The use of disease modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis (RA) is essential in order to achieve and maintain adequate disease control, and thus preventing irreversible functional damage. However, the rate of adherence to drug therapy has been reported to be between 20% and 107%. This variability may be due to the measurement methods used in the different studies.
ObjectiveTo test the overall medication adherence to oral treatment with DMARD in patients with RA using the self-report Spanish version Compliance-Questionnaire-Rheumatology (CQR) and to identify potential factors associated with non-adherence.
MethodsA cross-sectional descriptive study was conducted that included patients older than 18 years with RA diagnosed according to the ACR-EULAR 2010 criteria. They also had to have been prescribed oral DMARD for the previous 3 months, and had been seen by a rheumatologist in the last year. Patients completed the CQR to assess adherence, and were asked about disease knowledge, perception about treatment, side effects, Charlson Comorbidity Index, global index of social support, number of medications and DMARD prescribed, access to health resources, and disease activity measured by DAS 28 or CDAI. Good adherence was defined as a cut-off point of CQR > 80 or non-activity (remission or low activity). In the search for factors associated with adherence, the data were analysed using means of median and interquartile range, as well as frequencies and proportions. The comparison between adherent and non-adherent groups was performed using absolute comparisons, with the Mann-Whitney test for continuous, and chi-squared (for expected values> 5), or Fisher (for expected values <5) tests for categorical variables, taking as a level of significance a value of p < 0.05. OR and their respective 95% confidence intervals (95% CI) were used.
ResultsOf 170 participants included, 50% (n = 85) had a value greater than 80% (good drug adherence). Most patients had remission (60.6%) or low disease activity (17%). The subsequent analysis showed statistically significant association between adherence measured by CQR and the number of friends (P = .0012). An association was also found between disease activity as an indirect indicator of adherence and the global social support index (P = .004).
ConclusionThis study found a similar level of adherence to that reported in other populations, which could be due to the behaviour of our population, although the authors perceived difficulties reported by patients in understanding the statements of the questionnaire at all levels of education. Only the social support variables had a statistically significant relationship with adherence, which had also been described in the literature. Further studies are required to evaluate the operational characteristics of the CQR in our population.
el uso de los fármacos modificadores de la enfermedad (FAME) en pacientes con artritis reumatoide (AR) es esencial para alcanzar y mantener un control adecuado de la enfermedad y prevenir un daño funcional irreversible. Sin embargo, la tasa de adherencia a la terapia farmacológica varía entre el 20% y el 107%. Esta variabilidad puede deberse a los métodos de medición utilizados en los diferentes estudios.
Objetivoevaluar la adherencia global al tratamiento oral con FAME en pacientes con AR mediante el autodiligenciamiento del cuestionario Compliance Questionnaire on Rheumatology (CQR) y la actividad de la enfermedad e identificar los factores potenciales asociados con la baja adherencia.
Métodosestudio descriptivo transversal que incluyó pacientes mayores de 18 años con AR clasificados por criterios ACR-EULAR 2010 y con prescripción de FAME durante al menos tres meses y control con reumatólogo en el último año. Los participantes llenaron el cuestionario CQR y se les indagó acerca del conocimiento de la enfermedad, la percepción sobre el tratamiento, los efectos adversos, el índice de comorbilidad de Charlson, el índice global de apoyo social, el número de medicamentos y FAME prescritos, el acceso a los servicios de salud y la actividad de la enfermedad por DAS 28 o CDAI. Se definió como buena adherencia un punto de corte de CQR > 80 y ausencia de actividad (remisión o actividad baja). Para la búsqueda de factores asociados con adherencia se analizaron los datos por medio de mediana y rango intercuartílico, así como frecuencias y proporciones. La comparación entre los grupos de adherentes y no adherentes se hizo con comparaciones absolutas, por medio de test de Mann-Whitney para las variables continuas y chi-cuadrado (para valores esperados >5), o Fisher (para valor esperado < 5) para variables categóricas, tomando como nivel de significancia un valor de p < 0,05. Se utilizaron OR y sus respectivos intervalos de confianza al 95% (IC 95%).
Resultadosde los 170 participantes incluidos, el 50% (n = 85) tuvo un valor de CQR mayor a 80 (buena adherencia). La mayoría de los pacientes se encontraba en remisión (60,6%) o baja actividad de la enfermedad (17%). El análisis posterior únicamente encontró asociación estadísticamente significativa entre adherencia medida por CQR y el número de amigos (p = 0,0012) y entre adherencia medida por actividad de la enfermedad y el índice de soporte social global (p = 0,004).
