Monckeberg's sclerosis is a rare and low prevalence disease of unknown cause in which small and medium size arteries suffer calcification of the middle layer, leading to a reduction in caliber.
Clinically, the disease manifests by the appearance of arterial type ulcers on the skin of upper and lower extremities. Its diagnosis is difficult, and requires histopathological studies. Some rheumatic conditions, such as polyarteritis nodosa and giant cell arteritis may mimic this process. Treatment of the disease is not well established. Although the use of calcium chelators such as sodium thiosulfate has been proposed, the few available studies have failed to show significant results.
La esclerosis de Monckeberg es una enfermedad de baja prevalencia y causa desconocida, en la cual arterias de mediano y pequeño calibre sufren calcificación de la capa media generándose una reducción de la luz.
Suele manifestarse por la aparición de úlceras de tipo arterial en la piel de extremidades superiores e inferiores. Su diagnóstico requiere de estudios histopatológicos, con diagnósticos diferenciales como la poliarteritis nodosa y la arteritis de células gigantes. No existen guías de tratamiento, se han propuesto quelantes de calcio como el tiosulfato de sodio, pero los pocos estudios disponibles no han logrado demostrar resultados significativos.
Described for the first time in 1903 by Johann Georg Mönckeberg,1 it is a disease of unknown etiology, which consists in medial arterial calcification. This calcification is usually circumferential and may affect the vessel either focally or diffusely.2 Its incidence is higher in diabetics and elderly people, and its appearance predicts the risk of cardiac and peripheral vascular diseases, increasing the rates of limb amputations.3
Its pathophysiology is unclear, it is believed that the lesion will be produced by fatty degeneration of the smooth muscle cells of the middle layer, forming a mass that undergoes a hyaline degeneration and then it becomes calcified. In general, the clinical repercussion is scarce because the reduction of the lumen is minimal, unless it is overlapped with a process of atherosclerosis, where the clinical manifestations become more evident and serious.4,5
Below is reported the case of a patient with multiple complications of Monckeberg's sclerosis, which simulates a vasculitis of medium-sized vessels, and a literature review is carried out.
Case reportA 46-year-old woman with a history of arterial hypertension and type 2 DM since the age of 22 years, with poor metabolic control demonstrated by an HbA1C of 11%, without adequate adherence to medical treatment and clinical follow-up, and also with chronic kidney disease stage 5 in the last 4 years, requiring renal replacement therapy of hemodialysis type, without a history of associated dyslipidemia.
She entered a hospital of third level of complexity in the city of Medellin, Colombia, after 6 months of alteration of the sensitivity of the 4 extremities, Raynaud's phenomenon, weight loss and abdominal pain, with subjective fever prior to admission. There were necrotic ulcers in the distal phalanges of the upper limbs and a holosystolic heart murmur in the aortic area. Of relevance she had negative serologies for B hepatitis, C hepatitis, HIV and VDRL, negative antinuclear antibodies, anti-dsDNA, antineutrophil cytoplasmic antibodies, extractable antinuclear antibodies, anticardiolipin IgG and IgM, anti-B2 glycoprotein and rheumatoid factor. The complement and the protein electrophoresis were normal. The corrected calcium was 9.6mg/dl and the serum phosphorus 5.4mg/dl, for which she received calcium carbonate 1800mg daily.
Electromyography plus conduction velocities of the 4 extremities was performed, evidencing a chronic axonal sensorimotor polyneuropathy, denervation with increased amplitude of motor unit action potentials and neuropathic recruitment in distal muscles. A transesophageal echocardiogram was carried out, which reported segmental contractility disorders and valve leaflets without the presence of vegetations, but with multiple atheromatous plaques in the entire aortic trajectory.
The patient was taken to heart catheterization due to the high suspicion of vasculitis of medium-sized vessels with myocardial involvement, finding a stenosis of the middle and distal thirds of the anterior descending artery with stenosis of 65% in its long branch, and for this reason cardiology considered medical management.
