Systemic sclerosis (SSc) is a systemic autoimmune disease characterized by fibrosis, inflammation, and microcirculatory alterations.
ObjectiveTo evaluate abnormalities in the sublingual microcirculation of SSc patients and to establish any differences compared to healthy controls.
MethodsThe sublingual microcirculation was determined using a Sidestream dark-field (SDF) imaging device (MicroScan; MicroVision Medical, Amsterdam, the Netherlands) in patients with SSc and controls.
ResultsTwelve patients with SSc (75% with diffuse cutaneous SSc) were evaluated (mean age: 52.08±2.08 years). A group of 20 volunteers was used as the control. Significantly lower total capillary density (TCD) (9.2 [8.5–9.7] vs. 10.9 [9.8–12.5]) and functional capillary density (FCD) (7.0 [6.8–7.5] vs. 8.6 [7.5–9.8]) were observed in SSc patients than in healthy controls.
ConclusionsSSc is related to significantly lower capillary density in the sublingual microcirculation, and the SDF imaging technique could be an alternative to nailfold videocapillaroscopy for diagnosing and following-up patients with SSc.
La esclerosis sistémica (SSc) es una enfermedad autoimmune sistémica caracterizada por fibrosis, inflamación y alteraciones en la microcirculación.
ObjetivoEvaluar anormalidades en la microcirculación sublingual de pacientes con diagnóstico de esclerosis sistémica y establecer diferencias en comparación con controles sanos.
MétodosExploramos la microcirculación sublingual utilizando un dispositivo de imágenes de campo oscuro Sidestream (SDF) (Micro Scan, MicroVision Medical, Amsterdam, Holanda) en pacientes con SSc y controles.
ResultadosSe evaluaron 12 pacientes con SSc estable (75% con cutánea difusa) (edad media: 52.08±2.08). Un grupo de 20 voluntarios se utilizó como control. Se observó una disminución significativa en la densidad vascular total (TCD) (9.2 [8.5–9.7] vs. 10.9 [9.8–12.5]) y densidad capilar funcional (FCD) (7.0 [6.8–7.5] vs. 8.6 [7.5–9.8]) observado en pacientes con esclerosis sistémica en comparación con controles sanos.
ConclusionesLa SSc se relaciona con la disminución significativa de la densidad capilar en la microcirculación sublingual, esta técnica podría ser una alternativa en pacientes críticos con esclerosis sistémica o utilizarse para seguimiento durante la hospitalización.
Systemic sclerosis (SSc) is a systemic autoimmune disease characterized by fibrosis, inflammation, and microcirculatory alterations.1 Skin fibrosis is the principal manifestation (>90%); however, the pathological changes in the lungs, gastrointestinal tract, kidneys, and heart determine the clinical outcome. Raynaud's phenomenon (RP) is present in the majority of SSc patients (90%), and could be the first manifestation of SSc and its associated microcirculation alterations. The microvascular injury and endothelial cell activation that result in vascular damage are the earliest events in SSc, and the progressive vascular damage causes a reduction in the number of capillaries, thickening of the vessel wall because of intimal and smooth muscle cell proliferation, and luminal narrowing, leading to tissue hypoxia and oxidative stress.1,2 Activated endothelial cells recruit inflammatory cells and secrete endothelin 1 and connective tissue growth factor, along with other profibrotic factors that stimulate vascular smooth muscle cell proliferation and extracellular matrix production.3 One of the main objectives of treating rheumatic diseases is early diagnosis, and in SSc, nailfold videocapillaroscopy (NVC) is an effective and reliable tool that can distinguish between primary and secondary RP by identifying the early pattern of SSc.4 The “early” pattern refers to the presence of a few giant capillaries and hemorrhages, along with normal-shaped capillaries and relatively well-preserved capillary distribution, without the loss of capillaries; the “active” pattern is mainly characterized by the detection of several giant capillaries, along with mild loss of capillaries; and the “late” pattern is characterized by the loss of capillaries, with extensive avascular areas, ramified capillaries, and disorganization of the vascular array.4,5 In patients with RP, the combination of pathognomonic scleroderma-type changes on capillaroscopy with the presence of SSc-specific antibodies can predict progression to definite SSc in 47%, 69%, and 79% of patients over 5, 10, and 15 years of follow-up.6 The identification of microcirculation abnormalities with the loss of capillaries is also associated with pulmonary arterial hypertension (PAH), interstitial lung disease, peripheral vascular disease severity, and heart and lung involvement.7 However, objectivizing disease activity and SSc severity remains a challenge. Indeed, new classification criteria8 for SSc include NVC, because altered microvascular patterns have been related to the extent of organ involvement. New microcirculation imaging techniques aside from NVC could reveal more detailed microcirculatory blood flow alterations during SSc evolvement. Experience in septic patients has shown that sepsis is associated with decreased capillary density accompanied by increased heterogeneous perfusion in visualized capillaries. Several methods can be used to evaluate microcirculation in septic patients, with two techniques currently in use: the Sidestream dark-field (SDF) image technique and near-infrared spectroscopy. In SDF imaging, the most evaluated area is the sublingual microcirculation.9,10 Taking into account our experience in sepsis, these abnormalities could be extrapolated to SSc patients. Our objective was to evaluate abnormalities in the microcirculation of SSc patients using another anatomical area, such as the sublingual microcirculation, because this areas the most frequently studied in critical illness,10,11 and to establish the differences between SSc patients and healthy controls.
