.5 (5–20) |
| CRP, (n=65) mean (SD), mg/dL | 4.85 (6.03) | 3.24 (6.77) | 1.52 (2.86) |
| ESR, (n=66) mean (SD), mg/dL | 23.03 (13.52) | 13.76 (14.10) | 9.93 (9.49) |
Values expressed as median and interquartile range (IQR) unless otherwise mentioned. Comparisons of all determinations between baseline and 3 or 6 months were statistically significant (p<0.05). DAS 28: Disease Activity Score 28, TTJC28T: total tender joint count on 28 joints score, TSJC28T: total swollen joint count on 28 joints score, GADA: Global Assessment of Disease Activity, SDAI: Simple Disease Activity Index, CDAI: Clinical Disease Activity Index, CRP: C-Reactive Protein, ESR: Erythrocyte sedimentation rate, SD: standard deviation.
Likewise, the Simple Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI) indicated that more than 90% of the patients had a high disease activity score at the time of study entry. In both indexes, a considerable score decrease was observed at three months of treatment, however, for this period a higher percentage reached clinical remission according to the SDAI. At 6 months, both determinations showed similar percentages of patients with a low disease activity score or in remission. With respect to baseline values, the two indicators showed statistically significant reductions (p=0.01) at 3 and 6 months (Table 2).
The EULAR (European League Against Rheumatism) response criteria showed that 71.4% and 74.4% of the patients presented a good response at 3 and 6 months of treatment respectively, while the response rate was moderate in approximately 20% of the patients for both periods. Despite the fact that about 83.3% of the patients achieved a good or moderate EULAR response at three months and was maintained at 6 months, only 18 patients underwent this evaluation in all visits. During TCZ treatment, important changes were observed in the ACR20 and ACR50 response criteria, and it was clear that the percentage of patients who achieve ACR response increases over time. At 6 months, approximately 25% more patients reached ACR20 response, this increase was 16% for ACR50, 24.5% for ACR70 and greater than 10% for ACR90 (Fig. 2B).
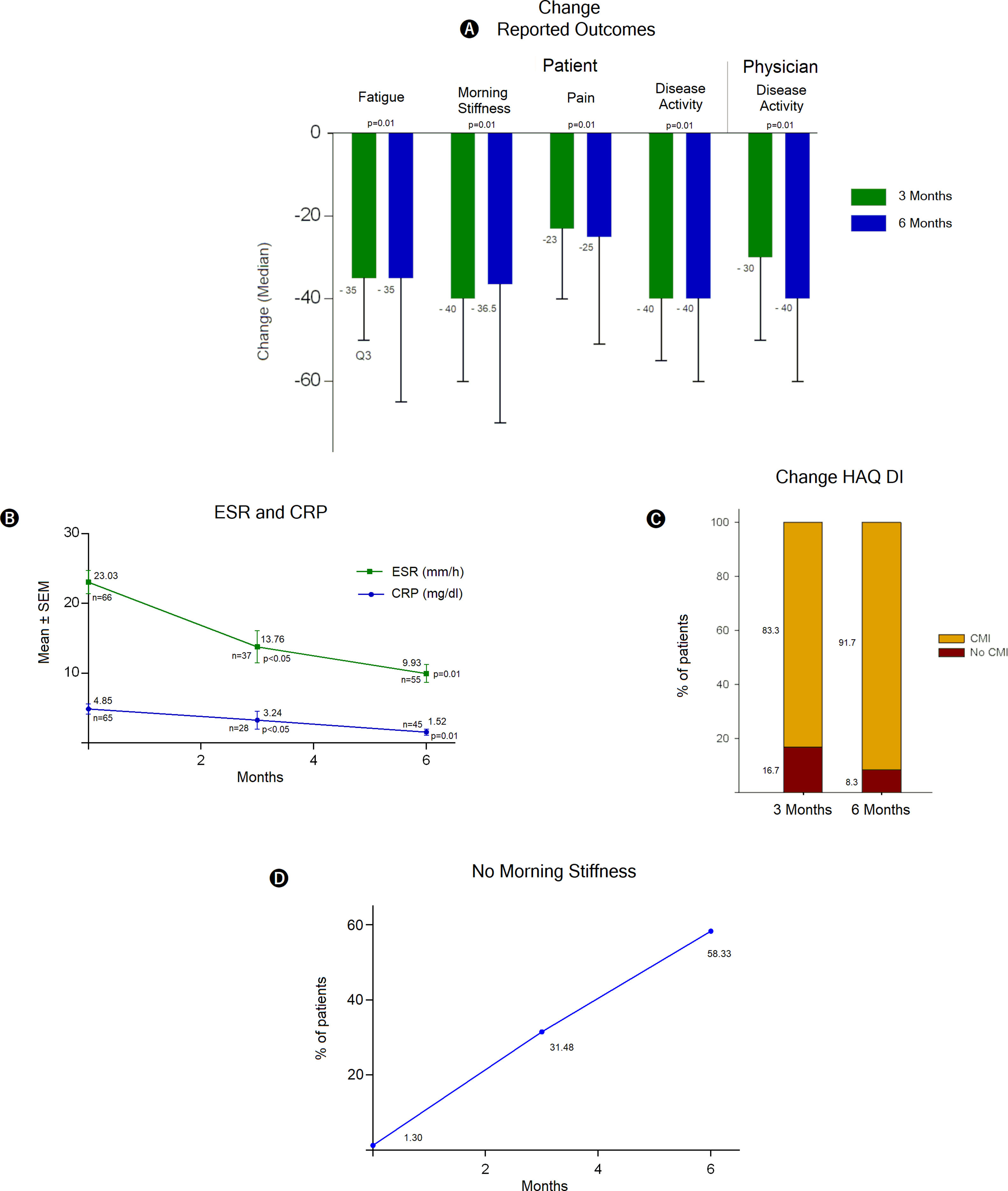
