To develop a cost minimization and a budget impact analysis of viscosupplementation with hylan G-F 20 1 × 6 mL for the treatment of knee osteoarthrosis in patients who are not suitable for pharmacological treatment or surgery in El Salvador and Panama.
Materials and methodsThe cost minimization and budget impact analyses were developed from the perspective of the public health system, with a 1-year and 5-year analysis horizon, respectively. The main parameters of the models were acquisition costs, administration, and the need for retreatment. For the budgetary impact, quantification of the population was based on published epidemiological information and local databases. Costs were reported in US dollars at 2020 prices.
ResultsIn El Salvador, the savings derived from its use were $ 35.0 (10%) vs. hylan G-F 20 (2 mL) and $ 202.2 (39%) vs. hyaluronic acid. In Panama, the savings derived from its use were $154.6 (28%) vs. hylan G-F 20 (2 mL) and $567.7 (58%) vs. hyaluronic acid. In the budget impact analysis, considering a gradual substitution over 5 years, the introduction of hylan G-F 20 (6 mL) would be associated with savings of $138,513 (2%) in El Salvador, and $290,728 (3.6%) in Panama.
ConclusionsViscosupplementation with hylan G-F 20 (6 mL) in patients with knee osteoarthrosis is a cost-saving alternative when compared to hylan G-F 20 (2 mL) and low molecular weight hyaluronic acid derivatives available in El Salvador and Panama.
Llevar a cabo un análisis de minimización de costos e impacto presupuestal de la viscosuplementación con hylan G-F 20 1 × 6 mL para el tratamiento de la osteoartrosis de rodilla en El Salvador y Panamá.
Materiales y métodosLos análisis de minimización de costos y de impacto presupuestal se desarrollaron desde la perspectiva del sistema público de salud, el horizonte de análisis fue de un año y de cinco años, respectivamente. Los principales parámetros de los modelos fueron costos de adquisición, administración y necesidad de retratamiento. Para el impacto presupuestal, la cuantificación de la población se basó en información epidemiológica publicada y en bases de datos locales. Los costos se expresaron en dólares americanos a precios del 2020.
ResultadosEn El Salvador, el ahorro fue de $ 35,0 (10%) en comparación con hylan G-F 20 (2 mL) y de $ 202,2 (39%) con respecto al ácido hialurónico. En Panamá, este ahorro fue de 154,6 dólares (28%) en relación con hylan G-F 20 (2 mL) y de 567,7 dólares (58%) comparado con el ácido hialurónico. En el análisis de impacto presupuestal, considerando una sustitución gradual durante 5 años, la introducción de hylan G-F 20 (6 mL) se asociaría con un ahorro de 138.513 dólares (2%) en El Salvador y 290.728 dólares (3,6%) en Panamá.
ConclusionesLa viscosuplementación con hylan G-F 20 (6 mL) en pacientes con osteoartrosis de rodilla es una alternativa costo-ahorradora en comparación con hylan G-F 20 (2 mL) y los derivados del ácido hialurónico de bajo peso molecular disponibles en El Salvador y Panamá.
Osteoarthrosis (OA) is defined as a symptomatic or significant loss of cartilage in the usual load-bearing area of a joint, associated with subchondral sclerosis and osteophyte formation.1 Given that the articular cartilages are avascular, alymphatic and aneural, OA does not meet the standard definition of inflammation, there is no redness or extravasation of fluids and inflammatory cells, and no sensation of pain is identified in early stages.2
One of the joints commonly affected by OA is the knee, particularly in overweigth patients,3 women under estrogen therapy for the treatment of postmenopausal syndrome4 and patients with symptoms related to metabolic abnormalities.5 About 10% of the population over 55 years of age present OA with painful knee, and 10% of men and 13% of women aged 60 years or older have symptomatic OA.6
OA is one of the leading causes of global disability, ranked as the eleventh cause of global disability, and the thirty-eighth in DALY, although it has been established that it is underestimated.7,8 In countries such as El Salvador it represents 135.5 DALYs per 100,000 inhabitants, while in Panama the figure is 141.5 DALYs per 100,000 ihabitants.