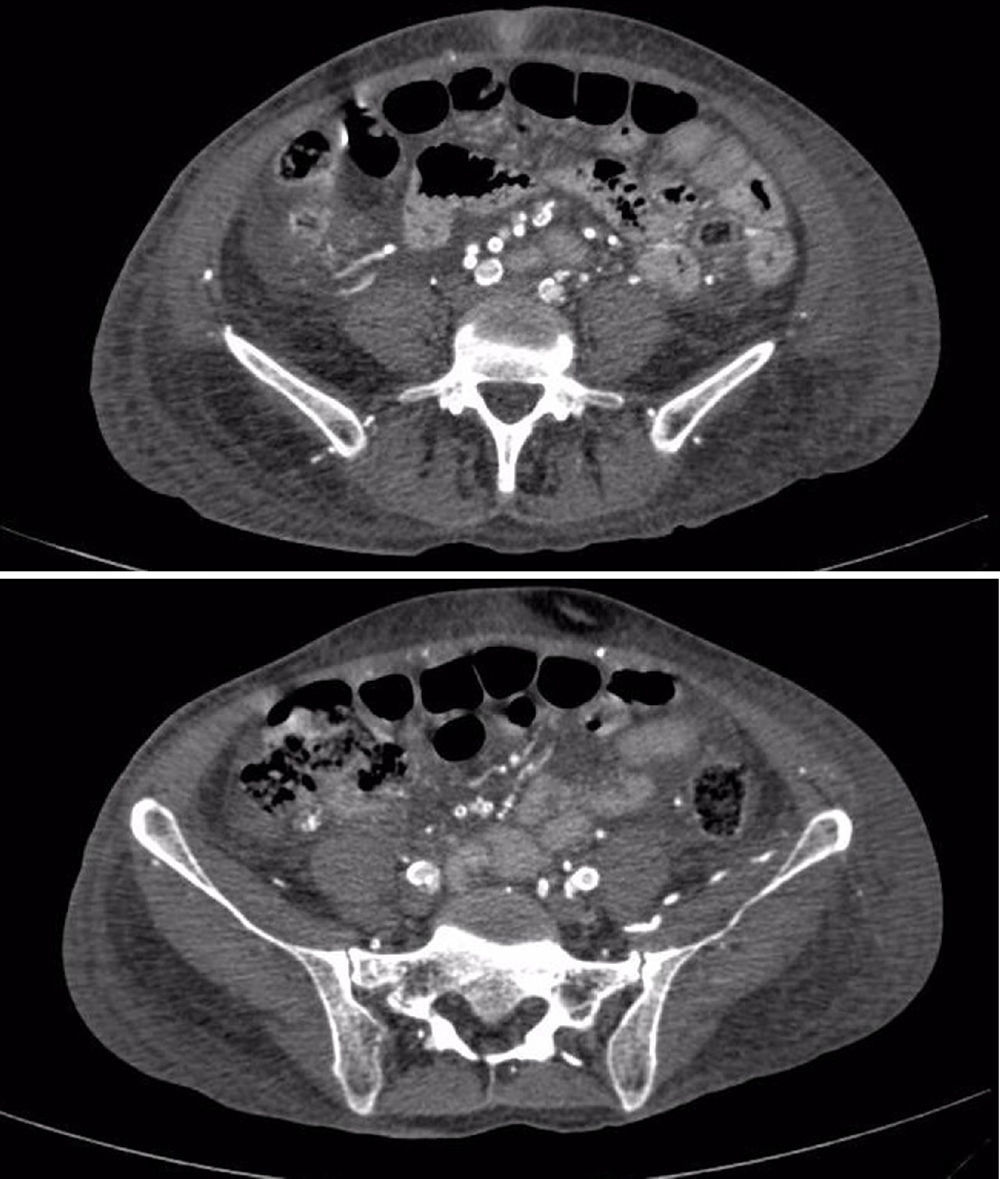
The patient was deteriorating clinically, with hemodynamic instability despite management, worsening of the digital lesions associated with greater necrosis and intense pain, requiring infusion of fentanyl for symptomatic control; vascular studies were performed, evidencing severe bilateral atherosclerotic changes with peak velocities between 50 and 60cm/s, with greater infrapopliteal commitment, reaching up to 18cm/s in the Doppler of the lower limbs, with a computerized tomography angiogram of thoracic and abdominal vessels that evidenced significant calcifications in the coronary arteries, abdominal aorta and its branches, shown in Fig. 1. It was decided to start treatment with bosentan for the management of distal vasospastic phenomena and multisystemic compromise; methylprednisolone pulses were started suspecting medial vessel vasculitis. Biopsies of the sural nerve and adjacent muscle were performed, which were reported as normal. There was little clinical response to management, and subsequently the patient started having sudden respiratory distress ruling out PTE, but with hypotension and persistent fever, and for this reason blood cultures were carried out which evidenced Klebsiella pneumoniae bacteremia, needing to stay in the intensive care unit for vasopressor support and targeted antibiotics. The patient continued hemodynamically unstable, with important ischemia of lower limbs, quite possibly worsened by the use of vasopressors and the hypoperfusion state, so she required finally infracondylar amputation of the left lower limb.