Treatment with TCZ showed a statistically significant decrease between baseline total tender joint count on 28 joints score (TTJC28J) and total swollen joint count on 28 joints score (TSJC28J) determinations and those made at 3 and 6 months; showing a greater reduction between 0 and 3 months in both assessments (Fig. 2C). The global assessments for disease activity registered at 3 and 6 months, both by the physician and self-reported by the patient, showed a significant decrease with respect to baseline evaluations. These determinations are comparable between clinicians and patients at 6 months (Fig. 2D). The assessments regarding fatigue, morning stiffness, pain and disease activity, reported by the patients show significant reductions both at 3 and 6 months. The extent of the decrease was similar for both periods (Fig. 3A).
Assessments and determinations regarding treatment response to TCZ. DA: disease activity, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, HAQ DI: Health Assessment Questionnaire Disability Index, CMI: clinically meaningful improvement, Q1 and Q3: first and third quartile respectively, SEM: standard error of the mean.
The population analyzed showed substantial decreases in acute phase reactants such as ESR and CPR, in both cases the values at 6 months were 50% lower than the baseline value, specifically in CPR a sustained decrease was observed (Fig. 3B). According to the Health Assessment Questionnaire Disability Index (HAQ DI) a clinically meaningful improvement was evident at 3 and 6 months of TCZ treatment, the proportion of patients who reported health enhancement was 83.3% and 91.7% respectively (Fig. 3C). Likewise, the benefit is noticeable regarding morning stiffness, where there was a pronounced improvement over time, at 6 months the number of patients free of stiffness throughout the whole day was close to 60% (Fig. 3D).
Considering normal limits (LSN) for the SGPT and SGOT values, approximately 85% of the patients did not show changes regarding liver function tests from their initial classification. At 3 months of treatment, 10.7% of study subjects showed the highest variation in SGOT values from their initial classification. Around 10% of patients reported an increase in total cholesterol levels after 3 months of TCZ treatment, a similar percentage was identified at 6 months. HDL cholesterol, LDL cholesterol, triglyceride and hemoglobin levels showed no significant changes and most patients maintained the same initial classification according to the total neutrophil and platelet count.