Conclusioneseste estudio muestra un nivel de adherencia similar al reportado en otras poblaciones, lo cual puede deberse a comportamientos propios de nuestra población, aunque los autores percibieron dificultades reportadas por los pacientes en el entendimiento de los enunciados del instrumento en todos los niveles de escolaridad. Únicamente las variables de soporte social tuvieron una asociación estadísticamente significativa con la adherencia, asociación descrita en la literatura. Se requieren más estudios para evaluar las características operacionales del CQR en nuestra población.
Rheumatoid arthritis (RA) is a chronic inflammatory disease, of autoimmune etiology. Failure to provide proper and timely management leads to functional limitation, disability and declining quality of life.1 Currently, early treatment and the availability of a broad therapeutic armamentarium allow for achieving the goal of remission or low disease activity.2 Hence, the used of disease modifying drugs (DMARDs) as the cornerstone therapy, reduces radiographic progression and improves prognosis and disability. However, just as with other chronic diseases, a delayed effective treatment implementation, in addition to low patient compliance with medical therapy, increases the morbidity and mortality, and limits the response to traditional medications. This makes it necessary to use second line interventions that provide a different effectiveness and are frequently more costly.3
Consequently, assessing the adherence to pharmacological and non-pharmacological therapy is essential for all the stakeholders involved in the management of the disease. While there is agreement with regards to the definition of compliance,4,5 there is a shortage of evidence published with regards to measurement of compliance in patients with RA and this limits a better understanding of the factors associated with the low rates of adherence reported to date.6 The identification of these factors will enable the development of strategies with a positive impact on the care models for RA, achieving higher rates of remission, reduced healthcare costs, and finally improved quality of life for our patients.
The Compliance Questionnaire on Rheumatology (CQR) is a 19-item, self-administered questionnaire, used to measure treatment compliance, identifying the factors that contribute to suboptimal adherence. It was specifically developed for rheumatology in RA, rheumatic polymyalgia, and gout at the Maastrich University Hospital.7 The original trial showed a sensitivity of 98%, specificity of 67% and kappa Cohen of 0.71 for the identification of low compliance. In a subsequent trial validated in patients with rheumatological inflammatory diseases, with electronic monitoring devices as a comparator, in 6 months of follow-up, the sensitivity was 62%, and the specificity was 95%; the expected k was 0.78 for the detection of non-compliance. The likelihood ratio to identify low compliance was 11.6.8 The CQR was culturally adapted and validated in Spanish by a Colombian group in 233 patients with RA. In this group, the cutoff point for the CQR was 80.7, and the sensitivity was 80.2% (95% CI: 71.9-86.9%), while the specificity was 72.3% (95% CI: 63.1-80.4%) to define good treatment compliance.9
Currently, the measurement of RA activity using clinimetrics scales is part of the standard clinical practice. It has been shown that treatment compliance impacts the activity of the disease,10 and therefore disease activity has been used by other authors as a method to measure compliance.11 In our trial, the RA activity was measured using DAS-28 or CDAI, on the same day of the interview.
In this paper, based on a group of patients with RA, adherence to oral DMARDs was measured directly using the CQR scale, and indirectly, measuring the activity of the disease. Additionally, the factors that could be associated with compliance with those medications were described. These two approaches to measure compliance are very different and have not been validated jointly, as part of one same definition; therefore, the were analyzed separately.
Patients and methodsDescriptive study with an analytical component, including patients periodically followed at a specialized outpatient clinic for the management of chronic diseases in Bogotá. The clinic has a special program for patients with RA and provides education activities and clinical pharmacology follow-up, inter alia. The participants had to be over 18-years old, meeting the RA classification criteria according to ACR-EULAR 2010,12 who had been prescribed oral DMARDs, regardless of receiving parenteral modifying therapy, for at least 3 months, and a minimum of 2 rheumatology control visits reported in the medical record, during the past two months. Patients with other autoimmune concomitant diseases, except for Sjögren, were excluded.