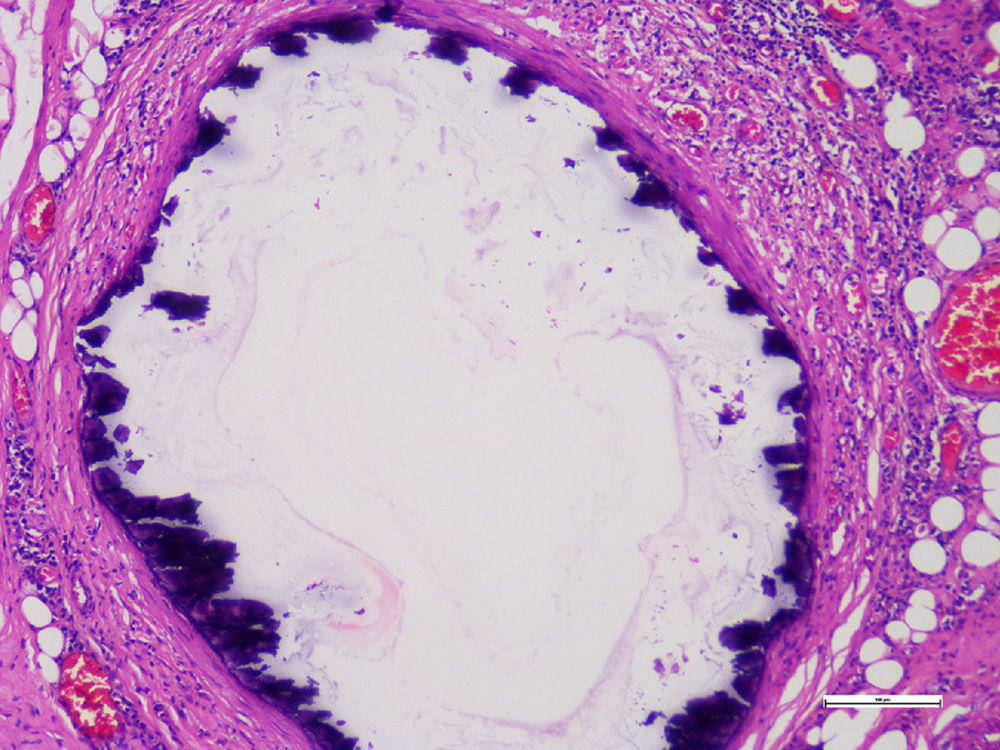
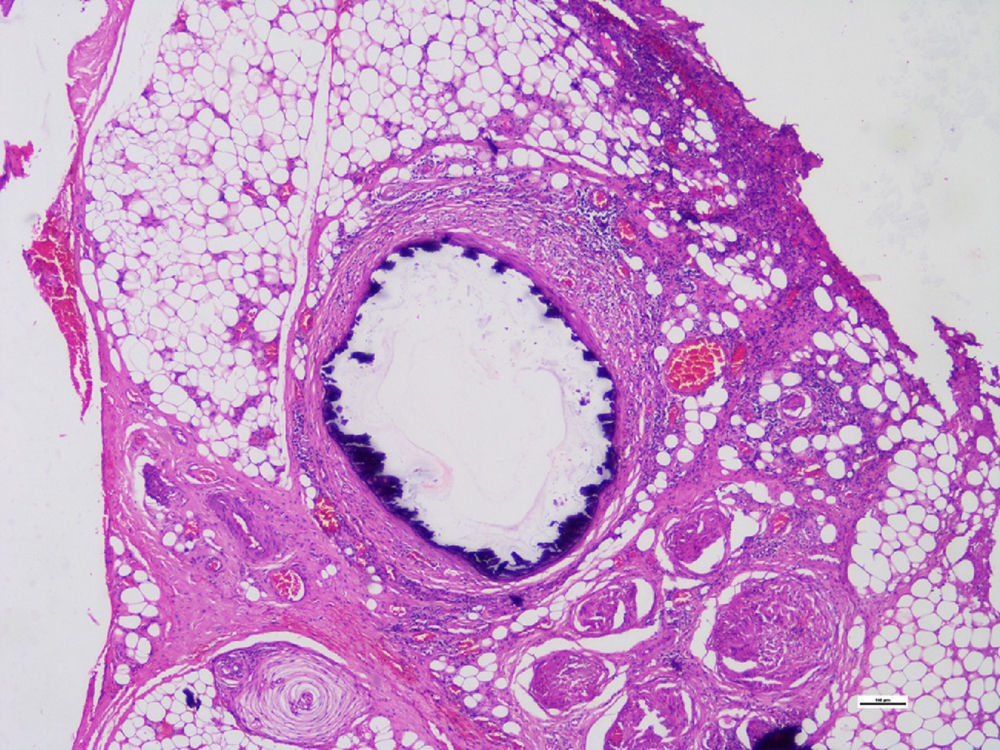
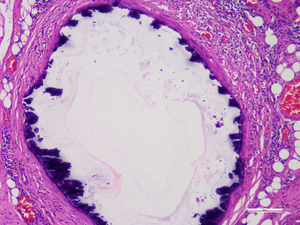
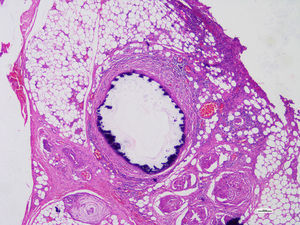
Histopathological studies of the amputated limb were performed describing, in sections of the blood vessels, smooth muscle cells of the middle layer, focally replaced by hyalinized fibrous tissue which presented concentric dystrophic calcification and osseous metaplasia associated with changes compatible with Monckeberg's disease (Figs. 2 and 3).
After a prolonged stay in the intensive care unit, the patient was transferred to hospitalization, where steroid clearing was started and she died 8 days later due to ventilatory failure, for which no confirmatory clinical necropsy was performed.
DiscussionSystemic vasculitis are serious and life-threatening pathologies, however, the heterogeneity of their clinical manifestations makes it difficult to establish an accurate diagnosis. It is here where becomes important a large list of etiologies that can simulate their clinical characteristics at the time of making differential diagnosis, since their treatment and prognosis will depend on it.6
Monckeberg's sclerosis stands among the differential diagnoses of vasculitis, however, its clinical relevance has been undervalued due to the low clinical impact attributed to it and the low reported incidence, being considered as a condition secondary to a process of deposit of inert calcium.7,8 The broad relationship between chronic kidney disease and hyperphosphatemia, as for the deposition of calcium phosphate crystals,9,10 causes the accumulation of these minerals in the blood vessels at the level of the intima or middle layers.1,11 This becomes and important comorbidity for the vascular disease,12,13 which is usually accelerated as the kidney disease progresses14 and increases with the requirement of hemodialysis as renal replacement therapy.2,15–17 For this reason, it has traditionally been proposed that the medial vascular calcification is a consequence of a simple deposition of crystals with vascular hardening.