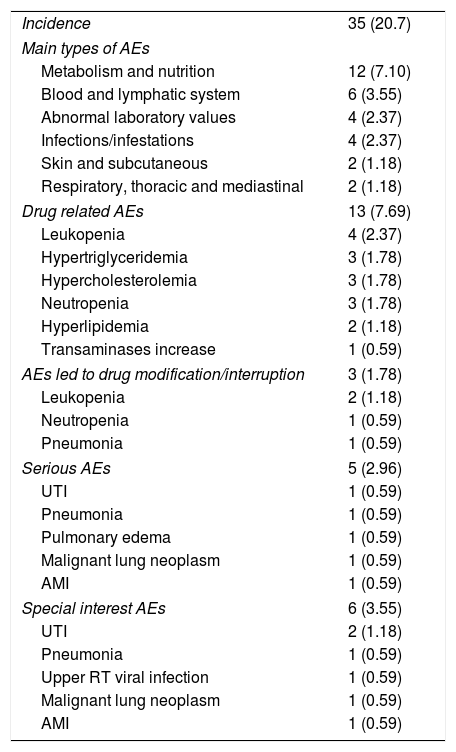
During the study period, 35 (20.7%) patients presented at least one adverse event. The intensity was reported as mild in 16.0% (n=27), moderate in 3.6% (n=6) and potentially fatal in 1.2% (n=2). Metabolism and nutrition alterations were the most common adverse events (EAs) reported with a frequency greater than 5%. Five patients presented a serious adverse event and causality with study drug was confirmed in 13 (7.7%) cases. Drug related EAs were mainly metabolism and nutrition alterations (4.7%), and blood and lymphatic system disorders (4.2%) (Table 3).
Adverse events reported during tocilizumab treatment.
| Incidence | 35 (20.7) |
| Main types of AEs | |
| Metabolism and nutrition | 12 (7.10) |
| Blood and lymphatic system | 6 (3.55) |
| Abnormal laboratory values | 4 (2.37) |
| Infections/infestations | 4 (2.37) |
| Skin and subcutaneous | 2 (1.18) |
| Respiratory, thoracic and mediastinal | 2 (1.18) |
| Drug related AEs | 13 (7.69) |
| Leukopenia | 4 (2.37) |
| Hypertriglyceridemia | 3 (1.78) |
| Hypercholesterolemia | 3 (1.78) |
| Neutropenia | 3 (1.78) |
| Hyperlipidemia | 2 (1.18) |
| Transaminases increase | 1 (0.59) |
| AEs led to drug modification/interruption | 3 (1.78) |
| Leukopenia | 2 (1.18) |
| Neutropenia | 1 (0.59) |
| Pneumonia | 1 (0.59) |
| Serious AEs | 5 (2.96) |
| UTI | 1 (0.59) |
| Pneumonia | 1 (0.59) |
| Pulmonary edema | 1 (0.59) |
| Malignant lung neoplasm | 1 (0.59) |
| AMI | 1 (0.59) |
| Special interest AEs | 6 (3.55) |
| UTI | 2 (1.18) |
| Pneumonia | 1 (0.59) |
| Upper RT viral infection | 1 (0.59) |
| Malignant lung neoplasm | 1 (0.59) |
| AMI | 1 (0.59) |
Values expressed as total quantity and percentage. AEs: adverse events, UTI: urinary tract infection, AMI: acute myocardial infarction, RT: respiratory tract.
Three patients presented AEs that required dose modification or treatment discontinuation. Dose reduction was performed in the presence of leukopenia and/or neutropenia, while interruption was carried out in case of pneumonia. The incidence regarding special interest AEs was less than 5% (Table 3) and no adverse reactions were identified during the infusion. Two deaths were reported, one during the time of the study and one outside the study period, both were considered not related to study medication.
