To determine the time Colombian patients with rheumatoid arthritis (RA) are treated with non-biological disease-modifying antirheumatic drugs (DMARDs) before changing to biological therapy.
MethodsA retrospective cohort study that collected information about the start of antirheumatic treatment in patients of all ages with a diagnosis of RA until the change to biological DMARD therapy. Survival analysis using Kaplan–Meier curves, from 1 January 2007 until 31 December 2013 by SPSS 23.0 for Windows, was made.
ResultsA total of 3880 patients (75.3% women) with a mean age of 51.3 years started non-biological DMARDs. After 5 years, 234 patients (6.0%) initiated biological DMARD therapy in 17.5±13.9 months. The use of glucocorticoids (OR: 2.49; 95% CI: 1.658–3.732), having any comedication (OR: 1.83; 95%CI: 1.135–2.966) and being treated in the city of Bogota (OR: 2.30; 95%CI: 1.585–3.355) or in the cities of the Colombian Atlantic coast (OR: 2.848; 95%CI: 1.468–5.524) were associated with a higher likelihood of biological DMARD initiation. Whereas the initiation of therapy with methotrexate (OR: 0.04; 95% CI: 0.014–0.108; p<0.001) or chloroquine (OR: 0.13; 95% CI: 0.092–0.187; p<0.001) or receiving antihypertensive medication (OR: 0.64; 95% CI: 0.421–0.960; p=0.031) was associated with a significant reduce in likelihood.
ConclusionAfter 5 years of non-biological DMARD therapy, 6.0% of people with RA started biological DMARDs. Receiving glucocorticoids, having any comedication, being treated in Bogota City or cities of the Colombian Atlantic coast affected the probability of switching to biological therapy in these patients.
Determinar el tiempo transcurrido desde que pacientes de Colombia con artritis reumatoide (AR) en tratamiento con fármacos antirreumáticos modificadores de enfermedad no biológicos (FAMEs) cambian a terapia con biológicos.
Materiales y métodosEstudio de cohorte retrospectiva que recogió información sobre inicio de tratamiento antirreumático en pacientes de todas las edades con diagnóstico de AR hasta que pasaron a terapia con FAMEs biológicos. Se hizo un análisis de sobrevida, utilizando curvas de Kaplan–Meier, desde el 1 de enero de 2007 hasta el 31 de diciembre de 2013 mediante SPSS 23.0 para Windows.
ResultadosUn total de 3880 pacientes iniciaron terapia con FAMEs no biológicos, (75,3% fueron mujeres) con una edad media de 51,3 años. Tras cinco años de seguimiento, 234 pacientes (6,0%) iniciaron FAMEs biológicos en promedio a los 17,5±13,9 meses. El uso de corticoides (OR: 2,49; IC95%: 1,658–3,732; p<0,001), recibir alguna comedicación (OR: 1,83; IC95%: 1,135–2,966), ser tratado en Bogotá (OR: 2,30; IC95%: 1,585–3,355), en las ciudades de la costa Atlántica (OR: 2,848; IC95%: 1,468–5,524) estuvieron asociados con una mayor probabilidad de inicio de biológicos mientras que el uso de metotrexate (OR: 0,04; IC95%: 0,014–0,108) o cloroquina (OR: 0,13; IC95%: 0,092–0,187) o recibir medicación antihipertensiva (OR: 0,64; IC95%: 0,421–0,960) redujeron la posibilidad.