The patient data was collected from the institutional electronic medical records and from an individual interview during which the patient completed a questionnaire specifically designed for the trial (Appendix Banexo 1, in the additional material). The questionnaire included information regarding the insurance system, the disease and treatment (knowledge about the disease, treatment perception, adverse effects), as well as the CQR compliance scale (Appendix Banexo 2, in the additional material), comorbidities and social support assessment. The Naranjo algorithm was used to record any adverse effects, (Appendix Banexo 3, in the additional material); this tool is used in pharmacovigilance for the causal analysis of the relationship between the use of the medication and adverse reactions.
To assess the social support, the MOS questionnaire was used (Medical Outcomes from Social Support) (Appendix Banexo 4, in the additional material); this tool measures the social support received by patients with chronic diseases, was developed in the United States and validated in Colombia. The Charlson index was used for the assessment of comorbidities (Appendix Banexo 5, in the additional material); this is a very popular instrument as an indicator of comorbidities. The Banexos Appendix includes all the instruments used in this trial.
The study was approved and monitored by an independent ethics committee and complied with all the regulations applicable for handling of information and anonymity of the data, in addition to the acceptance of each patient to participate, as expressed by signing the informed consent.
Patients with a score in the CQR scale of >80 were considered compliant, and ≤80 points were considered non-compliant. An indirect clinimetrics evaluation was conducted (DAS28, CDAI), and good compliance was indicated by patients in remission or low level of activity, while the non-compliant were patients with a moderate to high level of disease activity.
The data collected were analyzed using the median and interquartile range, as well as frequencies and proportionalities. The comparison between the compliant and the non-compliant groups based on the CQR score and on the activity of the disease for each variable, was conducted with absolute comparisons using the Mann-Whitney test for continuous variables and chi-square (for expected values of >5), or Fisher (for expected values of <5) for categorical variables, with a significant p value of p < 0.05. ORs and their respective 95% confidence intervals (95% CI) were also used.
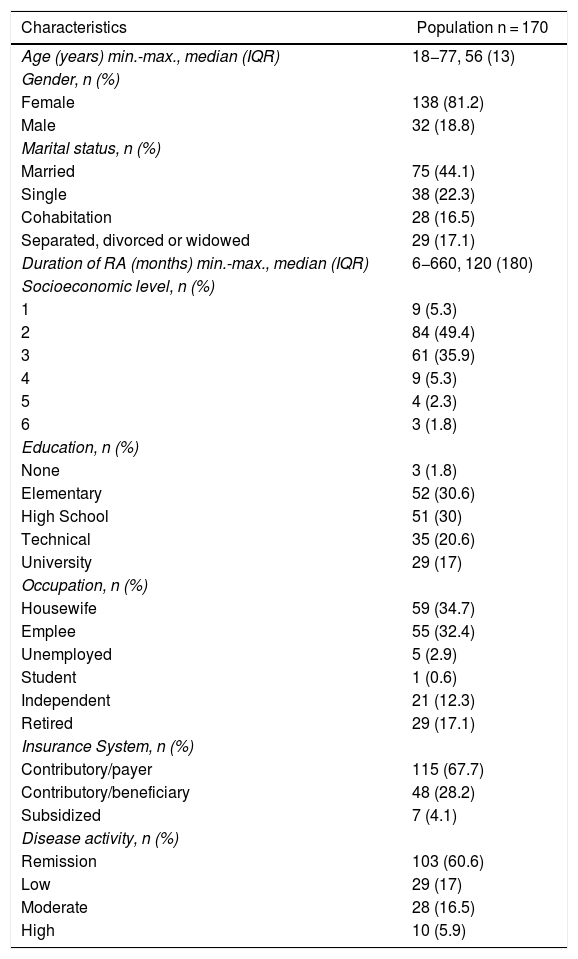
Results171 patients were enrolled, with a mean age of 55 years. 81.2% of the participants were females, with a male to female ratio of 1:4.3 and an average disease duration of 149 months. Most of the patients were in remission or had a low level of disease activity (60.8% and 16.9%, respectively) (Table 1). Among the group of patients with low disease activity or in remission, 55% were only being managed with oral DMARDs and 45% was also receiving a biologic.