Non-surgical treatment options for knee OA include the use of anti-inflamatory drugs,9 weight loss,10 intra-articular injections11 and physical therapy.12 Intra-articular injections, also known as viscosupplementation, are simple procedures that can be performed on an outpatient basis, for which functional benefits and in relation to pain treatment have been identified, with a positive impact on the progression of the disease.11,13,14
To carry out viscosupplementation, two techniques are usually used: the use of hylan G-F 20 (derivatives of high molecular weight hyaluronic acid) or use of low molecular weight hyaluronic acids (LMWHA).14 In terms of effectiveness, hylan G-F 20 has a positive effect higher than LMWHA in periods of 2–3 months post-infiltration.15 In terms of safety, it is important to mention that the risk of presenting adverse events is similar with both techniques,14,15 but the first is recommended for patients who are intolerant or resistant to non-steroidal anti-inflammatory drugs.
Panama and El Salvador are countries in demographic and economic transition, which is why they continue having a high rate of infectious and immunopreventable diseases and, simultaneously, have an increase in the prevalence of persistent chronic diseases, with an increase in life expectancy.16,17 The health system in both countries consists of a public system (governed by the ministry of health of each country) and a private system (financed with the personal funds of the inhabitants).16,17 In terms of health resources the number of doctors per 1,000 inhabitants is the same (1.57) for both countries, however, Panama has a higher rate of hospital beds/1,000 inhabitants (2.25) when compared with El Salvador (1.2).18
Despite having health systems that are similar in their composition, the level of coverage and the entities that regulate each country differ. In Panama, it is estimated that 60% of the population is covered by the Social Security Fund (CSS) and 40% by the Ministry of Health, which is responsible for providing, regulating and implementing health policies at the national level; as well as for maintaining the financing of 70% of the health system; the remaining funding comes from private funds.16 On the other hand, in El Salvador, the Ministry of Health (MinSal) provides health care free of charge to 80% of the population, among which the Salvadorian Institute of Social Security provides healthcare services to 18.4%, MinSal to 79.5% and the private funds to 2.1% of the population. The health sector in this country consists of the public sector, made up of MinSal, the Salvadorian Institute of Social Security, the Military Health System, the Magisterial Welfare Institute, the Salvadorian Institute for the Rehabilitation of People with Disabilities, the High Council of Public Health and the private sector.17
Despite seeking an equitable health system that guarantees comprehensive and quality health care, both countries present inequities that affect dispersed areas from a sociodemographic point of view, urban areas, and special populations, which worsens the problems derived from poverty, malnutrition, geographical dispersion and food shortages, among others.16,17
Recently, hylan G-F 20 was introduced, in a presentation of 6 mL, which requires fewer injections than the presentation of 2 mL and maintains the attributes in terms of effectiveness and safety. The purpose of this study was to perform a cost-minimization analysis to compare hylan G-F 20 of 6 mL with the presentation of 2 mL and other available hyaluronic acid-derivatives. The similarities between Panama and El Salvador in terms of available options, market dynamics and health systems allow the approach of a similar economic analysis in both countries. In this publication, this economic evaluation of the technology was carried out in Panama and Salvador, with the aim of determining the economic impact that the use of viscosupplementation has in both countries. This type of exercises provides valuable information for institutional decision makers who seek to achieve efficiencies in the use of health resources, a particularly important objective in countries with limited resources and great health needs. This is especially important considering that it is a prevalent pathology, with a high disease burden in terms of disability and costs. In addition, a budget impact analysis was developed, from the perspective of the third-party payer in these two countries. The results for each country were reported independently.
Materials and methodsIntervention and comparatorsThe technology assessed was hylan G-F 20 of 6 mL, a high-molecular-weight elastoviscous fluid that contains hylans and is biologically similar to hyaluronate. Hylan G-F 20 contains hylan A and hylan B (48 mg/6 mL).19 Hylan A has a molecular weight of approximately 6 million daltons and hylan B is a hydrated gel.19 The treatment regimen is an injection of 6 mL into the synovial space. The comparators for the evaluation were hylan G-F 20 of 2 mL and the products of sodium hyaluronate currently used in each country. For El Salvador, sodium hyaluronate of 25 mg/2.5 mL of 0.8 Mda (Olter) was considered,20 and for Panama, sodium hyaluronate of 20 mg/2 mL, whose molecular weigth is 3 Mda (Hyruan Plus).21
Cost minimization modelIn the cost minimization analysis of health technologies, the premise is that the evaluated technologies do not present significant differences in clinical effectiveness or safety, so the purpose of the analysis is to evaluate which health technology presents a lower cost in the management of the disease according to the perspective that is being evaluated.