ConclusionesDespués de cinco años de terapia antirreumática convencional, un 6,0% de pacientes con AR inició terapia con FAMEs biológicos. Recibir corticoides, recibir comedicación, ser tratado en Bogotá o la costa Atlántica afectan la probabilidad de cambiar a terapia biológica.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease, with a prevalence of 0.5–0.8% in the adult population,1 which predominates in females and has an annual incidence of 40 cases per 100,000 inhabitants.2 In Latin America and Colombia the prevalence is 1.6% and 0.15%, respectively.3,4
RA has a considerable impact on the quality of life and functionality of the patient, as well as on society, especially for loss of productivity due to illness or permanent disability and a high expenditure of resources for any health system. Life expectancy in patients with RA is 3–10 years lower than the general population.5
Treatment is usually done with non-biological disease-modifying antirheumatic drugs (DMARDs) alone or associated with glucocorticoids, but the addition of a biological DMARD can be necessary.6 Biological drug therapy is usually prescribed following failure to achieve remission of the morbidity with one or more non-biological DMARDs. However, there is the possibility of using them as a first line in the initial phase, taking into account the concept of window of opportunity, defined as an early stage of a disease during which there is the possibility of potentially altering its course or even reverting it to normality, as they have shown a greater effectiveness to stop progression and to induce remission of the inflammatory activity compared with therapies used in more advanced stages.6
Some studies suggest that the early treatment of RA translates to reduction of the cost of this pathology to society.3,6 It should be noted that other strategies such as the simultaneous combination of a non-biological and a biological DMARD have found higher rates of remission and less radiological progression of the disease compared to the use of any of the separate therapies.7–10
The General System of Social Security in Health (SGSSS in Spanish) of Colombia offers universal coverage to people through two regimes, one paid by the worker (contributory) and the other subsidized by the State, which has a benefits plan with a list of medicines which includes some of the biological and non-biological DMARDs. When a patient requires a DMARD outside the list, he or she has the option to obtain it through a special request made by their doctor (called the CTC) or through the legal guardianship tool. Given the economic, quality of life and morbidity impact of this group of drugs on patients with RA, we seek to determine the time elapsed from the start of therapy with non-biological DMARDs to the change to therapy with biological DMARDs, and the variables associated with this change in patients with RA affiliated to the Colombian SGSSS.
MethodsA retrospective cohort study was conducted with a survival analysis to determine the time elapsed between the initiation of therapy with non-biological DMARDs in patients diagnosed with RA and the change to therapy with biological DMARDs in patients affiliated to six different insurance companies called Health Promotion Entities (EPS) of the contributory regime of the SGSSS.
Patients with RA of all ages, of any sex and from any city, who had started therapy with non-biological DMARDs from January 1, 2007 until December 31, 2008, were included. Then, a monthly follow-up of the DMARD medication received for a period of 5 years was made for each subject until the time they started any biological DMARD.
The information was obtained and reviewed from the drug dispensation records of the company (Audifarma SA) by a pharmacologist physician. This corresponds approximately to 16.0% of the population affiliated to this regime in the country and 7.2% of the Colombian population. Data from individuals to whom these types of drugs were dispensed were included in the study. The variables included were:
- 1.
Sociodemographic: age, sex, city of origin.
- 2.
Pharmacological: (a) non-biological DMARDs (methotrexate, chloroquine, leflunomide and sulfasalazine); (b) biological DMARDs (abatacept, adalimumab, certolizumab pegol, etanercept, infliximab, golimumab, rituximab and tocilizumab). Their use was quantified by the amount dispensed per month and the dose was expressed in defined daily doses (DDD), established by the World Health Organization (WHO) as an international standard for future comparisons. In addition, the use of non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids was considered.
- 3.
Comedication was used as a substitute variable for the following comorbidities: (a) antihypertensive and diuretic/high blood pressure; (b) insulin and antidiabetic/diabetes mellitus; (c) analgesics/pain; (d) antiplatelet agents/ischemic heart disease or cardiovascular risk; (e) antiulcer drugs/peptic acid disease; (f) hypolipidemic/dyslipidemia; (g) antidepressants/depression; (h) thyroid hormone/hypothyroidism.
The start of follow-up was considered as the moment when the patient began non-biological DMARD therapy (time 0, t0); monthly time scales were used and biological DMARD therapy initiation was defined as the survival analysis event (time k, tk). Survival analysis was performed to assess the time elapsed from t0 (non-biological DMARD therapy initiation) to tk (initiation of biological DMARD therapy), where survival was the difference between tk and t0. The outcome variable was the onset of biological DMARD therapy in RA treatment. An Excel database was developed and included all the cases that participated, with the date of initiation of therapy with a non-biological DMARD, the date of initiation of therapy with a biological DMARD, the time elapsed in months and a variable denominated biological DMARD received or not at the 60-month follow-up time (5 years) for each person. At the end of the follow-up, subjects that did not initiate biological therapy were categorized as censured and those who withdrew from the insurance company before the completion of the follow-up time as lost in follow-up. Patients that continued non-biological DMARD therapy despite biological therapy initiation were also included. Subsequently, the same analysis was performed only for patients who had started therapy with biological DMARDs until the time they changed to another drug of the same group.
