Pyomyositis, defined as a suppurative muscle infection, is a rare entity that is classified as tropical or non-tropical according to the geographical region.
The case of a patient with a history of severe tophaceous gouty arthritis, coming from tropical wet climate in Colombia, who presented with suggestive cellulitis, with a torpid evolution despite standard dual management. An associated abscess was documented, and initially abscessed cellulitis and septic arthritis were suspected, the latter was finally ruled out, once the muscle origin of the abscess becames evident by means of computed tomography and ultrasonography. Intraoperatively, an abscess with fascia involvement and intramuscular tophaceous disease was observed. Surgical drainage and repeated washes were required. Carbapenem resistant Klebsiella pneumoniae was isolated, requiring last-line antibiotic therapy for a prolonged period of time.
La piomiositis es una infección supurativa del músculo estriado, rara, que se clasifica según la región geográfica de presentación, en tropical y no tropical.
Se presenta el caso de un paciente con antecedente de artritis gotosa tofácea severa, proveniente de zona tropical, quien comienza con cuadro sugestivo de celulitis, con evolución tórpida pese a manejo estándar dual; se documenta un absceso de ubicación no clara, sospechando en principio celulitis abscedada y artritis séptica, finalmente descartándose esta última y haciéndose evidente origen muscular del absceso por medio de tomografía computarizada y ultrasonografía. Quirúrgicamente se evidenció absceso con compromiso de fascia, enfermedad tofácea intramuscular; requirió drenaje quirúrgico y lavados, documentándose germen Klebsiella pneumoniae resistente a carbapenémicos, requiriendo antibioticoterapia de última línea por un periodo prolongado.
Pyomyositis is the primary suppurative infection of skeletal muscle. It is classified as “tropical” and “non-tropical” according to the geographical place of occurrence, with a different epidemiology for each type. In a hospital in the Amazonian region of Brazil, tropical pyomyositis accounted for 1% of hospitalizations, with an annual incidence between 52 and 62 cases per 100,000 inhabitants. These patients were predominately young men, and affected only one muscle in the lower extremities, with a mortality rate of 2%.1
The incidence is substantially lower in non-tropical areas. At a pediatric referral center in Brisbane, a sub-tropical region of Australia, pyomyositis accounted for 2 per 10,000 admissions, increasing to 8 per 10,000 admissions 10 years later. These cases were predominantly in boys and in the lower limbs.2 In another retrospective study at a referral center in Chandigarh, a subtropical region in India, pyomyositis accounted for 3 per 10,000 admissions. The patients were mostly men, with half in the age range of 25–50 years and a mortality rate of 16%.3
The classic description of tropical pyomyositis as an infection of more or less well-defined phases is classically attributed to Chiedozi et al.4 However, the pathogenesis of the non-tropical form in tropical regions is an event that must be considered, in addition to predisposing risk factors that are low in frequency but plausible, such as severe tophaceous gout.
The following is the case of a patient with pyomyositis in the context of severe tophaceous gout with intramuscular involvement, a condition that is proposed as a causal event for the development of the infection.
Case reportA 49-year-old man from San José del Guaviare, a Colombian municipality located at sea level with a tropical wet climate, who was in Bogota (2.600m over the sea level) for a week prior to admission, was hospitalized for severe right calf pain. The patient reported an eight-day course of symptoms characterized by severe dull pain in his right calf associated with erythema, edema, and nausea. Patient did not report any episodes of fever or other associated symptoms. He referred a history of hyperuricemia and difficult-to-control gouty disease with secondary deformities and functional limitation, due to a decrease in mobility of large and small joints, without impeding gait. He reported previous tophi resection in both elbows. He managed his symptoms with diclofenac. Additionally, he had a history of controlled hypertension.
On examination, patient was oriented and hydrated, with a heart rate of 82 beats per minute, without pulse deficits, blood pressure of 123/75mmHg, respiratory rate of 18 breaths per minute, and oxygen saturation of 91% while breathing ambient air. Multiple deformities were observed in all four limbs, with tophi in all articulations of both hands and in extension zones. In the medial aspect of the proximal right calf, warmth, erythema and tenderness upon palpation was observed. A distal capillary filling of 2s was present in all extremities. A reducible umbilical hernia was also palpable, with no other abnormal findings on physical examination.
Deep venous thrombosis and cellulitis were considered as differential diagnoses; deep and superficial Doppler ultrasonography did not identify thrombosis. Initially, oxacillin (2g intravenous every 4h) and clindamycin (600mg intravenous every 6h) were used and acetaminophen, colchicine and allopurinol, and amlodipine were also given. In addition, azotemia was observed and regarded as acute deterioration of chronic renal disease secondary to nephrocalcinosis (Table 1).
