Case reports or case series reports are descriptive studies, although their results are not used to make inferences, they are the first line of evidence for new therapies and a preamble to research with a stronger methodological design. This type of study is essential to describe new or unusual diseases, adverse events, or complications of known pathologies. They have become an elementary and accessible means of communication for health professionals. They are also pedagogical material to promote writing and intervention skills, mainly for novice researchers. This type of study strengthens academia, stimulates the spread of knowledge, and helps to resolve the dilemmas of clinical practice. In addition, they are a basic tool for the initiation and maintenance of research, so they will remain updated as a means of communication and linkage to medical knowledge. Therefore, it is important to know their definition, structure and role in the medical literature and research. This review provides the elements to consider when publishing a methodology for this type of study.
El informe de caso o los informes de series de casos son estudios descriptivos. Pese a que su resultado no se emplea para realizar inferencias, son la primera línea de evidencia de las terapias nuevas y un preámbulo de investigaciones con diseño metodológico idóneo. Este tipo de estudio es fundamental para describir enfermedades nuevas o inusitadas, eventos adversos o complicaciones de las patologías conocidas. Se han convertido en un medio de comunicación elemental y accesible para los profesionales de la salud. De igual forma, son un material pedagógico adecuado para fomentar habilidades de escritura e intervención, principalmente para los investigadores novicios. Además de fortalecer la academia, estimulan la difusión del conocimiento y ayudan a resolver los dilemas de la práctica clínica. También son una herramienta básica para el inicio y el mantenimiento de la investigación, por lo que seguirán vigentes como medio de comunicación y de enlace del conocimiento médico. En consecuencia, es importante conocer su definición, su estructura y su papel en la literatura médica y en la investigación. En esta revisión se proporcionan los elementos necesarios para tener en cuenta cuando se va a publicar una metodología de este tipo de estudio.
The case report or case series report is defined as a detailed narration about medical or scientific experiences, characterized by being innovative and informative and because it generally describes contents on clinical manifestations of rare diseases, epidemics, new diagnostic methods, drug effectiveness and safety, among other topics of medical practice. Based on these narrations it is possible to make hypotheses to develop studies with the adequate methodological design according to the research question that is formulated.1–4 This narrative review of the medical literature seeks to develop the methodology for writing a case report or a case series report.
Materials and methodsFor the development of this review, a literature search was conducted in the PubMed, EMBASE and LILACS databases, as well as in the grey literature Greylit, Opengrey, Trypdatabase and Google Scholar, in English and Spanish languages, for articles that had the Mesh terms: «Case reports» [Publication Type]; EMTREE: «Case report» [used for original items for reporting clinical work on not more than 4 individual patients]; and DeCS: «Informes de casos [Palabras]». Bibliographic citations published until January 2017 were included, and the search was updated on March 3, 2021. The authors obtained 38 articles and based on this information they wrote the guidelines for this article.