Characteristics of the population.
| Characteristics | Population n = 170 |
|---|---|
| Age (years) min.-max., median (IQR) | 18−77, 56 (13) |
| Gender, n (%) | |
| Female | 138 (81.2) |
| Male | 32 (18.8) |
| Marital status, n (%) | |
| Married | 75 (44.1) |
| Single | 38 (22.3) |
| Cohabitation | 28 (16.5) |
| Separated, divorced or widowed | 29 (17.1) |
| Duration of RA (months) min.-max., median (IQR) | 6−660, 120 (180) |
| Socioeconomic level, n (%) | |
| 1 | 9 (5.3) |
| 2 | 84 (49.4) |
| 3 | 61 (35.9) |
| 4 | 9 (5.3) |
| 5 | 4 (2.3) |
| 6 | 3 (1.8) |
| Education, n (%) | |
| None | 3 (1.8) |
| Elementary | 52 (30.6) |
| High School | 51 (30) |
| Technical | 35 (20.6) |
| University | 29 (17) |
| Occupation, n (%) | |
| Housewife | 59 (34.7) |
| Emplee | 55 (32.4) |
| Unemployed | 5 (2.9) |
| Student | 1 (0.6) |
| Independent | 21 (12.3) |
| Retired | 29 (17.1) |
| Insurance System, n (%) | |
| Contributory/payer | 115 (67.7) |
| Contributory/beneficiary | 48 (28.2) |
| Subsidized | 7 (4.1) |
| Disease activity, n (%) | |
| Remission | 103 (60.6) |
| Low | 29 (17) |
| Moderate | 28 (16.5) |
| High | 10 (5.9) |
Max: maximum; min: minimum; IQR: Interquartile range.
60.2% of the participants had a partner (43.8% married and 16.3% cohabitation), while 23% were single and 17% separated, divorced or widowed. Most of the participants (95%) were in the contributory healthcare system (67% as payers and 28% as beneficiaries). Most of the population were from a low socioeconomic level (86%) and a significant percentage had low levels of formal education (32.1% no schooling or just elementary school, and 30.4% high school education). Most of the participants were housewives (35%), while the percentage of employees was 32.1%, retired, 16.9%, and independent workers, 12.2%.
84.2% of the participants had a maximum global support index as measured by the MOS questionnaire13 and most of them experienced low comorbidity (98.7%).
84.2% of the participants had a reasonable knowledge of the disease; most agreed in saying that the main source of information about their condition was the healthcare personnel (91,8%), followed by Internet (2.92%), television (1,75%), and relatives (1.75%).
In average, the participants used 5.8 general drugs and 1.4 DMARDs. 98.2% of the patients said they clearly understood the instructions given by the healthcare staff about the right way to take their medications; 44% received biologics concomitantly.
When asked about the issues regarding lack of continuity in the delivery of the oral medications, 83% of the participants had collected their medications uninterruptedly over the past 6 months; and among those who didn’t, the most frequent reason was non-availability of appointments (n = 9), and shortages of the medication (n = 6).
30.4% of the patients reported some adverse reaction. According to the Naranjo’s algorithm, those reactions were considered as questionable (8.1%), possible (11.7%) or probable (10.5%). None were established as a definitive adverse reactions.
With regards to the perception of efficacy, 88,8% considered that the pharmacological therapy controlled their disease, which was supported by 88.3% of the participants that felt confident with their medical management. Although, around half of the patients (49.1%) expressed fear of experiencing side effects with the use of DMARD, only 22.8% felt that some of the oral medications prescribed were unnecessary. And just 24% reported concomitant use of alternative medicines, while 74.2% said that they never used unprescribed medicines and the rest said they did it only occasionally.