The patient presented a torpid evolution, with persistent signs of local inflammation, ipsilateral knee pain, considerable C reactive protein (CRP), without signs of hypoperfusion or shock, lactate at 1.3mmol/L, and overall a poor response to antibiotic management during 48h. Methicillin-resistant Staphylococcus aureus (MRSA) was suspected. Antibiotic therapy was adjusted with vancomycin (1g every 24h intravenously) and piperacillin tazobactam (4.5g every 6h intravenously). Vancomycin was subsequently switched to linezolid (600mg intravenously every 12h) because acute deterioration of chronic renal disease.
A soft tissue ultrasound of the calf revealed a collection of 39mm×28mm×39mm of hypoechoic predominance in an unspecified location. Ultrasound of the knee demonstrated heterogeneous intra-articular fluid and inflammation of soft tissues of the knee. This collection was not considered susceptible to puncture. Arthrocentesis was performed and 60cc of liquid with abundant whitish crystals was collected.
Despite broad-spectrum antibiotic management, the patient persisted with tachycardia, intermittent fever of nocturnal predominance between 38 and 39°C, local inflammatory reaction, an increase of CRP to 408mg/dL, and a negative synovial fluid culture at 72h. It became evident in the physical examination zone of renitence in medial aspect of the right infrapatelar region. Second ultrasound of soft tissues demonstrated an increase of the echogenicity of the skin and the subcutaneous cellular tissue, with interstitial fluid of laminar disposition. Likewise, in the muscular plane a heterogeneous hypoechoic collection of 120mm×70mm×70mm was observed. Pyomyositis was considered, it was decided to continue with linezolid and other antibiotics switched to meropenem (2g IV ad 8h for 10 days) and gentamicin (240mg IV day).
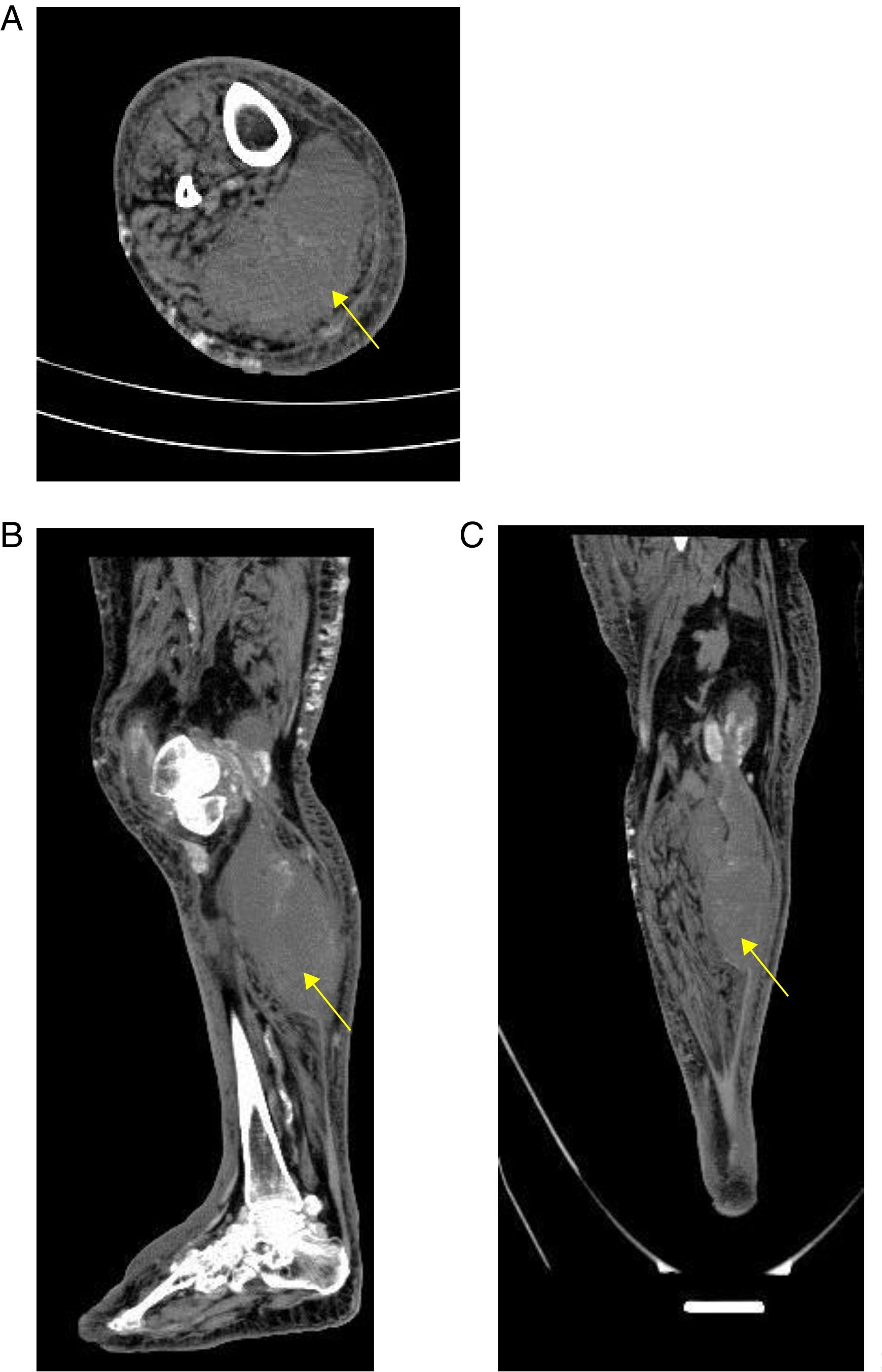
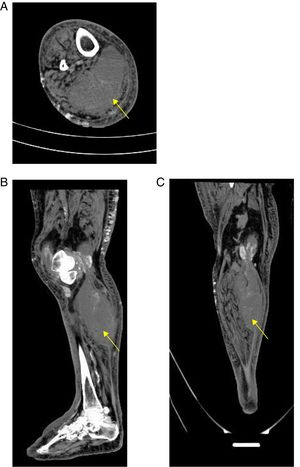
A simple computed tomography result was obtained, showing an organized collection in the medial gastrocnemius (see Fig. 1). Abdominal and pelvic tomography showed inflammatory soft tissue changes in the gluteal and right thigh region with reactive inguinal adenopathies. A final synovial fluid culture result was obtained and was positive for carbapenem resistant Pseudomonas fluorescens, which was initially considered a contamination (Fig. 1).
Surgical drainage of infective focus was performed by orthopedic service. In the posterior compartment of the leg an organized collection of fluid was observed, obtaining 200cc of purulent secretion with abundant gouty tophi. Excisional debridement was performed and samples were sent to culture.
