Psoriatic arthritis (PsA) is a chronic inflammatory disease that can lead to a reduction of function and quality of life. Early diagnosis could be important to prevent damage and improve patient-reported outcomes (PROs), such as pain and disease consequences.
ObjectiveThe objective of this study was to evaluate the differences in the PROs in PsA patients with early diagnosis of PsA with respect to patients with late diagnosis.
Methodology and methodsIn this cross-sectional analysis of a longitudinal cohort, patients with PsA receiving treatment with conventional and/or biological disease-modifying antirheumatic drugs (DMARDs) for at least 6 months were recruited.
Patients with less than 2 years of disease duration prior to their diagnosis were identified as having early PsA. Clinical, demographic, and disease activity data, as well as different PROs (including PtGA, pain VAS, PASS, HAQ-DI, and PSAID-12) were obtained at the last visit in our unit.
ResultsOf 135 patients with complete data, 49 had an early diagnosis (<2 years from symptom onset) and 86 had a late diagnosis (>2 years). No differences were found between groups in terms of PtGA, VAS pain, PASS, and PSAID-12. However, HAQ-DI was found to be significantly higher, [median (IQR): .5 (.125–1) in late-diagnosis patients compared to patients with early diagnosis [median (IQR): .3 (0–.5)].
ConclusionOur findings suggest that early diagnosis of PsA patients may have a major impact primarily on their function and quality of life.
La artritis psoriásica (PsA) es una enfermedad inflamatoria crónica que puede producir un deterioro de la función y de la calidad de vida. El diagnóstico temprano podría ser importante para prevenir daño y mejorar los resultados reportados por los pacientes (PRO), como el dolor y las consecuencias de la enfermedad. El objetivo de este estudio fue evaluar las diferencias en los PRO que acuden a nuestra unidad con diagnóstico precoz de PsA con respecto a los pacientes con diagnóstico tardío.
MetodologíaEn este análisis transversal de una serie longitudinal se reclutaron pacientes con PsA que estaban recibiendo tratamiento con fármacos antirreumáticos convencionales y/o biológicos modificadores de la enfermedad (DMARD) al menos por menos 6 meses. Los pacientes que tenían menos de 2 años de duración de la enfermedad previamente a su diagnóstico se identificaron como con PsA temprana. Se obtuvieron datos clínicos, demográficos y de actividad de la enfermedad, así como diferentes PRO (incluyendo PtGA, dolor en VAS, pPASS, HAQ-DI y PSAID-12) en la última visita en nuestra unidad.
ResultadosDe 135 pacientes con datos completos, 49 tuvieron un diagnóstico temprano (<2 años desde el inicio de los síntomas) y 86 tuvieron un diagnóstico tardío (>2 años). No se encontraron diferencias entre los grupos en cuanto a PtGA, dolor en VAS, PASS y PSAID-12; sin embargo, se encontró que el HAQ-DI fue significativamente superior (mediana [IQR]: 0,5 [0,125-1] en pacientes con diagnóstico tardío, comparados con los pacientes con diagnóstico temprano [mediana (IQR): 0,3 (0-0,5)]).
ConclusiónNuestros hallazgos sugieren que un diagnóstico temprano de los pacientes con PsA puede tener un impacto, principalmente en su funcionalidad y calidad de vida.
Psoriatic arthritis (PsA) is a complex and chronic inflammatory disease that affect about 30% of patients with skin psoriasis. It could be recognized as a “syndrome” in which different manifestations such as psoriasis, peripheral and axial joint involvement, enthesitis, extra-articular manifestations and comorbidities can develop during the disease course.1–4 Due to the complexity of the disease, unidimensional and multidimensional disease activity indices that include the assessment of all disease domains, or that are mainly focused on joint involvement, were developed.5–10 Furthermore, in daily clinical practice, the evaluation of the so-called patient's reported outcome (PROs) has become important to capture the disease impact on patients.11 In PsA, PROs have been used both in clinical trials and clinical practice. PROs are key components of efficacy end-points and are incorporated with physician-based measures in composite disease activity indices, including the primary outcome in PsA randomized controlled trials (RCTs), the American College of Rheumatology 20% improvement response criteria (ACR20), the Minimal Disease Activity (MDA)5 and the Disease Activity index for Psoriatic Arthritis (DAPSA)9 that represent the current achievable treatment targets.12,13 As a part of the OMERACT PsA Core Domain Set,14 PROs representing patient global assessment, pain, physical function, and health related quality of life are expected to be measured in all PsA studies in addition to physician assessments of joints and skin. Beyond these domains, PROs are used to capture work productivity, fatigue, psychological endpoints and other symptoms.15
In the last years, a greater attention was focused on the concept of “early” psoriatic arthritis. As in rheumatoid arthritis, early diagnosis may lead to better outcomes in terms of early introduction of effective treatment, reduction of disease progression, better possibility to achieve remission and even a better impact on PROs.16 Unfortunately, a shared and validated definition of what is early PsA is lacking, and different cut-offs were proposed.16,17
The aim of this study was to evaluate the differences in different PROs in patients attending our unit with a diagnosis of “early” PsA in respect to patients with delayed diagnosis, in order to assess the possible benefits of early diagnosis on these important outcome measures. As secondary aim, we evaluated the differences in disease activity scores.