Discussion and conclusionsBaseline demographic and clinical characteristics for the population analyzed are comparable with those previously described in other Latin American studies.23 Although the identified percentage of patients who initiate TCZ as monotherapy in the clinical practice is lower than what has been published in Patient Disease Registries and studies from other regions,24 nevertheless is consistent with the first open trials that resemble routine practice.23,25 This situation could be related to specific aspects in the Central American and Caribbean region such as limited access to the drug and the need to develop experience with the administration scheme. These same reasons would also apply to explain why the percentage of naive patients who start monotherapy with TCZ is lower than what has been reported.18,23
Although the identified percentage of adherence to treatment at 6 months was high (74.0% CI95% (66.7–80.4%)), this is lower than what has been estimated and reported in other studies.19,23 It is important to identify specific factors that could be affecting these numbers, since according to the results obtained in this investigation, the difference does not appear to be related to a lack of efficacy or safety aspects of the study drug; main factors implicated in early treatment suspensions.18 The identified causes for study treatment interruption before 6 months are mainly related to aspects regarding follow-up or failure in the administration of study drug within the observation period, conditions which have also been described in other Latin America studies.23 It is also necessary to expand the analysis to evaluate patient adherence rate considering the type of treatment scheme: monotherapy or combined therapy, since other studies report differences in treatment continuity according to the type of therapy scheme used.26
The concomitant use of DMARDs and medications to treat RA, as well as their modifications during treatment with TCZ are similar to those reported in other real-life studies.23,27 As reported in the pivotal studies and other investigations from the usual practice clinical care setting, results regarding efficacy identified for Central America clearly shows the noticeable improvements experienced by patients during treatment with TCZ.28 Further, this study confirms the usefulness of this therapeutic alternative in the region, demonstrating superior efficacy when compared to what has been already reported in other studies. Similar situations have been reported in trials that include heterogeneous populations. Specifically, there is a greater decrease in the change of DAS28 scale values at 6 months with respect to the TAMARA study,29 a greater improvement by meeting ACR criteria than the one reported in other real-life studies25 and a better EULAR response when compared to the GISEA registration.30 However, the data of the latter is limited, due to the small number of patients who underwent such determination at all visits.
The safety profile identified in this observational study was very similar to what has been previously reported in controlled studies and real-life studies; particularly a low AEs incidence rate related to study drug during a 6 month treatment. In turn, the safety information from other studies, although not totally comparable, such as TOZURA and ACT-MOVE,31,32 is consistent with what was identified. Furthermore, the present investigation did not identify new AEs or a change in their severity.
It is important to mention that unlike what was reported in other research from the ACT UP project and in other real-life studies,18,33 where infections were the most frequently reported AEs, in this study the most prevalent AEs were hypertriglyceridemia and hypercholesterolemia followed by decreases in leukocyte and neutrophil count. Alterations in metabolism and nutrition have also been the main AEs identified in other usual clinical practice setting studies in the region.23 In accordance with other studies34 infections were part of the serious and special interest EAs identified.
The main limitation for this study is related to its nature and design, since it is an observational study in which the dose of TCZ, frequency and duration of study drug administration were not established by a study protocol, it is inevitable the incorporation of bias. Moreover, the selection of the drug was not carried out randomly. However, these limitations constitute at the same time, the main strengths of this study, since they reliably reflect the context of the usual clinical practice, the main aspect to be evaluated. The possible incorporation of patients undergoing treatment with subcutaneous TCZ and a longer period of observation are factors to take into account.
This research shows that the adequate management of RA requires the complex interaction between training, experience, research, adherence to international guidelines and judgment. This medical management should not only be based on data from traditional randomized controlled clinical trials, but also on data or studies that reflect existing usual clinical practice care in the region. Thus, evidence from studies of usual practice should be combined with that from clinical studies to provide a more complete picture of the results and the effectiveness of the intervention.
FundingThis multicenter study was funded by Roche Servicios SA, who participated in the design of the study, interpretation of data, and review and approval of the manuscript. Roche Servicios SA also funded medical writing support and article processing charges. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interestsAll authors were coinvestigators and received payment in this sponsored non-interventional study. Guerra-Bautista has given lectures and has received support for medical education activities participation from Novartis, Pfizer, Roche and Janssen. López-Barquero is part of Roche Medical Affairs Staff. Méndez-Rodríguez has given lectures and has received support for medical education activities participation from Pfizer, Roche, Aurinia, Astra Zeneca, Pfizer, Roche, Menarini, Novartis, Sanofi Aventis and Abbvie. Muñoz-Louis has been advisor and lecturer for Abbvie, Roche, Lilly, Pfizer and Janssen. Ortega-Gómez is part of Roche Medical Affairs Staff. Sanabria-Castro has been scientific advisor for Novartis, medical writer for Roche and has received support for medical education activities participation from Merck, AG.