Data analysis was conducted using IBM SPSS Statistics version 23.0 for Windows (IBM, USA). Student's t-tests or ANOVA were used for comparison of quantitative variables and X2 for categorical variables. Binary logistic regression models were used using the onset of biological DMARDs as a dependent variable and as covariables those that were significantly associated with the dependent variables in the bivariate analysis. Survival analysis was performed using Kaplan–Meier survival curves and differences among groups were estimated through a log-rank test (Mantel–Cox). Statistical significance was predetermined to be p<0.05 (95% confidence interval, CI).
The protocol was reviewed by the Bioethics Committee of the Universidad Tecnológica of Pereira (Pereira, Colombia); it was approved as “research without risk” and guaranteed the anonymity of the patients, following the principles of the Declaration of Helsinki.
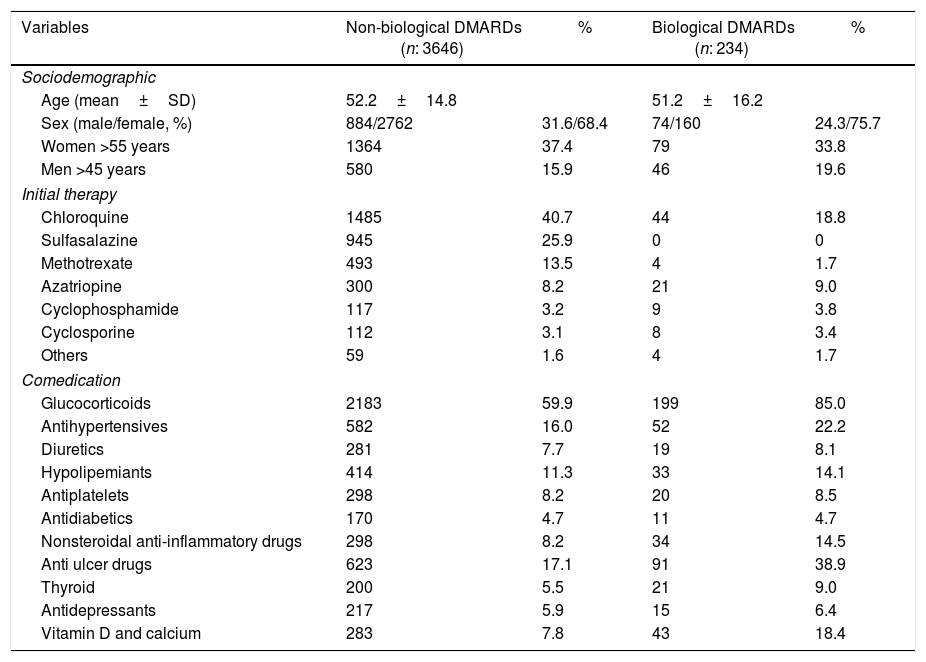
ResultsDuring the observation period in 38 Colombian cities, 3880 patients with RA that initiated therapy with non-biological DMARDs were found; 2922 (75.3%) were female and the mean age at the initial time of the observation period was 51.3 years (range: 7–101 years). During the 5-year follow-up, 234 patients (6.0%) started therapy with biological DMARDs, of which 160 (68.4%) were female. A total of 62 (26.5%) patients required a change of therapy for another biological DMARD during the course of follow-up. Sociodemographic characteristics, initial therapy and comedications can be seen in Table 1.