MethodologyPatient selectionIn this cross-sectional analysis of a longitudinal cohort, patients were enrolled at Rheumatology Unit, Department of Medicine and Health Science – University of Molise, Italy. We collected the patients’ data at last visit performed in our unit during the period of time (1st January 2021–1st January 2022) and who were on at least 6-months follow-up treatment with conventional and/or biologic disease modifying anti-rheumatic drugs (DMARDs).
Early PsA was identified when patients have less than 2 years disease duration before the diagnosis, according to literature.17
Inclusion criteria were:
- 1)
PsA classified with the ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria.18
- 2)
age≥18 years,
- 3)
at least 6 months follow-up at the study visit (patients had to have been treated for at least 6 months in our centres).
The study protocol was in compliance with the declaration of Helsinki and written consent was obtained from each participant. The study was approved by the Institutional Review Board of the University of Molise (protocol n. 0001-09-2017).
Data collectionA detailed medical history and physical examination were performed in all patients. Demographics and disease characteristics including gender, age, disease duration, level of education, and pattern of articular manifestations were carefully collected. For each patient, we evaluated the date of symptoms onset (including the development of arthritis, enthesitis, dactylitis or axial manifestations but not skin involvement) and the data of the diagnosis.
Subset of disease (axial, mono, oligo, polyarticular, enthesitic and predominant distal interphalangeal joint involvement) was also evaluated.
Laboratory parameters were also collected. The clinical assessment encompassed the number of tender joints (of the 68 assessed joints) and swollen joints (total of 66 joints), enthesitis and dactylitis. Enthesitis was assessed by using the Leeds Enthesitis Index (LEI),19 and dactylitis as present/absent/past. Skin assessment was performed using the body surface area (BSA).
We collected, for each patient, different PROs including the HAQ-DI,20 the Patient Global Assessment (PtGA)21 and pain assessment on VAS (0–10cm), the patient's acceptable symptoms state (PASS)22 and the Psoriatic arthritis Impact of Disease (PsAID).23
Disease activity indices and remission/low disease activity indices (MDA and DAPSA)MDA was defined according to Coates et al.6 Patients were considered in MDA when they satisfied 5/7 of the following criteria: tender joint count≤1; swollen joint count≤1; BSA≤3%; patient pain visual analogue scale (VAS) score of ≤15; PtGA VAS score of ≤20; HAQ score≤0.5; and tender entheseal points≤1.
Very low disease activity was satisfied when all 7 criteria were met. DAPSA score was calculated by adding the number of tender and swollen joints, VAS pain, PtGA and CRP (mg/dl).7 DAPSA score≤4 identified remission while DAPSA≤14 a condition of low disease activity.
Statistical analysisStatistical analysis was performed using SPSS software (version 26). Demographic and clinical characteristics were summarized using descriptive statistics. Non normally distributed continuous variables were reported by median and Interquartile Range (IQR) and categorical variables by number and percentage. Patients were divided in two groups based on disease duration before the diagnosis: ≤2 years and >2 years. The Mann–Whitney U-test and the chi-square test were used to compare continuous variables and categorical data between these two groups, respectively. Finally, multivariate logistic regression analysis was performed in order to evaluate potential confounders which include sex, BMI, smoking status and subset of articular involvement. Goodness of fit was estimated using the adjusted R2. Odds ratio (OR) and confidence interval (CI) 95% were calculated when appropriate. We categorized PROs as follow: PtGA and VAS pain <= or > than 20, PsAID <= and > than 4 and HAQ <= or > than 0.5.
A statistical significance was defined as a 2-tailed p value ≤0.05.