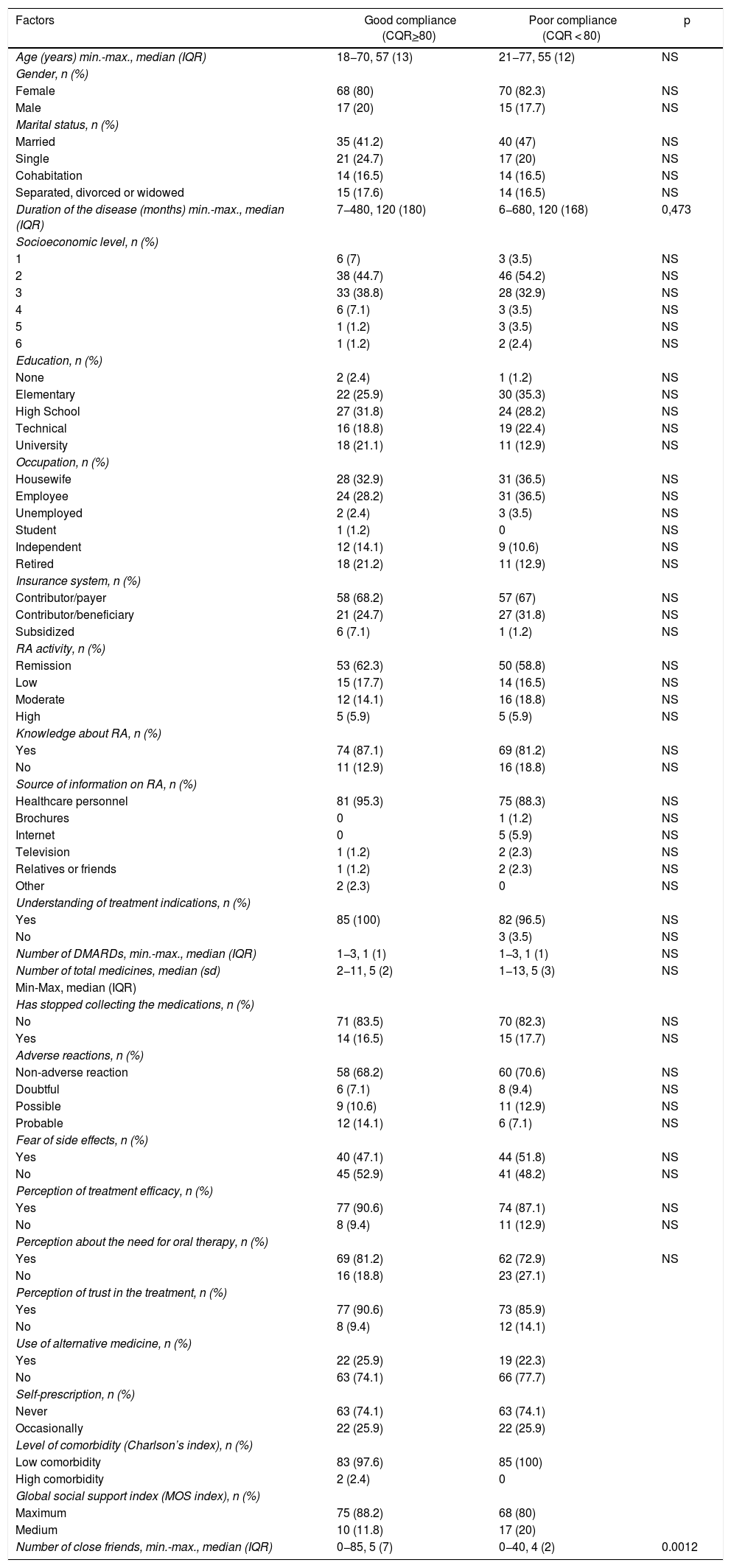
Compliance measurement and potential factors associated with adherence to therapy85 of the patients enrolled (50%) scored 80 or more in the CQR (good compliance). The only statistically significant relationship identified was between CQR-based compliance and the number of friends (p = 0.0012), and between compliance measured by disease activity and social support index (p = 0.004). The different factors evaluated associated with compliance are shown in Table 2.
Factors associated with compliance measured with CQR.