The potential cost reductions associated with the use of hylan G-F 20 in the treatment of knee osteoarthrosis were estimated in this analysis. The cost minimization analysis is supported by a systematic literature review that was previously carried out, in which no statistically significant differences between the intervention and the comparators in terms of safety and tolerability were found.22 Both schemes of hylan G-F (6 and 2 mL) were considered equivalent23 and their effect was longer than that obtained with low molecular weight sodium hyaluronate products.24
The model takes into account that, in order to maintain a similar effectiveness profile as measured by pain relief, a higher proportion of patients who receive low molecular weight sodium hyaluronate require to repeat the treatment course before 12 months. This approach is based on another systematic review, according to which high molecular weight sodium hyaluronate products are more effective than those with a molecular weight lower than 3 Mda.25 Therefore, the costs of acquisition and administration, as well as the differences in duration of the therapeutic effect, are the main elements of the economic evaluation.
Perspective, horizon and discount rateThe perspective of analysis was that of the third payer, which corresponds to the Salvadorian Institute of Social Security and the CSS in the case of El Salvador and Panama, respectively. Both entities were selected given that in their respective countries they are responsible for providing health coverage to the majority of the population,16,17 which allows to make a global analysis of the impact on the health system of each country. The analysis horizon considered in the economic evaluation was one year, which is deemed sufficient to observe the differences between the alternatives in terms of clinical costs and benefits, since it is consistent with the evaluation period of the clinical studies.26–28 No discount rate was applied, taking into account the selected.
ModelThe analysis was performed using a decisión tree (see Supplementary material, Annex 1). The model was developed in Microsoft Excel, considering that a patient receives a first course of treatment and during the analysis horizon may experience recurrence of symptoms or increase in pain, which implies the administration of an additional course of treatment.
Probability of retreatmentThe probabilities of retreatment with each alternative were obtained from the clinical studies identified in the systematic review previously developed. For hylan G-F 20 of 6 mL and 2 mL, the probabilty of retreatment was obtained from the study conducted by Pal et al.,26 in which patients with osteoarthrosis of the knee grades I to III according to the classification of Kellgren and Lawrence were included. A sensitivity analysis that considered the study by Waddell et al.,27 that included patients with severity grades II to IV and a worse scenario, in which it is assumed that retreatment can be up to 40%, was performed. For sodium hyaluronate products, the probability of retreatment was calculated based on the study by Petrella et al.28 According to the study, the average retreatment time in the patients with sodium hyaluronate was 27 ± 7 weeks (range: 12–84 weeks). This event was modeled with a Log-normal distribution29 to obtain the probability of retreatment at 52 weeks. The probabilities of retreatment considered in the analysis are presented in Table 1.
Probability of retreatment, prices and dosing regimens.
| [0,1–5]General parameters | ||||
|---|---|---|---|---|
| Drug | Base case | Sensitivity scenario 1 | Sensitivity scenario 2 | Source |
| Hylan G-F 20, (6.0 mL and 2 mL) | 0.0305 | 0.2530 | 0.4000 | Pal et al.26Waddell et al.27Assumption |
| Sodium hyaluronate 1%, 0.8 Mda | 0.9965 | 0.9982 | 0.9982 | Petrella et al.28 |
| [0,1–5]Base case - El Salvador | ||||
|---|---|---|---|---|
| Drug | Number of injections per series | Cost per unit | Course of treatment | Source |
| Hylan G-F 20, 6.0 mL | 1 | $ 290.5 | $ 290.5 | Manufacturer |
| Hylan G-F 20, 2.0 mL | 3 | $ 96.8 | $ 290.5 | Tender |
| Sodium hyaluronate 1%, 0.8 Mda | 5 | $ 35.0 | $ 175.0 | Tender |
| [0,1–5]Base case – Panama | ||||
|---|---|---|---|---|
| Drug | Number of injections per series | Cost per unit | Course of treatment | Source |
| Hylan G-F 20, 6.0 mL | 1 | $ 316.7 | $ 316.7 | Manufacturer |
| Hylan G-F 20, 2.0 mL | 3 | $ 107.2 | $ 316.7 | Tender |
| Sodium hyaluronate1%, 3 Mda | 5 | $ 87.2 | $ 261.5 | Tender |
| [0,1–5]Sensitivity analysis - El Salvador | ||||
|---|---|---|---|---|
| Drug | Minimum | Maximum | Distribution | Source |
| Hylan G-F 20, 6.0 mL | $ 217.9 | $ 363.2 | Gamma | Manufacturer |
| Hylan G-F 20, 2.0 mL | $ 72.6 | $ 121.1 | Gamma | Tender |
| Sodium hialuronate 1%, 0.8 Mda | $ 26.3 | $ 43.8 | Gamma | Tender |
| [0,1–5]Sensitivity analysis - Panama | ||||
|---|---|---|---|---|
| Drug | Mean | Minimum | Distribution | Source |
| Hylan G-F 20, 6.0 mL | $ 237.5 | $ 395.9 | Gamma | Manufacturer |
| Hylan G-F 20, 2.0 mL | $ 79.2 | $ 132.0 | Gamma | Tender |
| Sodium hialuronate 1%, 3 Mda | $ 65.4 | $ 109.0 | Gamma | Tender |
It is assumed that patients cannot receive more than one retreatment (full course of 1, 3 or 5 injections, depending on the health technology) per year, and it is further assumed that retreatment is administered in a timely manner in all cases that require it.