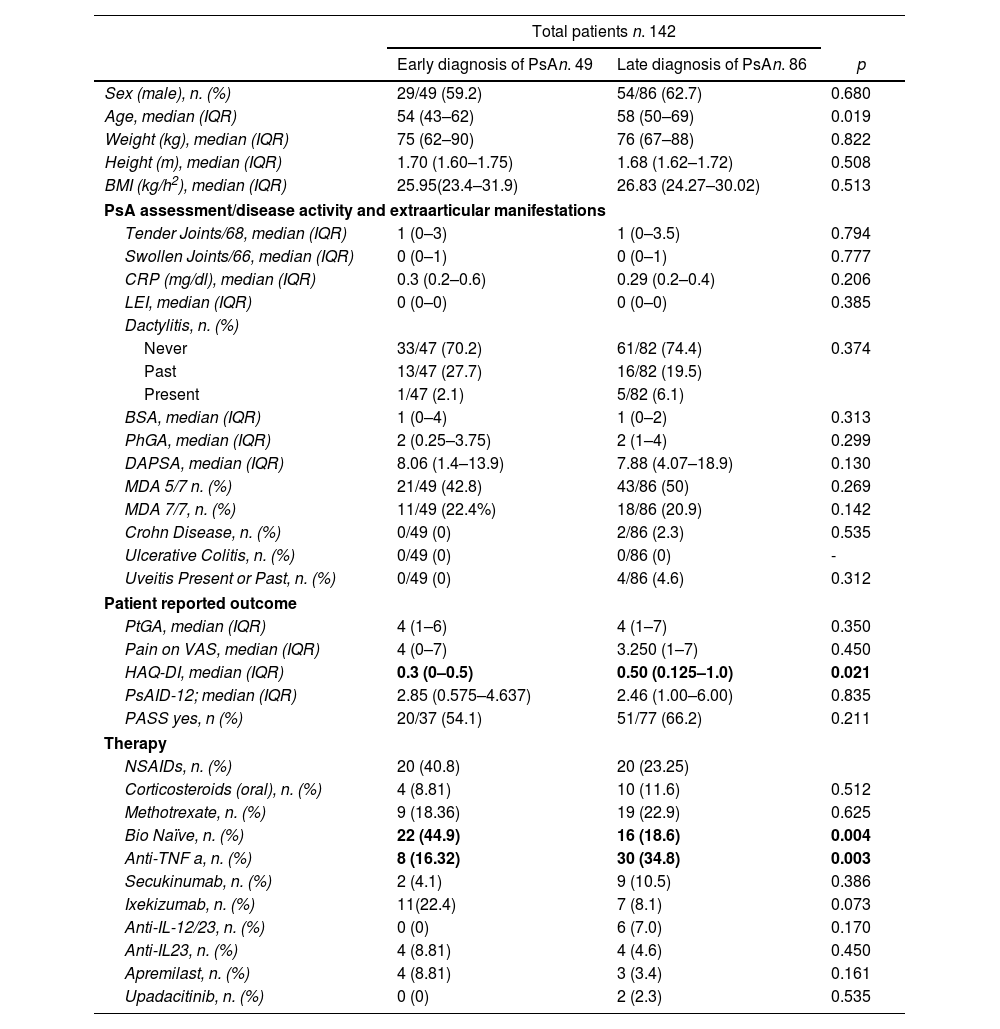
ResultsA total of 142 patients (83 male and 59 female) with PsA who met the inclusion criteria were consecutively recruited. Of these, 7 patients (2 patients with early disease and 5 patients with late diagnosis) were excluded had missing data on disease symptoms onset and were excluded from the analysis. Of the remaining 135 patients, 49 had an early diagnosis within 2 years from onset and 86 had a delayed diagnosis (>2 years). Table 1 summarized the clinical, demographic and disease characteristics between the two groups of patients.
Demographic, clinical and disease characteristics of the enrolled PsA patients.