What are a case report and a case series report?The case report is an observational study in which one to four clinical cases are reviewed; however, there is no unified criteria.4–7 In its content, the information of the patient or patients is required: demographic data, symptoms and other relevant clinical characteristics, vital signs and detailed physical examination related to the health problem. This methodology is a tool to report rare or unknown diseases, inform unusual presentations or complications of known diseases. In addition, it communicates the diagnostic approaches and the anecdotal surgical and therapeutic procedures that have not been subjected to studies with adequate methodology due to a lack of a sufficient sample, or that have appeared recently,6,8–11 which makes them the first line of evidence. Therefore, they are useful as an introduction or starting point for research with greater methodological rigor.9,12
The case series report is a descriptive study, in which more than four clinical cases (usually four to ten cases) are typically documented, but it should be clarified that in the reviewed literature there is heterogeneity regarding this criterion. The main characteristic of the patients is that they share common clinical or therapeutic aspects.4,6,7,13–15 The report of case series is useful for the study of an infrequent disease or to develop a hypothesis and thus start a research more solid in evidence.10,11 From the pedagogical perspective, the case report and the case series report can be used as didactic material, strengthening the learning of diseases and motivating students to generate debates about the clinical process, the diagnostic approach and the therapeutic decisions.11
What is the history of the case report and case series report?The case report dates from the time of Hippocrates (460 B.C.),who described in one of his most outstanding books, with the title of Epidemics II (see Fig. 1), the cardiovascular diseases, and for this purpose he used histories of cases related to physical disease, without ignoring the mental aspects of the patients. The author reviewed retrospective clinical cases in chronological order and highlighted key aspects of the patient’s clinical signs and symptoms. He considered that the narrator physician should have an observer role, and therefore, the clinical records were focused on objective findings and on the outcomes of the clinical condition.16
In ancient Egypt, by the year 1600 B.C., a treatise of 48 clinical cases was brought to light (the Edwin Smith Papyrus), which is considered a classical medical document and which apparently was written by scribes of the XVII dynasty. The writing made reference to war injuries, as well as to head and upper chest injuries. Each report presented a defined structure as follows: a title (the region or organ affected), the physical examination (anamnesis, inspection, palpation and auscultation), the diagnosis, the description of the medical or surgical management (the decision was in accordance with the severity) and the final comments.17 This document is one of the first contributions of the medical task to scientific knowledge that was based on clinical experience. It stands out for leaving aside the magical managements or classical spells of the epoch (see Fig. 2).
Extract of the Edwin Smith papyrus.
Source: Rison.17
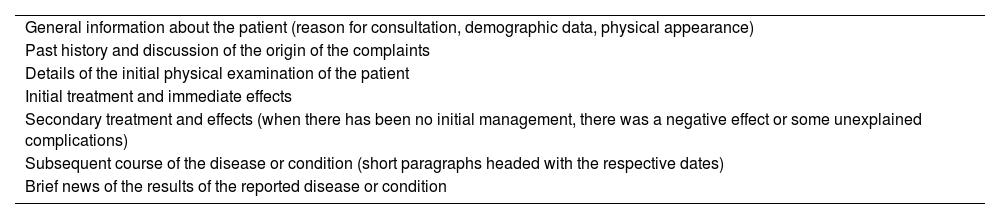
Subsequently, in 1992, Professor Atkinson published an analysis on the medical writing of The Edinburgh Medical Journal (considered the most ancient of England) which comprised several volumes. This journal was published between 1835 and 1985. Atkinson points out that towards the end of the eighteenth century (more precisely in the year 1775) a conventional description of the case report was made, based on an outlined structure (see Table 1).18
Structure of a case report from the year 1775.
| General information about the patient (reason for consultation, demographic data, physical appearance) |
| Past history and discussion of the origin of the complaints |
| Details of the initial physical examination of the patient |
| Initial treatment and immediate effects |
| Secondary treatment and effects (when there has been no initial management, there was a negative effect or some unexplained complications) |
| Subsequent course of the disease or condition (short paragraphs headed with the respective dates) |
| Brief news of the results of the reported disease or condition |
Source: adapted from Atkinson.18
The case report and the case series report are important scientific publications that since their beginning have been instruments of communication and dissemination of knowledge, both for doctors and scientists, by means of which the experiences about one or more patients are communicated under defined conditions that have already been mentioned.19 Their level of evidence is considered low, however, they are the first line of research for new therapies and medical approaches.8,20 Other advantages of this type of publications are described below:
- •
They are useful to generate the first hypotheses of causality and then take them to research with studies of appropriate methodology.3,8,19,21
- •
They are means of scientific communication between the different actors of the health sciences.12
- •
They are a didactic tool to generate participation, discussion and controversy about one or several clinical situations or about ethical dilemmas.3,11,22
- •
They are inexpensive, compared to other types of studies, and easy for researchers to develop when they want to publish in medical journals.6,11,22
- •