The patient persisted with nocturnal febrile peaks up to 40°C. After 48h, a new surgical procedure was carried out, and an abscess of 150 cc of purulent secretion was observed, along with necrosis of the fascia of the medial and semitendinosus gastrocnemius. Debridement and lavage were performed, requiring a total of four surgical interventions for elimination of purulent secretion. Finally, a local flap closure was performed.
The fluid culture was positive for carbapenem resistant Klebsiella pneumoniae subsp. ozaenae, compatible with pyomyositis and fasciitis of the medial gastrocnemius. Linezolid, meropenem and gentamicin were continued. In order to rule out possible acquired immunosuppression, HIV ELISA test was repeatedly requested, which the patient refused.
Despite a favorable response to repeated surgical lavage, the patient persisted with febrile spikes. It was decided to discontinue gentamicin, to continue meropenem and to start polimixin B at a dose of 300,000IU intravenous every 12h (adjusted for renal function) and tigecycline (50mg IV every 12h). No new fever was reported. Once 10 days of effective treatment was completed, tigecycline was discontinued. Magnetic resonance imaging of the leg did not report fluid collections but did find muscle inflammation.
Due to an adequate clinical evolution, absence of new fever and resolution of the systemic inflammatory response, with modulation of basic pathologies, it was decided to end antibiotic management. The patient received 42 days of carbapenem and 27 days of polymyxin B. At outpatient follow-up, he remained stable at, with no new episodes of systemic inflammatory response or fever, with improvement in limb pain.
DiscussionClassically pyomyositis is considered a pathology of tropical areas, predominantly in children, young adults and healthy population. However, there were cases in non-tropical areas5 in association with immunosuppressive conditions such as HIV infection, diabetes mellitus, organ transplantation, malignancy, chemotherapy or rheumatologic diseases. This non-tropical form has been called by some authors “temperate” pyomyositis.6
Thus, in primary pyomyositis, the geographical context of presentation, tropical versus non-tropical, defines two distinct pathological entities in terms of epidemiology, pathogenesis of infection, risk factors and etiological agents.