Sociodemographic and pharmacological characteristics of 3880 patients with rheumatoid arthritis treated with disease-modifying antirheumatic agents, Colombia 2007–2013.
| Variables | Non-biological DMARDs (n: 3646) | % | Biological DMARDs (n: 234) | % |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age (mean±SD) | 52.2±14.8 | 51.2±16.2 | ||
| Sex (male/female, %) | 884/2762 | 31.6/68.4 | 74/160 | 24.3/75.7 |
| Women >55 years | 1364 | 37.4 | 79 | 33.8 |
| Men >45 years | 580 | 15.9 | 46 | 19.6 |
| Initial therapy | ||||
| Chloroquine | 1485 | 40.7 | 44 | 18.8 |
| Sulfasalazine | 945 | 25.9 | 0 | 0 |
| Methotrexate | 493 | 13.5 | 4 | 1.7 |
| Azatriopine | 300 | 8.2 | 21 | 9.0 |
| Cyclophosphamide | 117 | 3.2 | 9 | 3.8 |
| Cyclosporine | 112 | 3.1 | 8 | 3.4 |
| Others | 59 | 1.6 | 4 | 1.7 |
| Comedication | ||||
| Glucocorticoids | 2183 | 59.9 | 199 | 85.0 |
| Antihypertensives | 582 | 16.0 | 52 | 22.2 |
| Diuretics | 281 | 7.7 | 19 | 8.1 |
| Hypolipemiants | 414 | 11.3 | 33 | 14.1 |
| Antiplatelets | 298 | 8.2 | 20 | 8.5 |
| Antidiabetics | 170 | 4.7 | 11 | 4.7 |
| Nonsteroidal anti-inflammatory drugs | 298 | 8.2 | 34 | 14.5 |
| Anti ulcer drugs | 623 | 17.1 | 91 | 38.9 |
| Thyroid | 200 | 5.5 | 21 | 9.0 |
| Antidepressants | 217 | 5.9 | 15 | 6.4 |
| Vitamin D and calcium | 283 | 7.8 | 43 | 18.4 |
DMARDs: disease-modifying antirheumatic drugs.
The most frequently added biological DMARDs to conventional therapy were adalimumab (n=62; 26.5% of patients), etanercept (n=58; 24.8%), infliximab and rituximab (n=45; 19.2% each one), abatacept (n=19, 8.1%) and others (n=5; 2.1%).
When a change of therapy to biological DMARDs was required, the most used were: adalimumab (n=20; 32.3% of the 62 patients), then etanercept (n=12; 19.4%), tocilizumab (n=8; 12.9%), abatacept (n=7; 11.3%), rituximab (n=6; 9.7%), infliximab (n=5; 8.1%) and others (n=4; 6.5%). A total of 13 patients required a third change and of these, three moved to a fourth biological DMARD.
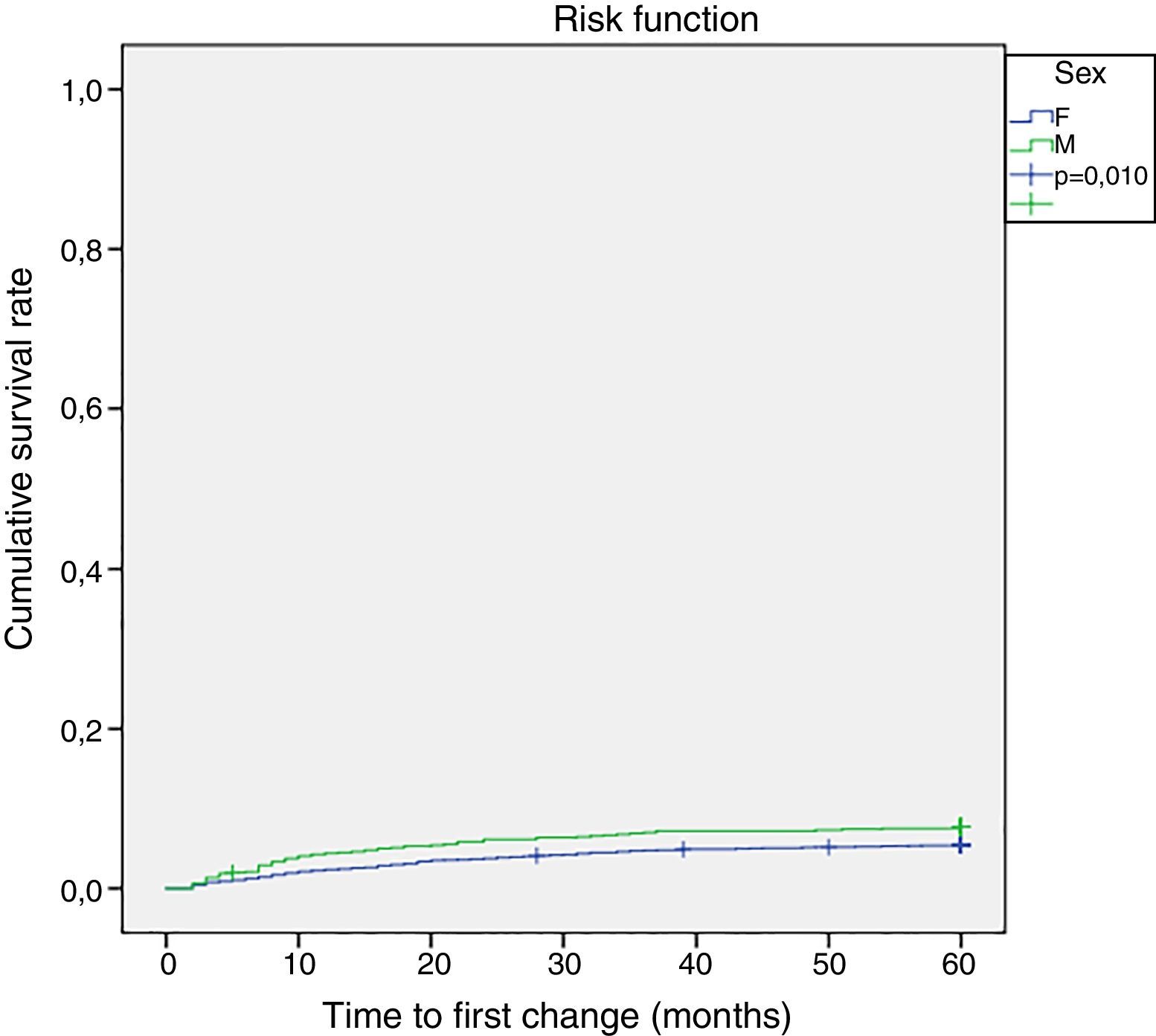