| Total patients n. 142 | |||
|---|---|---|---|
| Early diagnosis of PsAn. 49 | Late diagnosis of PsAn. 86 | p | |
| Sex (male), n. (%) | 29/49 (59.2) | 54/86 (62.7) | 0.680 |
| Age, median (IQR) | 54 (43–62) | 58 (50–69) | 0.019 |
| Weight (kg), median (IQR) | 75 (62–90) | 76 (67–88) | 0.822 |
| Height (m), median (IQR) | 1.70 (1.60–1.75) | 1.68 (1.62–1.72) | 0.508 |
| BMI (kg/h2), median (IQR) | 25.95(23.4–31.9) | 26.83 (24.27–30.02) | 0.513 |
| PsA assessment/disease activity and extraarticular manifestations | |||
| Tender Joints/68, median (IQR) | 1 (0–3) | 1 (0–3.5) | 0.794 |
| Swollen Joints/66, median (IQR) | 0 (0–1) | 0 (0–1) | 0.777 |
| CRP (mg/dl), median (IQR) | 0.3 (0.2–0.6) | 0.29 (0.2–0.4) | 0.206 |
| LEI, median (IQR) | 0 (0–0) | 0 (0–0) | 0.385 |
| Dactylitis, n. (%) | |||
| Never | 33/47 (70.2) | 61/82 (74.4) | 0.374 |
| Past | 13/47 (27.7) | 16/82 (19.5) | |
| Present | 1/47 (2.1) | 5/82 (6.1) | |
| BSA, median (IQR) | 1 (0–4) | 1 (0–2) | 0.313 |
| PhGA, median (IQR) | 2 (0.25–3.75) | 2 (1–4) | 0.299 |
| DAPSA, median (IQR) | 8.06 (1.4–13.9) | 7.88 (4.07–18.9) | 0.130 |
| MDA 5/7 n. (%) | 21/49 (42.8) | 43/86 (50) | 0.269 |
| MDA 7/7, n. (%) | 11/49 (22.4%) | 18/86 (20.9) | 0.142 |
| Crohn Disease, n. (%) | 0/49 (0) | 2/86 (2.3) | 0.535 |
| Ulcerative Colitis, n. (%) | 0/49 (0) | 0/86 (0) | - |
| Uveitis Present or Past, n. (%) | 0/49 (0) | 4/86 (4.6) | 0.312 |
| Patient reported outcome | |||
| PtGA, median (IQR) | 4 (1–6) | 4 (1–7) | 0.350 |
| Pain on VAS, median (IQR) | 4 (0–7) | 3.250 (1–7) | 0.450 |
| HAQ-DI, median (IQR) | 0.3 (0–0.5) | 0.50 (0.125–1.0) | 0.021 |
| PsAID-12; median (IQR) | 2.85 (0.575–4.637) | 2.46 (1.00–6.00) | 0.835 |
| PASS yes, n (%) | 20/37 (54.1) | 51/77 (66.2) | 0.211 |
| Therapy | |||
| NSAIDs, n. (%) | 20 (40.8) | 20 (23.25) | |
| Corticosteroids (oral), n. (%) | 4 (8.81) | 10 (11.6) | 0.512 |
| Methotrexate, n. (%) | 9 (18.36) | 19 (22.9) | 0.625 |
| Bio Naïve, n. (%) | 22 (44.9) | 16 (18.6) | 0.004 |
| Anti-TNF a, n. (%) | 8 (16.32) | 30 (34.8) | 0.003 |
| Secukinumab, n. (%) | 2 (4.1) | 9 (10.5) | 0.386 |
| Ixekizumab, n. (%) | 11(22.4) | 7 (8.1) | 0.073 |
| Anti-IL-12/23, n. (%) | 0 (0) | 6 (7.0) | 0.170 |
| Anti-IL23, n. (%) | 4 (8.81) | 4 (4.6) | 0.450 |
| Apremilast, n. (%) | 4 (8.81) | 3 (3.4) | 0.161 |
| Upadacitinib, n. (%) | 0 (0) | 2 (2.3) | 0.535 |
IQR: interquartile range; BMI: body mass index; CRP: C reactive protein; LEI: Leeds Enthesitis Index; BSA: body surface area; PhGA: physician global assessment; DAPSA: disease activity score for Psoriatic arthritis; MDA: minimal disease activity; PtGA: patient global assessment; HAQ-DI: health assessment questionnaire-disability index; PsAID: psoriatic arthritis impact of disease; PASS: patient's acceptable symptoms score; NSAIDs: non steroidal anti-inflammatory drugs.
Comparing the two groups, based disease onset, we found that PtGA, pain on VAS, PASS and PsAID-12 were not different between the two groups.
However, HAQ-DI were statistically significant higher, [median (IQR): 0.5 (0.125–1) in patients with a late diagnosis>2 years, in respect to patients with early diagnosis [median (IQR): 0.3 (0–0.5)] (see Table 1).
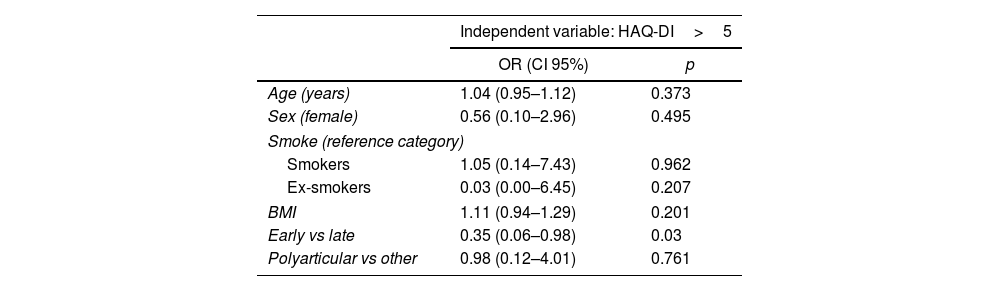
We also perform a multivariate logistic regression analysis in which we analyzed potential predictors and confounders (see Table 2).