The exact incidence and prevalence is not known, since the information in the literature is restricted to case series and because it is a little known entity, there is delay in diagnosis and treatment. In warm tropical regions, it accounts for between 1% and 4% of hospital admissions.1,7,8 It is more frequent in healthy males with a male to female ratio of 2.5:11,9 and although it appears in all age groups, the tropical form presents incidence peaks between the ages 2 and 5 years and between 35 and 40.7,9
In countries with temperate climates, such as Australia, an annual incidence of 0.5 cases per 100,000 people over the age of 22 is estimated.10 Most cases occur in adults and, of these, 60% are immunosuppressed.7
The most frequent areas of involvement are the large muscles of the lower limbs, especially the femoral quadriceps, gluteal and trunk muscles, usually the iliopsoas.9,10 It affects multiple muscles in 12–43% of tropical cases and in temperate climates up to 60%.6 Mortality varies from 0.5 to 2%7 but in a case series in India, mortality was reported at 16%.3 Cultures of abscess secretions are positive only in 21–41% and blood cultures are positive between 16 and 38%.11
In 90% of the cases in tropical areas9 and in 75% of temperate areas,7S. aureus is the causative etiological agent, detected in cultures of purulent secretion and blood cultures. The molecular characterization of S. aureus in a tropical case series in the Amazonian region of Peru showed that 90% were carriers of Panton-Valentine leukocidin genes, of which almost half belonged to the multilocus sequence type 25.8 The second causative agent is group A streptococcus in 1–5% of cases. Mycobacteria, fungi and anaerobes such as Salmonella, Vibrio, Enterococcus, and Stenotrofomona spp. are anecdotal in case reports. In the non-tropical form, Gram-negative bacteria have a higher incidence associated with underlying diseases. Staphylococcus spp., Bartonela spp. and Salmonella spp. are more common in HIV patients.
In Nigeria, Dr. Chiedozi described and documented in 1979 three progressive stages in the natural history of pyomyositis in its tropical form4:
- -
“Invasive” stage: The initial, with 2% of the patients in the Chiedozi series classifying in this stage. It starts with acute pain in the affected muscle, followed by a week of edema, increased pain, and low grade fever (nine out of ten). Inflammatory signs are usually scarce, with induration of the affected region (all cases). The symptoms are accompanied by leukocytosis and mild eosinophilia. Needle puncture does not usually obtain purulent material.
- -
“Suppurative” stage: This stage develops between 10 and 21 days of onset. The inflammatory signs are evident, with swelling, allodynia and hypersensitivity. Fever and leukoctosis are usually substantial, associated with eosinophilia of more than 10%. The majority of punctures in this case evidence purulent secretion. More than 90% of the cases in the Chiedozi series were found at this stage.
- -
“Late” stage: Final stage of infection that does not autoresolve if not treated. It involves a critically ill patient. Severely painful fluctuating swelling in the affected area, substantially high fever, bacteremia, shock, altered state of consciousness. Five percent of patients in the Chiedozi series present at this stage, with the only death in that series being in this group.
On the other hand, in the non-tropical form, the development by stages is not clear, the clinical presentation can be varied and its differentiation with cellulitis or as complication of cellulitis can be difficult. For example, in a case of cellulitis complicated by gastrocnemial pyomyositis caused by Pasteurella multocida from a cat bite, the skin infection lasted for more than a month without treatment at the time of diagnosis of pyomyositis.12 In another case, pyomyositis in the quadriceps and iliopsoas caused by S. aureus isolated from blood and resistant to methicillin debuted clinically by mimicking a cellulite of the underlying skin, presenting a rapid onset (less than a week). In a reported case of cellulitis complicated by left sternoclavicular arthritis and pyomyositis of the right sternocleidomastoid muscle by Streptococcus agalactiae, the clinical presentation associated with cellulitis was the predominant one, that is, pain, rubor, and warmth.13
In addition, in the non-tropical form many cases may be associated to a loss of skin barrier. A series of cases of patients diagnosed with pyomyositis by ultrasound, in Buenos Aires (non-tropical region), reported that of 32 cases, 25 were associated with a loss of soft tissue integrity, 19 cases (60%) due to cutaneous pathologies and 6 cases (19%) associated with recent hospitalization with puncture site in the region of pyomyositis.14
In the case presented here, the presence of pyomyositis was diagnosed in the context of an initial suspicion of cellulitis. It is not entirely clear whether or not the patient had a cutaneous infection, and if he did, if this was the cause or consequence of pyomyositis. The patient presented classic clinical skin infection, but as has been seen, pyomyositis can clinically debut as cellulitis.15 Similarly, if only pyomyositis was developed, in context of the patient's geographical place of origin, a hot region in low latitude, it should in principle be considered of the tropical type, although the isolated microorganism is not frequent in this form of pyomyositis. Additionally, this does not fit into any of the classical stages of pyomyositis as described by Dr. Chiedozi,4 because there was marked leukocytosis in the first eight days of the disease, and the abscess zone was not yet clinically evident despite the marked systemic inflammatory response. Cases of primary pyomyositis caused by K. pneumoniae that have been reported have been associated to an immunosuppressive factor such as diabetes mellitus and most are in non-tropical areas.16–19
It should be noted that in areas located in low latitudes, such as Colombia, altitude determines drastically temperature,20 being both tropical and temperate, generally less than 2300 meters above sea level areas considered as truly “tropical”. The patient came from an area at sea level, so he presented the geographic and epidemiological context (age and sex) to present with the tropical form. However, the presentation of pyomyositis in tropical areas should not rule out the causes that have been described in non-tropical areas, including potentially immunosuppressive pathologies. By refusal of the patient, HIV infection could not be ruled out. However, it was possible to rule out upper gastrointestinal, pulmonary and abdominal neoplastic pathology.