Survival analysisThe measured time for the initial biological DMARD therapy was 17.5±13.9 months (15.9 months for males and 19.2 months for females; range 2–57 months). Fig. 1 shows the progression of the initiation of any biological DMARD. Males were more likely to initiate therapy with these new drugs (p=0.010) (Fig. 1).
On the other hand, the most frequent periods of initiation of biological DMARD therapy were the second, third and seventh follow-up months. Almost half (47.4%, n=111) of the patients that required a change or addition of a biological DMARD had it in the first year of follow-up, accounting for 2.9% of the total cohort.
Multivariate analysis for the initiation of a biological DMARDThe multivariate analysis showed that being male (OR: 1.74; 95% CI: 1.24–2.42; p=0.001), receiving glucocorticoids (OR: 2.49; 95% CI: 1.65–3.73; p<0.001), having any comedication (OR: 1.83; 95% CI: 1.13–2.96; p=0.013) and being treated in the city of Bogota (OR: 2.30; 95% CI: 1.58–3.35; p<0.001) or in the cities of the Colombian Atlantic coast (OR: 2.84; 95% CI: 1.46–5.52; p=0.002) were statistically associated with an increased likelihood of initiation of therapy with biological DMARDs, whereas the initiation of therapy with methotrexate (OR: 0.04; 95% CI: 0.01–0.10; p<0.001) or chloroquine (OR: 0.13; 95% CI: 0.09–0.18; p<0.001) or receiving antihypertensive medication (OR: 0.64; 95% CI: 0.42–0.96; p=0.03) was associated with a significant reduce in risk.
Multivariate analysis for change of biological DMARDWhen analyzing by logistic regression, it was found that being male (OR: 1.89; 95% CI: 1.07–3.33; p=0.027), receiving treatment with glucocorticoids (OR: 2.64; 95% CI: 1.23–5.65; p=0.012) and having some comedication (OR: 3.65; 95% CI: 1.64–8.09; p=0.001) were statistically associated with a higher likelihood of a change in therapy to another biological DMARD, whereas initiation of chloroquine (OR: 0.07; 95% CI: 0.02–0.16; p<0.001) was associated with a significant decrease in likelihood of DMARD use.