Material and methodsWe explored the sublingual microcirculation using an SDF imaging device (MicroScan; MicroVision Medical, Amsterdam, the Netherlands) in patients with SSc and controls at Fundación Valle del Lili Hospital (Cali, Colombia). Twenty healthy, age-matched volunteers who were workers at the hospital were invited to participate in the image acquisition. After the purpose of the study was explained to them and signing the informed consent, 12 patients with SSc underwent sublingual microcirculation evaluation over a period of 2 months. Videos and images were obtained in our office by a staff member who was trained in the use of the equipment. The videos were labeled in a blind manner, and were then evaluated blind by two researchers trained to read microcirculation findings. The data were stored in a database, and were later added to the clinical data of each patient at the time of evaluation: age, type of SSc, and pulmonary manifestations. Vessels were classified as large or small using a cutoff value of 20μm. Microvessels with continuous flow were considered as normal, whereas sluggish, intermittent, and stopped flow were considered abnormal. According to consensus and validated criteria,10 we calculated the proportion of small-perfused vessels (PPV), the microvascular flow index (MFI), the total capillary density (TCD), and the functional capillary density (FCD; vessels <20μm in diameter).
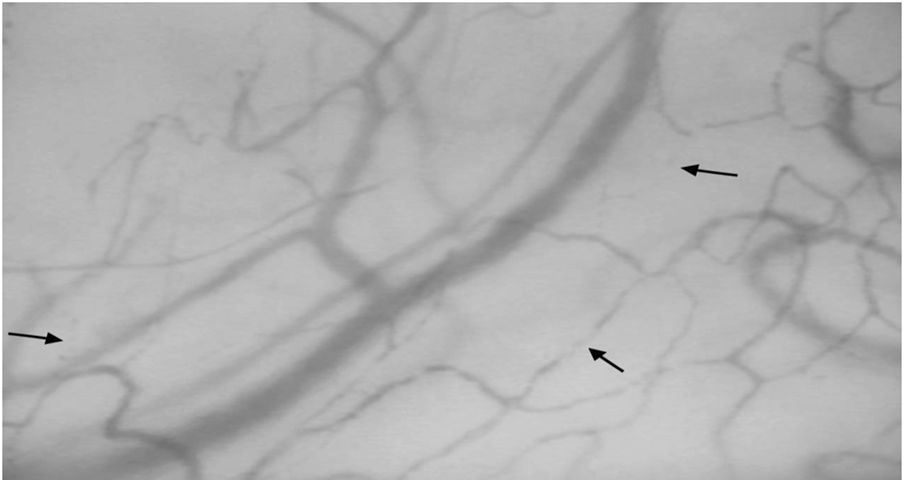
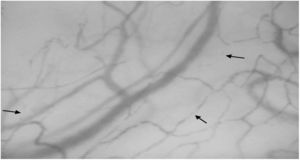
ResultsTwelve patients with SSc were evaluated. The median age was 52 years, 11 patients were female, 9 patients (75%) had diffuse cutaneous SSc, and 3 patients (25%) had limited cutaneous SSc (Table 1). A group of 20 volunteers was used as the control. Significantly lower TCD (9.2 [8.5–9.7] vs. 10.9 [9.8–12.5]) and FCD (7.0 [6.8–7.5] vs. 8.6 [7.5–9.8]) were observed in the SSc patients than in the healthy controls (Fig. 1). Conversely, PPV and MFI did not significantly differ between the two groups (Table 2). No relationship was found between microvascular abnormalities and calcium antagonist use.