They are important for the art of medicine and from the pedagogical point of view, since they help to strengthen teaching and learning.3
- •
They can play a protagonic role in the detection of epidemics by reporting one or more cases or outbreaks of a new disease or a previously known disease that was controlled.6,8
- •
They are an easily accessible tool to make possible the start of the production of scientific knowledge by beginning researchers.3,12,19,23
- •
They are useful to describe the characteristics of very rare diseases and the initial step for investigation thereof.3,6,8,20
- •
They are essential for the elaboration of post-marketing records in order to report adverse effects of drugs and thus help to better establish their safety.3,6,15
- •
They are a means of communication to inform about the results of therapies for rare or orphan diseases.3,6,11
- •
In clinical practice, they guide the individualization and personalization of treatments.13,24
These types of publications are not free of disadvantages and it is convenient to take them into account at the time of using them:
- •
They present a high risk of biases, such as selection and publication biases, which could be controlled if the reports were recorded in a general registry of clinical studies in which there was a repository of clinical cases.6,8,10,25,26
- •
The conclusions are generated based on few cases, therefore, there is no representative sample of the study population, which prevents the adoption of outcome measures such as ratios, percentages, incidences, or prevalences.6,8,26
- •
Causality cannot be inferred because it is an uncontrolled study.5,10,11
- •
In case reports, the findings cannot be generalized to a sample or to a population of subjects.5,10,11
- •
They are longitudinal studies, but not prospective or analytical, therefore, they do not have a control group.8,15,26
- •
In the literature reviewed, a bad concept is identified because the level of evidence is low, which is why these studies have little acceptance to be published in the journals.6–8 The latter means that in certain media this type of study is not accepted to consider it as promotion in the academic ladder.
To postulate a case report or a case series report is convenient to review and follow the instructions of the journal to which is desired to submit the study. A basic structure that must be taken into account for this type of methodology is presented below. These recommendations are mainly based on the CARE guidelines (acronym for Case Reports), prepared in 2013 by an international group of experts. The CARE guidelines were developed to encourage the accuracy, transparency, and usefulness of the case reports, and have been endorsed by several medical journals and publishers in many languages. Table 2 shows the checklist that must be followed for this methodology according to the CARE guidelines, while Table 3 describes the requirements of some journals that publish them. The key elements that need to be included are listed and described below:
- 1.
Title: it should be concise, striking, informative, relevant, and easy to locate by the reader. It is classified into two categories: nominal and compound. The first must contain only one sentence and the second is made up by two or more sentences. In the reviewed literature, some authors do not recommend the use of the words “case report” associated with the title. The CARE guidelines recommend writing them at the same time as the clinical topic to be published. On the other hand, some authors consider that expressions such as “single case” or “first report” are “ostentatious”, and they recommend not adding them to the title.19,27–29
- 2.
Keywords: the general recommendation is to mention two to five words that will help the reader to carry out an easy search of the related literature. Mesh terms can be used.27,29
- 3.
Abstract: two types of summary style are mentioned in the analyzed literature: the narrative summary, in which a short version of the article which includes a flowing text with its main ideas and without having headings is carried out, and the structured summary, which has subheadings that describe the corresponding topics29; a) the introduction: in which the clinical case is described in one or two sentences and a description of the main idea of the article is made; b) the case presentation: relevant data on symptoms, signs, diagnoses, and main outcomes; and finally c) the discussion: which reflects the learning from the respective case report or case series report. This style of abstract is frequently used in scientific and clinical publications.2,19,27
- 4.
Introduction: the antecedents referenced in the medical literature are summarized,27 what is relevant in the publication and the expected impact on the scientific community are highlighted (in one or two paragraphs in length). The social and historical context is indicated in this section, which is one of the main axes of the design of the case report and case series report. In addition, it is required to concisely mention if there are previous or similar cases, as well as to highlight the novel characteristics compared to previous studies.2,19,29
- 5.
Description of the case or cases: the clinical history is presented, using a narrative style, annotating the chronology of the disease and presenting only the information relevant to the diagnosis. It begins with the demographic data, the reason for consultation, the current disease, the review by systems, and the personal and family antecedents.19,27,29,30
- 6.
Clinical findings: the clinical findings of the physical examination that are relevant to the diagnosis are described.27
- 7.