| Factors | Good compliance (CQR>80) | Poor compliance (CQR < 80) | p |
|---|---|---|---|
| Age (years) min.-max., median (IQR) | 18−70, 57 (13) | 21−77, 55 (12) | NS |
| Gender, n (%) | |||
| Female | 68 (80) | 70 (82.3) | NS |
| Male | 17 (20) | 15 (17.7) | NS |
| Marital status, n (%) | |||
| Married | 35 (41.2) | 40 (47) | NS |
| Single | 21 (24.7) | 17 (20) | NS |
| Cohabitation | 14 (16.5) | 14 (16.5) | NS |
| Separated, divorced or widowed | 15 (17.6) | 14 (16.5) | NS |
| Duration of the disease (months) min.-max., median (IQR) | 7−480, 120 (180) | 6−680, 120 (168) | 0,473 |
| Socioeconomic level, n (%) | |||
| 1 | 6 (7) | 3 (3.5) | NS |
| 2 | 38 (44.7) | 46 (54.2) | NS |
| 3 | 33 (38.8) | 28 (32.9) | NS |
| 4 | 6 (7.1) | 3 (3.5) | NS |
| 5 | 1 (1.2) | 3 (3.5) | NS |
| 6 | 1 (1.2) | 2 (2.4) | NS |
| Education, n (%) | |||
| None | 2 (2.4) | 1 (1.2) | NS |
| Elementary | 22 (25.9) | 30 (35.3) | NS |
| High School | 27 (31.8) | 24 (28.2) | NS |
| Technical | 16 (18.8) | 19 (22.4) | NS |
| University | 18 (21.1) | 11 (12.9) | NS |
| Occupation, n (%) | |||
| Housewife | 28 (32.9) | 31 (36.5) | NS |
| Employee | 24 (28.2) | 31 (36.5) | NS |
| Unemployed | 2 (2.4) | 3 (3.5) | NS |
| Student | 1 (1.2) | 0 | NS |
| Independent | 12 (14.1) | 9 (10.6) | NS |
| Retired | 18 (21.2) | 11 (12.9) | NS |
| Insurance system, n (%) | |||
| Contributor/payer | 58 (68.2) | 57 (67) | NS |
| Contributor/beneficiary | 21 (24.7) | 27 (31.8) | NS |
| Subsidized | 6 (7.1) | 1 (1.2) | NS |
| RA activity, n (%) | |||
| Remission | 53 (62.3) | 50 (58.8) | NS |
| Low | 15 (17.7) | 14 (16.5) | NS |
| Moderate | 12 (14.1) | 16 (18.8) | NS |
| High | 5 (5.9) | 5 (5.9) | NS |
| Knowledge about RA, n (%) | |||
| Yes | 74 (87.1) | 69 (81.2) | NS |
| No | 11 (12.9) | 16 (18.8) | NS |
| Source of information on RA, n (%) | |||
| Healthcare personnel | 81 (95.3) | 75 (88.3) | NS |
| Brochures | 0 | 1 (1.2) | NS |
| Internet | 0 | 5 (5.9) | NS |
| Television | 1 (1.2) | 2 (2.3) | NS |
| Relatives or friends | 1 (1.2) | 2 (2.3) | NS |
| Other | 2 (2.3) | 0 | NS |
| Understanding of treatment indications, n (%) | |||
| Yes | 85 (100) | 82 (96.5) | NS |
| No | 3 (3.5) | NS | |
| Number of DMARDs, min.-max., median (IQR) | 1−3, 1 (1) | 1−3, 1 (1) | NS |
| Number of total medicines, median (sd) | 2−11, 5 (2) | 1−13, 5 (3) | NS |
| Min-Max, median (IQR) | |||
| Has stopped collecting the medications, n (%) | |||
| No | 71 (83.5) | 70 (82.3) | NS |
| Yes | 14 (16.5) | 15 (17.7) | NS |
| Adverse reactions, n (%) | |||
| Non-adverse reaction | 58 (68.2) | 60 (70.6) | NS |
| Doubtful | 6 (7.1) | 8 (9.4) | NS |
| Possible | 9 (10.6) | 11 (12.9) | NS |
| Probable | 12 (14.1) | 6 (7.1) | NS |
| Fear of side effects, n (%) | |||
| Yes | 40 (47.1) | 44 (51.8) | NS |
| No | 45 (52.9) | 41 (48.2) | NS |
| Perception of treatment efficacy, n (%) | |||
| Yes | 77 (90.6) | 74 (87.1) | NS |
| No | 8 (9.4) | 11 (12.9) | NS |
| Perception about the need for oral therapy, n (%) | |||
| Yes | 69 (81.2) | 62 (72.9) | NS |
| No | 16 (18.8) | 23 (27.1) | |
| Perception of trust in the treatment, n (%) | |||
| Yes | 77 (90.6) | 73 (85.9) | |
| No | 8 (9.4) | 12 (14.1) | |
| Use of alternative medicine, n (%) | |||
| Yes | 22 (25.9) | 19 (22.3) | |
| No | 63 (74.1) | 66 (77.7) | |
| Self-prescription, n (%) | |||
| Never | 63 (74.1) | 63 (74.1) | |
| Occasionally | 22 (25.9) | 22 (25.9) | |
| Level of comorbidity (Charlson’s index), n (%) | |||
| Low comorbidity | 83 (97.6) | 85 (100) | |
| High comorbidity | 2 (2.4) | 0 | |
| Global social support index (MOS index), n (%) | |||
| Maximum | 75 (88.2) | 68 (80) | |
| Medium | 10 (11.8) | 17 (20) | |
| Number of close friends, min.-max., median (IQR) | 0−85, 5 (7) | 0−40, 4 (2) | 0.0012 |
RA: rheumatoid arthritis; CQR: Compliance Questionnaire on rheumatology; DMARD: Disease modifying anti-rheumatic drug; max: maximum; min: minimum; NS: non-significant; IQR: Interquartile range.