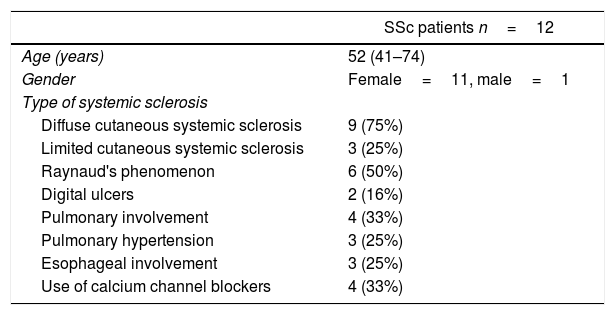
Clinical characteristics of systemic sclerosis (SSc) patients.
| SSc patients n=12 | |
|---|---|
| Age (years) | 52 (41–74) |
| Gender | Female=11, male=1 |
| Type of systemic sclerosis | |
| Diffuse cutaneous systemic sclerosis | 9 (75%) |
| Limited cutaneous systemic sclerosis | 3 (25%) |
| Raynaud's phenomenon | 6 (50%) |
| Digital ulcers | 2 (16%) |
| Pulmonary involvement | 4 (33%) |
| Pulmonary hypertension | 3 (25%) |
| Esophageal involvement | 3 (25%) |
| Use of calcium channel blockers | 4 (33%) |
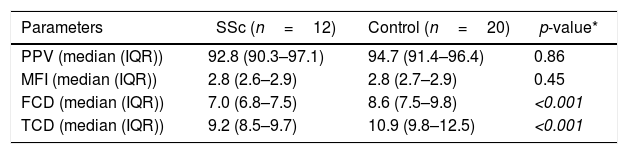
Sublingual microcirculatory abnormalities in patients with systemic sclerosis. Functional capillary density and total capillary density were lower in patients compared to controls (*Mann–Whitney test).
| Parameters | SSc (n=12) | Control (n=20) | p-value* |
|---|---|---|---|
| PPV (median (IQR)) | 92.8 (90.3–97.1) | 94.7 (91.4–96.4) | 0.86 |
| MFI (median (IQR)) | 2.8 (2.6–2.9) | 2.8 (2.7–2.9) | 0.45 |
| FCD (median (IQR)) | 7.0 (6.8–7.5) | 8.6 (7.5–9.8) | <0.001 |
| TCD (median (IQR)) | 9.2 (8.5–9.7) | 10.9 (9.8–12.5) | <0.001 |
SSc: systemic sclerosis; PPV: small-perfused vessels; MFI: microvascular flow index; FCD: functional capillary density; TCD: total capillary density.
Our study shows a significant lower capillary density in the sublingual microcirculation in SSc patients. SSc is an autoimmune disease that has a high burden of morbidity and mortality, with a mortality rate in the USA of 3.9 per million people per year.12
The only tool that is used to objectivize disease activity and microvasculature abnormalities in SSc is NVC; however, NVC has some limitations, including the difficulty visualizing capillaries in some patients because of low skin transparency or darki pigmentation, as well as the possibility of coexisting mechanical or chemical causes of microangiopathy in the nail fold. Grassi et al.13 performed capillary microscopy on the lip of patients with SSc, and found significant microvascular changes relative to the controls; therefore, capillary microscopy is an alternative technique for patients in whom NVC is difficult to perform. The use of sublingual microcirculation has been of great help in the diagnosis and monitoring of critical patients with different types of shock,10 and therefore, sublingual microcirculation data may correlate with chronic microcirculation abnormalities, such as in patients with SSc. Additionally, sublingual microcirculation could be followed in “real time” in SSc patients. Taking into account that pulmonary hypertension is very common in SSc (10–40%),1 findings in patients with PAH, or those at risk of developing it, could be extrapolated to SSc patients, because PAH patients exhibit changes in sublingual microcirculation. Similar findings were described by Dababneh et al.14 in 26 patients with PAH versus 14 healthy subjects; the authors found that patients with PAH showed a lower flow index in the sublingual microvasculature and higher tortuosity than healthy controls.
ConclusionsIn conclusion, SSc is related to significantly lower capillary density in the sublingual microcirculation. A larger sample of patients should be considered to increase the evidence showing SDF as an alternative for evaluating the microcirculation as a predictor of activity or severity.
- -
Strengths: novel, non-invasive method that provides information on the central microcirculation.
- -
Weaknesses and limitations: special equipment, not available in all centers, number of patients.
- -
Interpretation: new methods can complement the findings of NVC.
SSc is related to significantly lower capillary density in the sublingual microcirculation.
Conflicts of interestThe authors have no conflicts of interest to declare.