Cost estimationAccording to the perspective of analysis, only direct medical costs were taken into account. All costs were expressed in U.S. dollars at 2020 prices. The costs considered in the model were those of acquisition and administration. Acquisition costs include the cost of the initial treatment and the additional course of treatment. The dosing schemes were based on the drug technical sheets.30 The prices of the medicines were obtained from public tenders. Acquisition costs for each country are reported in Table 1.
The cost of administration corresponds to the intra-articular administration of the medications. In the case of El Salvador, a cost of $17 per application was considered and $75 for Panama. The information was obtained from consultation to health professionals in the public sector.
Sensitivity analysisA deterministic and a probabilistic sensitivity analysis were developed. The deterministic analysis was carried out on the intervention prices, the comparators and the probability of retreatment, while the probabilistic analysis was performed using Monte Carlo simulations, in which for each cycle, each distribution is randomly sampled to calculate the annual value of a treatment for a hypothetical patient. Gamma distributions were used for the drug costs. 1000 simulations were run and from them, the probability of the intervention being cost-saving compared with the comparator was calculated. The parameters considered in the sensitivity analyzes are presented in Table 1.
Budget impact analysisThe objective of this analysis was to evaluate the net impact of the adoption of hylan G-F 20 of 6 mL in the public sector of the health system of El Salvador and Panama. The model was developed in Excel. Based on different sources of information, the target population was projected for a 5-year analysis horizon. Only the acquisition costs of the initial course and the possible retreatment are taken into account. The costs per year and accumulated over the 5 years are reported.
Target populationThe population of adults over 45 years of age with a diagnosis of knee osteoarthrosis was quantified. Considering the perspective of analysis, the population was restricted to those affiliated to the public social security system. The population was refined taking into account the level of severity and the candidates for therapy. It was taken into account that viscosupplementation with the evaluated technologies was indicated for severity levels I–II in the case of El Salvador and I–III in the case of Panama. No study was found that reported the distribution of patients in the different levels of severity according to the Kellgren-Lawrence scale in the case of Panama, so the information was obtained from consultation with clinical experts. The candidates for therapy were calculated based on the purchase projection of hylan G-F 20 of 2 mL which today holds 100% of the market. In the year 2019, 11,645 units were reported in El Salvador and 7,427 in Panama. The details about the sources of information used to quantify the population in each country can be found in Table 2.
Target population for the budget impact analysis.
| [0,2–3]El Salvador | [0,4–5]Panama | |||
|---|---|---|---|---|
| Criterion | % (n) | Source | % (n) | Source |
| Population ≥45 years | (6,765,753) | 31 | (4,278,500) | 32 |
| Insured population | 27% (1,826,753) | 1 | 84% (3,593,940) | 33 |
| Prevalence of knee OA | 4.3% (78,958) | 1 | 4.5% (160,649) | 3 |
| Severity grade I–II /I–III | 70% (55,270) | 34 | 87.5% (140,568) | Experts |
| Candidates for therapy | 7% (3,869) | 2% (2,811) | ||
In the current scenario, patients receive viscosupplementation with the options available in the public sector, hylan G-F 20 of 2 mL has 100% of the market in the institutional market. For the budget impact, low molecular weight sodium hyaluronates considered in cost minimization were not taken into account, because no tenders have been awarded to them in recent years. The possibility of administering hylan G-F 20 in its presentation of 6 mL is added in the new scenario. In the base case, it was considered a gradual substitution in which, in the case of El Salvador, the technology starts at 10% and increases by 5 percentage points per year until capturing 30% in the fifth year. In the case of Panama, technology starts with 11% and increases one percentage point per year until it captures 15%.
Sensitivity analysisIt was developed a sensitivity analysis comparing different scenarios in which the size of the target population, the market capture, the prices of the drugs and the probability of retreatment were mainly modified.