Timeline: is the chronological description of the important data of the clinical history of the patient over a timeline. An example is presented in Fig. 3.27
- 8.
Diagnosis: the interpretation of the diagnostic methods, the diagnostic reasoning, the differential diagnoses, the key results and the prognosis according to the case is evidenced.27
- 9.
Therapeutic intervention: report details of the types of therapeutic interventions with their outcomes.27
- 10.
Follow-up and results: describes the course and clinical follow-up, the results of the paraclinical tests, the information on adherence and the safety of the treatment.27
- 11.
Discussion: the description of the findings is elaborated, the previous experience with the observed outcomes is included, and the results are compared with those of the other related studies of the medical literature reviewed. The strengths and limitations of the case are reported and it is mentioned whether or not there are proposals for future research.19,27,29
- 12.
Conclusion: it is usually the learning generated by the case report or case series report, and the expected contribution to clinical practice should be described in a few sentences.19,30
- 13.
Perspective of the patient: the opinion of the patient and the description of his/her pathology from his/her point of view, the possible interventions expected and the outcomes that could have been considered are taken into account.27
- 14.
Informed consent: describes whether the patient gave consent, if was informed that his/her case was for publication and that the information of his/her identity would be restricted.27
- 15.
Conflict of interest: mention if there is any conflict of interest on the part of the authors related with the publication.27
- 16.
Acknowledgments: the people who helped or participated are pointed out in a concise way. It is not necessary to write the gratitude to the patient
- 17.
Approval by the medical ethics committee: describes whether approval by a medical ethics committee was obtained and the generated document should be available.27
- 18.
References: describes and enumerates the references used by the authors for the elaboration of the article. A lower or upper limit number of articles to be reviewed is not described in the literature, however, it is useful to take into account the requirements requested by each journal.29
- 19.
Annexes: they are the photos, tables and graphs that must be described at the end of the manuscript. A lower or upper limit number of articles to be reviewed is not described in the literature, however, it is useful to take into account the requirements requested by each journal.29
Checklist of the guideline of the CARE (Case Reports) group.
| Item | Subject | Description of the item of the checklist |
|---|---|---|
| 1 | Title | Central clinical topic followed by the words “case report” |
| 2 | Key words | Two to five words |
| 3 (3a, 3b, 3c, 3d) | Abstract | 3a: Introduction (reason why it is novel and important). 3b: Main symptoms and signs. 3c: Main diagnoses, therapeutic interventions and results. 3d: Conclusion (moral) |
| 4 | Introduction | Antecedents referenced in the pertinent medical literature |
| 5 (5a, 5b, 5c, 5d) | Information of the patient | 5a: Demographic data. 5b: Main symptoms. 5c: Personal and family antecedents. 5d: Pertinent concomitant diseases. |
| 6 | Clinical findings | Findings relevant to the physical examination |
| 7 | Timeframe | Chart on important events according to the time of occurrence |
| 8 (8a, 8b, 8c, 8d) | Diagnostic assessment | 8a: Diagnostic methods. 8b: Differential diagnosis. 8c: Diagnostic reasoning. 8d: Prognostic characteristics where applicable |
| 9 (9a, 9b, 9c) | Therapeutic intervention | 9a: Types of intervention used. 9b: Administration of the intervention. 9c: Changes in the intervention |
| 10 (10a, 10b, 10c, 10d) | Follow-up and results | 10a: Results assessed by the doctor and by the patient. |
| 10b: Important results of the follow-up test. 10c: Adherence to the intervention and tolerability thereof. 10d: Adverse or unforeseen events | ||
| 11 (11a, 11b, 11c, 11d) | Discussion | 11a: Strengths and limitations in the management of this case. 11b: Discussion on the relevant medical literature. 11c: Justification of the conclusions. 11d: Main lesson that can be drawn from this case report |
| 12 | Patient perspective | Patient experience |
| 13 | Informed consent | Of the patient or of the institution |
Source: adapted from The CARE guidelines template.27
Requirements for case or case series reports of some journals of medicine and rheumatology.