DiscussionThis study identified the time elapsed between the initiation of non-biological DMARD therapy and the change to biological therapy in RA patients after a 5-year follow-up, information that had not been described in the Colombian or Latin American population.
During the follow-up period, 6% of patients started therapy with biological DMARDs, lower than the 23.1% found in a study conducted in the United States11 but comparable with the level reported in another study in that same country in which it was used in 7% of patients.12 This low percentage of change could be related to the severity of the disease or to clinical considerations of the patients about the aggressiveness of medication, side effects (i.e. infections, elevated liver enzymes), combination with other medications, route of administration and influence on fertility and pregnancy, among others13; however, research available with this study design is limited.
When comparing the mean time for initiation of therapy with biological DMARDs in this study (17.5 months), there is a wide difference from the one performed in the United States, in which an average time of 7.5 months was reported, a situation that could be explained by the economic impact that it could represent for the Colombian health system, as happens in other countries’ health systems,14,15 and the delay in referral to a rheumatology specialist.16,17
In the multivariate analysis of the variables that were associated with the initiation of therapy with biological DMARDs, the results were different to those reported by Kim et al. in the United States where men had a lower risk of using them. However, the use of methotrexate reduced the probability of initiating therapy with biological DMARDs in both studies.17 As in this study, Morgan et al. reported that previous use of glucocorticoids was associated with an increased risk of initiation of biological DMARDs, but did not find a statistically significant association with the comedication,18 it also might be explained as a possible indication bias since the disease activity is not known. On the other hand, the reduction of risk for biological DMARD initiation with the use of chloroquine could be due to its association with adequate control of the disease, reported by us in the Colombian population.19
The differences found regarding prescription behavior for biological DMARDs in the different Colombian cities of the study may be due to the variability in medical care, in particular of prescription habits, a constant finding in pharmacoepidemiological studies.19
On the other hand, the need for a change in biological therapy could be related to the low effectiveness in the control of the disease, as reported in another study in which a change was made more frequently to etanercept and adalimumab, and in agreement with these research results where the change was made to adalimumab.20 On the other side, the use of corticosteroids could support the relationship between the need for change and the effectiveness of treatment in difficult-to-control patients, as we found that these increased the risk of using biological DMARDs. The relationship found between the use of antihypertensive drugs and the initiation of biological therapy has not been characterized in the literature.
The information shown in this study is representative of treatment practices and therefore its interpretation should be made within the context of populations with similar health care conditions. In addition, the data include care of outpatients with RA and are therefore not applicable to hospital care. The limitations of this study include a lack of clinical data such as the rate of disease activity, the time from diagnosis to the start of the first DMARD and the presence of erosive disease or other clinical manifestations, which can determine the start of biological therapy.
We also have no information on the specific reason that led to the initiation and/or change of biological therapy. However, it has strengths that include the number of RA patients followed in the cohort who started pharmacological therapy in different cities in Colombia and the 5-year follow-up period that provides valuable information for the continuous monitoring of rheumatoid disease-modifying therapy.
In conclusion, this study showed that only 6% of patients with RA who were receiving treatment with non-biological DMARDs had to start therapy with biological DMARDs; of these, the most used were, in order, adalimumab, etanercept and infliximab, with a mean start time of close to 18 months. Methotrexate and chloroquine decreased the probability of initiation of therapy with biological DMARDs. Knowledge of the time elapsed until the beginning of therapy with these biological DMARDs provides valuable information that can be used by health administrators to make decisions aimed at improving the health care of patients with RA in Colombia.
Contributor statementsJorge Enrique Machado-Alba participated in the drafting, data collection, data analysis, description of results, discussion, critical revision of the article, and evaluation of the final version of the manuscript. Santiago García-Betancur participated in the drafting, data collection, data analysis, description of results and discussion. Luis F. Calvo-Torres participated in the drafting, data collection, data analysis, description of results and discussion. Diego Alejandro Medina Morales participated in the drafting, data collection, data analysis, description of results and discussion. Alejandra M. Bañol-Giraldo participated in the drafting, data collection, data analysis, description of results and discussion.
FundingUniversidad Tecnológica de Pereira, Audifarma S.A.
Conflict of interestThe authors have no conflict of interest to declare.
To Soffy Lopez and Viviana Orozco for the support in the database.