With respect to the diagnostic approach, simple radiography is a tool with little usefulness for the diagnosis of primary pyomyositis. It has a possible advantage in ruling out pathologies such as primary bone sarcoma or subacute osteomyelitis that may clinically mimic pyomyositis.11 On the other hand, given its high availability in relation to its acceptable diagnostic performance, computed tomography and ultrasonography are configured as the main diagnostic tools in pyomyositis, in addition to being used as guides for the drainage of muscular abscesses. Computerized tomography scans better delineate muscle mass and allow the visualization of abscesses. Ultrasound could eventually differentiate between cellulitis and pyomyositis, since pyomyositis, besides being a complication of cellulitis, could be a differential diagnosis.21 The great limitation of ultrasonography and tomography, as in the present case, is its low sensitivity in the early stages of infection and in children.11
If, in the presented case, it is considered that the patient was admitted in the early stage of pyomyositis, this case shows that ultrasound loses sensitivity at this stage because it was not able to discriminate topographically the origin of the abscess. It is possible that the tomography would have had a greater diagnostic value. The delay of the tomography could have delayed the aggressive surgical management that was necessary in this case.
The imaging exam by excellence in pyomyositis is the image by magnetic resonance, more precisely detailing the inflammatory lesions, as well as the abscesses. Magnetic resonance is more sensitive than the tomography and the ultrasound in the initial phase of the disease.22 Likewise, magnetic resonance allows differentiation with certainty between pyomyositis and necrotizing fasciitis.23
Finally, as a hypothesis derived from this case, it is proposed the tophaceous gout with severe intramuscular involvement as a pathogenic element in muscle infection. In both the imaging and surgical washes, it was evident that abscessed lesions were associated with intramuscular crystals and tophi. Chronic rubbing trauma may predispose entry sites for the implantation of bacterium, either by bacteremia, as suspected, or by continuity of infection in adjacent tissues. A case of intramuscular tophi mimicking clinical and imaging abscesses in the vastus medialis and intermedius muscles during a non-infectious gouty crisis has been described.24 Regarding the joint involvement of gout, the concomitance of urate crystals with septic arthritis has been evidenced, albeit with a low frequency.25 Likewise, it must be taken into account that in the tophaceous gout crises procalcitonin rises significantly.26 We found no literature associating tophaceous disease with pyomyositis.
ConclusionsDespite the classical differentiation of pyomyositis into tropical and non-tropical forms, the coexistence of both infectious sites in tropical regions should always be considered. It should not be forgotten that the clinical presentation can be atypical especially in the non-tropical form, being able to simulate the cellulitis. Thus the clinical suspicion of pyomyositis should be increased in cases of patients with lower limb cellulitis that do not improve despite adequate empirical management. Cases developed in non-tropical areas are associated with serious diseases with associated immunosuppression but also to traumatic events. Given the evidence of coexistence of septic arthritis with gouty arthritis, with this case we propose that severe tophaceous disease with intramuscular involvement could predispose to pyomyositis by the effect of chronic friction, resulting in implantation of microorganisms in areas affected by trauma, continuity of infection or by bacteremia.
Regarding the clinical case presented, we do not have finally microbiological and molecular tests to confirm the production of carbapenemics of the isolated bacteria.
Declaration of transparencyThe authors state that the clinical case presented is a complete and honest presentation, that the essential details of the course have not been omitted and that all limitations have been exposed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo direct funding was received.
Conflict of interestsAuthors have no conflict of interest to declare.
We thank the staff and administrative personnel of the Hospital de Suba, in Bogota for their assistance.