| Journal (reference number) | Characteristics of the clinical case | Structure of the clinical case | Number of words/references/author’s role/images |
|---|---|---|---|
| The Lancet31 | -Interesting | -Case presentation | <1000 words, |
| Does not specify recommendations or guidelines for case report(s) to make the manuscript | -A difficult diagnosis | -Anamnesis and physical examination | <5 references |
| -Useful for learning | -Research, management and result | The author must be involved in the care of the patient | |
| -A diagnostic dilemma | -The reader should be educated in the discussion | Do not use dark bars or similar to anonymize the patient | |
| Does not publish cases of effectiveness of medical interventions in individual cases | Respect the dignity of the patient | ||
| Informed consent of the patient or family member | |||
| Colombian Journal of Rheumatology32 | Document that presents the results of a study on a particular situation in order to make known the technical and methodological experiences considered in a specific case | Includes a systematic review of the literature on analogous cases | Not specified |
| Does not specify recommendations of guidelines or reports of cases to prepare the manuscript | Does not specify a structure for the case report or the case series | ||
| BMJ Case Reports33 | -Reminder of an important clinical lesson | -Title of the case | <2000 words |
| -Findings that shed new light on the possible pathogenesis of a disease or an adverse effect | -Abstract | Does not specify the role of the author | |
| Does not accept case seriesa | -Unusual presentation of a common disease | -Introduction | Images <500 words, see image template in the web page |
| -Rare or new disease | -Case presentation | ||
| Does not specify recommendations of guidelines of case report(s). Suggests a template which is available on the web page | -Novel treatment | -Investigations | |
| -Differential diagnosis | |||
| -Treatment | |||
| -Results and follow-up | |||
| -Discussion, lessons learned and messages to take away | |||
| -References, figures and videos | |||
| -Anonymous patient perspective | |||
| Informed consent of the patient or his/her guardian | |||
| International Journal of Surgery Case Reports34 | -They can be prospective or retrospective | -Title | <1500 words |
| -Abstract <250 words (introduction, case presentation, discussion, conclusion) | <20 references | ||
| Suggests CARE guidelines for the preparation of the manuscript | -Effect of an intervention on more than one patient | -Key words (<6) | <5 figures |
| -Informed consent of the patient or guardian. Approval by ethics committee |
The case report and the case series report are publications that allow the dissemination of knowledge in the medical community. They must be novel, have a clear focus, leave lessons learned, and enable the generation of a hypothesis for the development of future research. They are useful to inform new or rare diseases, describe adverse events or complications of already known pathologies. These studies are used as a pedagogical method for novice researchers thanks to their easy attainment and low cost. They have advantages and disadvantages. They are considered to have a low level of evidence, which may lead to not publishing them for fear of not achieving their acceptance, however, their importance is still valid. Knowing their role and following the recommendations on how to develop them will result in a good product to be published.
FundingThe authors did not receive funding or sponsorship for the preparation of this article.
Conflict of interestThe authors declare that they have no conflict of interest for the preparation of this article.
Thanks to doctors Renán A. Morales, Manuela Rubio, Gabriel Londoño, Manuel Puerta, Carlos Calderón and Federico Rondón for their valuable comments of substance and form.










![Epidemics II of Hippocrates. Source: available at http://www.schoyencollection.com/24-smaller-collections/medical-texts/hippocrates-epidemics-ms-2634-3 [Accessed 3 March 2021]. Epidemics II of Hippocrates. Source: available at http://www.schoyencollection.com/24-smaller-collections/medical-texts/hippocrates-epidemics-ms-2634-3 [Accessed 3 March 2021].](https://static.elsevier.es/multimedia/24444405/0000003000000002/v1_202307240956/S244444052300047X/v1_202307240956/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

![An example of the timeline. Source: available at http://data.carestatement.org/ [Accessed 15 April 2017]. An example of the timeline. Source: available at http://data.carestatement.org/ [Accessed 15 April 2017].](https://static.elsevier.es/multimedia/24444405/0000003000000002/v1_202307240956/S244444052300047X/v1_202307240956/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