Despite the foregoing, recent pathophysiological studies have suggested that there are inflammatory and genetic alterations closely related with the medial vascular calcification. It has been described how the deposition of these nanocrystals is capable to induce a phenotypic plasticity of the mesenchymal cells derived from the vascular smooth muscle cells, leading them to a transdifferentiation both in vitro and in vivo, gaining osteogenic characteristics that are directly related with medial vascular calcification.18 It has also been described how the CD73 deficiency, caused by the mutation of the CD73 gene that generates a loss of its activity, causes an increase in the activity of the tissue-nonspecific alkaline phosphatase, a key protein for the bone formation and main conductor of the medial vascular calcification.19
The above suggests that Monckeberg's sclerosis could be the manifestation of a vascular compromise mediated by immunological genetic and inflammatory alterations that complement a known disease such as atherosclerosis, since at the vascular level it can occur with calcification of the intima layer and this can be associated with atherosclerotic plaques, which result from the accumulation of modified lipids, proinflammatory cytokines and cell apoptosis, compromising the blood flow.20,21 The calcification of the tunica media favors episodes of remodeling and mineralization, with the consequent decrease in vascular elasticity.22–24
There are 2 theories to explain the described phenomenon: the first proposes the loss of expression of proteins associated with the inhibition of calcification such as: GLA, osteoprotegerin, fibrillin-I and carbonic anhydrase. The second theory proposes that the calcification is a consequence of metabolic changes due to necrobiotic lesion of the vessel wall. It is from these data that many authors consider the process as a calciphylaxis or calcinosis that can occur concomitantly with endovascular fibrosis; however, for others, Monckeberg's sclerosis is an advanced stage of arteriosclerosis without clear evidence to define that it is a completely independent condition.23,25,26 Currently, there are still disagreements about the associated involvement by calcification of the internal elastic lamina, which was not described in the original article of Dr. Monckeberg, but according to histopathologic studies, it seems to be equally implied.4,27
There is a broad spectrum of clinical manifestations, all derived from the vascular occlusion which is generated and that includes coronary vessels, the aorta and its branches, with special emphasis on peripheral vessels. It is frequent to found ulcers of arterial type in the upper and lower limbs of distal predominance, so serious that sometimes may require amputation of the extremity to control the symptoms.23,28
Differential diagnoses are mainly given by vasculitis affecting these vessels, among them, polyarteritis nodosa and giant cell arteritis.29 The symptoms could be so similar that, in view of the inability to determine the alteration in the calcium-phosphorus metabolism, it must be resorted to the biopsy of tissue as a means to achieve the definitive diagnosis.29
Specific measures for the treatment of Monckeberg's sclerosis are not currently available, and only are taken measures aimed to optimize the calcium metabolism, including calcium chelators such as sodium thiosulfate.17
ConclusionThe development of calcification of the arteries is a normal process of aging; risk factors such as diabetes mellitus and chronic kidney disease are directly related to its progression.30 The foregoing causes an earlier and more severe form of presentation in these groups, which represents the occurrence of cardiovascular events as the leading cause of death in these patients.1,2
Monckeberg's sclerosis should be suspected in those patients with the risk factors described; with signs and symptoms of peripheral arterial disease or signs of critical of acute obstruction in which an atherosclerotic, embolic or autoimmune phenomenon is ruled out.31 Considering also that its diagnosis is still an incidental finding in histopathological samples and there are no laboratory diagnostic aids or images specific of the disease; the clinical suspicion is the cornerstone of the diagnosis and medical management, to avoid an accelerated progression of the disease and unnecessary treatments.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Díaz Coronado JC, Uribe SH, González MR, Giraldo CP, Zuluaga MM. Manifestaciones clínicas de la esclerosis de Monckeberg. Reporte de caso y revisión de la literatura. Rev Colomb Reumatol. 2017;24:118–122.