ResultsCost minimization analysisIn the cost minimization analysis, the hylan G-F 20 of 6 mL turned out to be a cost-saving alternative, compared with the hylan G-F 20 of 2 mL and the low molecular weight hyaluronic acid derivatives previously used in the public sector. In the case of El Salvador, the cost reduction with respect to hylan G-F 20 of 2 mL was 10%, and compared with low molecular weight sodium hyaluronate it was 39%. In the case of Panama, the cost reduction in relation to hylan G-F 20 of 2 mL was 28%, and compared with low molecular weight sodium hyaluronate, 58%. The results are presented in Table 3.
Results of the cost minimization analysis.
| [0,1–4]El Salvador | |||
|---|---|---|---|
| Drug | Annual cost | Difference | % of change |
| Hylan G-F 20, 6.0 mL | $ 316.9 | --- | --- |
| Hylan G-F 20, 2.0 mL | $ 351.9 | −$ 35.0 | −10.0% |
| Sodium hyaluronate 1%, 0.8 Mda | $ 519.1 | −$ 202.2 | −39.0% |
| Panama | |||
| Drug | Annual cost | Difference | % of change |
| Hylan G-F 20, 6.0 mL | $ 403.7 | --- | --- |
| Hylan G-F 20, 2.0 mL | $ 558.2 | −$ 154.6 | −27.7% |
| Sodium hyaluronate 1%, 3 Mda | $ 971.3 | −$ 567.7 | −58.4% |
In the analysis by scenarios, the intervention is maintained as a cost-saving option with respect to its comparators. In the case of El Salvador, the savings with respect to sodium hyaluronate were $50 when excluding administration costs, of $133.8 when considering a retreatment of 25.3% and of $88.6 when considering a retreatment of 40%. In Panama, the savings in relation to sodium hyaluronate were $195.7 when excluding administration costs, of $ 480.5 if the retreatment was 25.3% and of $ 422.9, when considering a retreatment of 40%.
In El Salvador, according to the probabilistic sensitivity analysis, the probability that the intervention is cost-saving with respect to hylan G-F 20 of 2 mL and to sodium hyaluronate of 10 mg/mL is 74.8% and 100%, respectively. In Panama, the probability that the intervention is cost-saving with respect to hylan G-F 20 of 2 mL and to sodium hyaluronate of 10 mg/mL is 99.7% and 100%, respectively.
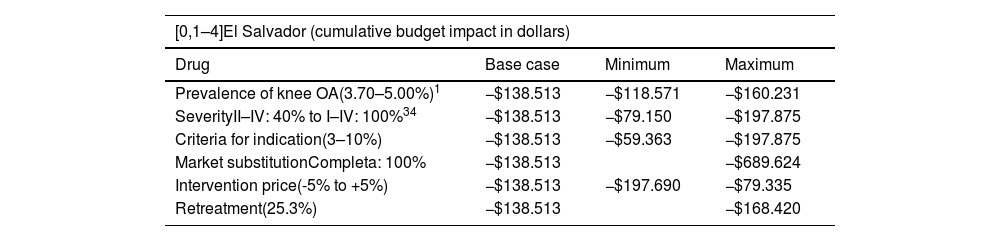
Budget impact analysisIn El Salvador the total number of projected patients for year was from 3,869 to 4,003 in the fifth year. The costs per year in the comparison scenarios are presented in Fig. 1. It was estimated that for a horizon of 5 years, the cot reduction associated with the introduction or hylan G-F 20 of 6 mL would be of $138,513. The cost reduction would be in the range between $13,556 in the first year and $42,079 in the 5th year.
In Panama, 2,811 patients were projected in the first year up to 2,963 in the fifth year. The costs per year in the comparison scenarios are presented in Fig. 2. It was estimated that, for a horizon of 5 years, the cost reduction associated with the adoption of hylan G-F 20 of 6 mL would be $290.728. The cost reduction would be within the range of $47,802 in the first year and $68,705 in the year 5.
According to the sensitivity analyzes (Table 4), the introduction of hylan G-F 20 of 6 mL leads to a cost reduction in all simulated scenarios.