Multiple logistic regression analysis evaluating potential confounder and factors associated to worst function assessed by HAQ-DI.
| Independent variable: HAQ-DI>5 | ||
|---|---|---|
| OR (CI 95%) | p | |
| Age (years) | 1.04 (0.95–1.12) | 0.373 |
| Sex (female) | 0.56 (0.10–2.96) | 0.495 |
| Smoke (reference category) | ||
| Smokers | 1.05 (0.14–7.43) | 0.962 |
| Ex-smokers | 0.03 (0.00–6.45) | 0.207 |
| BMI | 1.11 (0.94–1.29) | 0.201 |
| Early vs late | 0.35 (0.06–0.98) | 0.03 |
| Polyarticular vs other | 0.98 (0.12–4.01) | 0.761 |
OR: odd ratio; BMI: body mass index.
We evaluated the potential differences in disease activity (assessed by DAPSA) and disease state (assessed by MDA) between the two groups. Of note, no significant differences were found in median DAPSA score and no differences were found in the rate of patients achieving MDA.
DiscussionPsoriatic arthritis is a complex and multifaceted disease with the possible involvement of different domains. In the last 20 years, biologic treatment revolutionized the management of PsA: anti-TNF, anti-IL-17, anti-IL12/23, anti-IL23 agents and small molecules allowed the achievement of remission or low disease activity in almost all disease domains.7,24–33 PROs are an important part of the assessment of PsA patients, beyond the mere evaluation of disease activity. In our study, we did not find significant differences in PsAID score, pain, PASS and PtGA between patients with early diagnosis in respect to patients with delayed diagnosis, underlining how the impact of the diseases on patient's perception could be independent by the disease duration.
However, a delayed diagnosis may have a significant impact on the probability to have a worst function and quality of life in respect to early diagnosis. Of note, in those patients, even if there was a worst function, the impact of the disease, the levels of pain and the PtGA are not different. This could also be related to the different aspect of the disease evaluated by these indices. In particular, HAQ-DI may reflect the impact on functional derived from articular damage while the PsAID mainly assess the impact of disease.
Some other studies also suggest that a delay in diagnosis is associated with a worse outcome. In the Toronto cohort, PsA patients first seen after 2 years of diagnosis compared with those seen within 2 years, had a greater rate of joint damage.16 Furthermore, in the Bath cohort, authors demonstrated that a delay in diagnosis as well as smoking, female gender, and older age at onset were associated with a worse physical function measured by the HAQ-DI.34 Similar observations were reported in a Dublin cohort, in which patients with late referral having greater peripheral joint erosions and worse physical function.35
Finally, even the short duration of symptoms could be a factor potentially important to the achievement of minimal disease activity as show in a recent study.36
Some further evidences in support of early intervention comes from clinical trials. In the PRESTA study, in patients receiving etanercept 50mg once weekly, with PsA for less than 2 years, PROs and quality of life were generally superior in respect to patients with longer duration, after 24 weeks of treatment.37,38
Therefore, evidences suggest that early intervention may be important in decreasing the burden of disease. In our study, we did not find differences in other PROs in respect to PRESTA trial. However, this result could be related to the different study design and population.
The study has some limits, in particular the definition of what is “early” PsA, since it is still no clear how the disease developed in patients with psoriasis, with the possibility to have an “occult” joint involvement.39,40 Moreover, we did not assess the imaging of patients due to lack of sufficient data and, finally, the presence of different comorbidities that may have an impact on disease activity and PROs41–43 were not included in the analysis. Furthermore, the retrospective nature of the study, data collected, data entry, and data quality, not planned ahead of time, may be affected by different bias
In conclusion, our findings suggest that early diagnosis of PsA may have an impact on functional outcome assessed by HAQ-DI. However, due to the nature of the study, caution is recommended in the interpretation of results and further prospective studies should be performed to evaluate this intriguing topic.
ContributorshipAll listed authors equally contributed to the planning, conduct, collection of data and in the writing of the work.
FundingNone.
Conflict of interestsThe authors declare no conflict of interests.