There are two direct and indirect methods to assess treatment compliance. The direct methods include measurement of the drug via biological samples, and direct observation of adherence to medical indications, without patient awareness. However, these methods are invasive, expensive and impractical. So, the indirect methods based on interviews, self-administered questionnaires or tablet count, may identify good compliance with a 90% specificity, although these self-reported strategies usually overestimate compliance because of recall and notice bias.6
The rate of compliance measured with CQR is this trial was 50%. There is a broad range of rates reported with regards to oral DMARDs, estimated between 22% (underutilization) and 107% (overutilization), in part due to the different measuring methods used.14 When the method used is CQR, the studies with non-Latin American populations report a level of compliance ranging between 60 y and 70%.15–18
In Latin America few groups have used the CQR in Spanish. Two trials were conducted in Argentinian population, and one of them reported 47% compliance,19 while the second one reported 51.7%, but with a cutoff point above 60.20 The first study documented higher compliance when the patient feels the need to comply and has less concerns about the medication; compliance was lower in the higher education levels and among married patients. The second study correlated the lack of insurance and the lack of an effective and rapid response, with lower levels of compliance. The third Latin American trial was conducted in Colombian population and reported 43.8% compliance; no relationship was identified with adverse effects, age, gender or marital status. No other variables were analyzed.9
The World Health Organization (WHO) has listed 5 components associated with treatment compliance: socioeconomic factors, healthcare system-related factors and the medical team; factors related to comorbidities, factors related to the therapy and patient-related factors.5,6,21 Other authors have studied the activity of the disease, the physician-patient relationship, and age, as factors associated with overall compliance. Finally, a determining factor for compliance is what patients belief about the treatment.3,22,23
When exploring factors associated with low compliance, this study just identified the relationship between compliance measured with CQR and with the activity of RA, with the social support variable. Various authors have reported that living alone, or having a poor social support network, has a negative impact on treatment compliance.24,25
One of the major weaknesses of the study is that the patients enrolled belong to a RA management program, which means they have received education about the disease at least once, and are aware of the importance of compliance, which may have influenced the large number of patients who reported being knowledgeable about the disease, and expressed their interest in following the indications of the treating physician; however, it is impossible to know the impact of these activities on actual compliance in this study. Neither is it possible to conclude whether the fact that most patients were in remission or had low disease activity, really reflects better adherence to oral therapy, particularly since the use of biologic therapy is significant. The authors believe that a larger sample size would help to identify the differences in the factors present in the compliant versus the non-compliant groups.
It should be highlighted as well that whilst CQR was adapted culturally and validated in Colombia, the researchers realized the difficulty in administering the questionnaire to the participants, who were constantly complaining about the ambiguity of the statements and the difficulty to decide whether they agreed or disagreed with each statement, particularly with regards to the 5 items expressed as a negative assertion. While only one third of the group (32.1%) had elementary education or no formal education whatsoever, and most belonged to a low socioeconomic status (2 or 3), the complaints were also expressed by those with higher education and higher socioeconomic status, and even patients with PhDs.
ConclusionThe level of compliance in this study is similar to what has been reported in other populations, which is probably due to the behavior of our population, similar to other studies. Only the social support index showed an association when considering that the disease activity was an indirect measurement of compliance.
Further studies are needed to assess the operating characteristics of the CQR in Spanish, and possibly to design a specific tool to assess treatment compliance in rheumatic patients in Latin America.
FinancingEconomic assistance of the Colombian Association of Rheumatology for Research Projects in 2017.
Conflict of interestsThe authors have no conflict of interests to disclose.
Please cite this article as: Mora C, Beltrán A, Rincón J, Astudillo Y, Franco M, Jaimes D et al. Adherencia a medicamentos orales en pacientes con artritis reumatoide, una experiencia colombiana. Rev Colomb Reumatol. 2021;28:38–45.