Sensitivity analysis for budget impact evaluation.
| [0,1–4]El Salvador (cumulative budget impact in dollars) | |||
|---|---|---|---|
| Drug | Base case | Minimum | Maximum |
| Prevalence of knee OA(3.70–5.00%)1 | −$138.513 | −$118.571 | −$160.231 |
| SeverityII–IV: 40% to I–IV: 100%34 | −$138.513 | −$79.150 | −$197.875 |
| Criteria for indication(3–10%) | −$138.513 | −$59.363 | −$197.875 |
| Market substitutionCompleta: 100% | −$138.513 | −$689.624 | |
| Intervention price(-5% to +5%) | −$138.513 | −$197.690 | −$79.335 |
| Retreatment(25.3%) | −$138.513 | −$168.420 | |
| [0,1–4]Panama (cumulative budget impact in dollars) | |||
|---|---|---|---|
| Drug | Base case | Minimum | Maximum |
| Prevalence of knee OA(3.82–5.15%)1 | −$290.728 | −$248.452 | −$334.954 |
| SeverityII–IV: 55% to I–IV: 100% | −$290.728 | −$182.744 | −$332.260 |
| Criteria for indication(1–10%) | −$290.728 | −$145.364 | −$1.453.641 |
| Market substitutionComplete: 100% | −$290.728 | −$2.231.860 | |
| Intervention price(−5% to +5%) | −$290.728 | −$321.421 | −$260.037 |
| Retreatment(25.3%) | −$290.728 | −$353.499 | |
The results of the cost minimization analysis showed that in El Salvador and Panama, hylan G-F 20 of 6 mL is a cost-saving option, compared with the low molecular weigth hyaluronic acid derivatives. Compared with the presentation of 2 mL, it would also produce savings due to the fact that it requires fewer injections. Low molecular weight hyaluronic acid derivatives are not currently being used in the institutional market, so the prices reported in the last available tender were considered as a conservative scenario. However, even in a price parity scenario, savings would be expected with hylan G-F 20 due to the lower number of injections per course of treatment and the lower probability of retreatment. The results were robust to changes in prices and in the need for retreatment. In the sensitivity analyses, the probability of hylan G-F 20 of 6 mL of being a cost-saving option compared with the low molecular weight hyaluronic acid derivatives remained above 90%.
The budget impact analysis at 5 years showed that the introduction of hylan G-F 20 for the management of knee OA potentially eligible for treatment would result in savings of $138,513 (2.0%) in El Salvador and of $290,728 (3.6%) in Panama. The results are robust to changes in the target population, the price of technologies, the acquisition scenarios, and the probability of retreatment. The greatest savings are obtained if a complete market substitution is considered. Given the objective of this type of analysis, only the technologies used in the public sector were included in the current scenario and not those that could enter.
The potential savings that can be obtained in the health system represent an opportunity that implies being able to promote access for patients with knee OA who have not been treated, or to use these budget resources in other prioritized cohorts. These two countries, having similar health systems, population and demographic characteristics, health technology and access barriers, present a similar structure and characteristics that allow their analysis together, even though individual analyzes were performed for each country.
Economic evaluations that compared hylan G-F 20 with low molecular weight hyaluronic acid derivatives were not identified. The majority of the studies found correspond to cost-effectiveness evaluations in which high molecular weight derivatives are compared with conservative treatment that includes the use of non-steroidal anti-inflammatory drugs and other analgesics, physiotherapy, weight loss or even intra-articular corticosteroid injections.35–37 In these studies, the intervention was considered cost-effective from the perspective of the third-party payer. Among the Latin American studies, it was identified one developed in Colombia in which the cost-effectiveness of viscosupplementation with hylan G-F 20 versus conservative treatment was evaluated.38 In this analysis, hylan G-F 20 was considered a dominant alternative, since it produced an improvement in the symptoms of the disease, the joint function and the health-related quality of life, at a lower cost than its comparator.
In the study in question, the uncertainty about certain parameters considered was addressed through different sensitivity analyses. In terms of the budgetary impact that the substitution of hyaluronates could represent, it should be noted that the target population could be larger, since the refinement of the population was carried out based on the report of units sold to the institutional market. This implies a greater potential in cost reduction and savings to be perceived by the health systems of Panama and El Salvador. Likewise, although they are presented together, the results of each country are independent, considering the peculiarities that each one may have.
ConclusionsThe results obtained, both of cost minimization and budget impact, allow us to conclude that the adoption of hylan G-F 20 of 6 mL in El Salvador and in Panama can have a positive impact on the budget, taking into account its low cost compared with the presentation of 2 mL and with low molecular weight hyaluronic acid derivatives.
FundingThe funding for this project was provided by Sanofi. The research and the report of that investigation were not dictated or influenced in any way by Sanofi.
Conflict of interestThe authors declare that they have no conflict of interest.