Several studies have suggested a role for the vitamin B group in bone physiology. This article discusses a systematic review of the literature on the interaction of vitamin B with homocysteine and their relationship with bone metabolism and osteoporosis. Some studies have suggested that low levels of vitamin-B, particularly B12 and B9, have been associated with a low bone mineral density and an increased risk of fracture, in addition to participating in the metabolism of homocysteine; therefore, the deficit of these vitamins may lead to hyperhomocysteinemia. Recent publications suggest that hyperhomocysteinemia is associated with bone demineralization, low quality of bone mass, and increased levels of bone turnover biomarkers, since it affects the osteoclastic activity and the cross-linking of collagen molecules. Therefore, hyperhomocysteinemia could play a role in reduced bone density and quality. Further information is needed to establish whether each vitamin directly impacts bone health, or if their influence is merely through the homocysteine serum concentrations.
Se han propuesto varios estudios que sugieren que el grupo de vitaminas B posee un rol en la fisiología ósea. Se realizó una revisión bibliográfica sobre la interacción de este con la homocisteína y la relación de ambos con el metabolismo óseo y la osteoporosis. Algunos estudios han sugerido que los niveles de vitamina B, sobre todo las vitaminas B12 y B9, se han asociado a una baja densitometría ósea y a un aumentado riesgo a fractura; y que estos, a su vez, intervienen en el metabolismo de la homocisteína; por lo que, su déficit puede ocasionar un estado de hiperhomocisteinemia. Publicaciones recientes proponen que la hiperhomocisteinemia se encuentra asociada a desmineralización ósea, baja calidad de masa ósea y aumento de biomarcadores de recambio óseo, dado que influye en la actividad osteoclástica y en los enlaces cruzados de colágeno. Por lo tanto, la hiperhomocisteinemia puede ser un factor que reduce la densidad y la calidad ósea. Se necesita más información para determinar el papel que tiene cada vitamina directamente en la salud ósea, o si estas solo influyen a través de las concentraciones séricas de homocisteína.
Osteoporosis is a systemic skeletal disease, characterized by progressive bone demineralization and damaged microarchitecture, leading to a higher risk of fracture.1 The diagnosis of osteoporosis is based on the DXA T-score for bone mineral density (BMD) of the femoral neck and the spine, defined with a value ≤ 2.5 standard deviations of a young adult woman. There are various factors contributing to the risk of fracture, including age, gender, body mass index (BMI), previous fragility fractures, glucocorticoid therapy, smoking, and use of alcohol,2 in addition to modifiable risk factors such as weight, physical activity, use of medications, and nutrient deficiency that may accelerate bone loss and increase bone fragility.1,3 Biomarkers such as procollagen type 1 N-terminal pro-peptide, (P1NP) and C-terminal telopeptide of collagen type 1 (CTX) are markers of bone formation and bone resorption, with prognostic significance for fracture.2
The specific FRAX® per country uses many of these risk factors, together with BMD measured with dual energy X-ray absorptiometry to estimate the probability of fracture in 10 years. If the BMD is not available, it is possible to use FRAX® without the BMD value.2
Calcium and vitamin D have been broadly studied as essential nutrients in bone physiology; however, several reviews have reported that other substances may also play important physiological roles in promoting bone health, for instance vitamin B and homocysteine.4,5 It has been shown that hyperhomocysteinemia increases the risk of fractures, but its effects are less significant in BMD. A lot of reports attribute its adverse effects on bone quality to bone resorption and alteration in collagen cross-linking.5,6
There is a growing number of publications associating the high levels of homocysteine to low vitamin B concentration in adults with osteoporosis. Further research is needed about the mechanisms participating in bone health, in order to suggest new prevention approaches for this disease. The intent of this literature review is to offer a comprehensive view of the scientific information on the topic, for a better understanding of the relationship between these factors.
MethodologySearch strategyA thorough bibliography search using PubMed, Cochrane and Google Scholar databases was conducted, with a view to identify as many published trials as possible, about the relationship between vitamin B12 and levels of homocysteine in bone metabolism. The Medical Subject Headings used were: Folate, Vitamin B9, Cobalamin, Vitamin B12, Vitamin B, Riboflavin, Vitamin B6, Osteoporosis, Bone health, Homocysteine in humans and in vitro studies. PubMed was used as advanced search technique to identify the most recent articles and also the most referenced. The last systematized review was conducted in October 2018. The selection of titles was conducted in two steps; the first one was based on the title and content of the abstract; the second step was the selection of the full text of the article, with no time constraints.
Selection of studies and data collectionAll articles on the role of vitamin B and homocysteine were reviewed in full text. The selection languages were Spanish, English and Portuguese. The data were collected by a researcher and reviewed by other 2 specialists and included: primary author, journal, year of publication, country of origin, type and design of the study, number of patients included, inclusion and exclusion criteria, demographic information, associations and results.
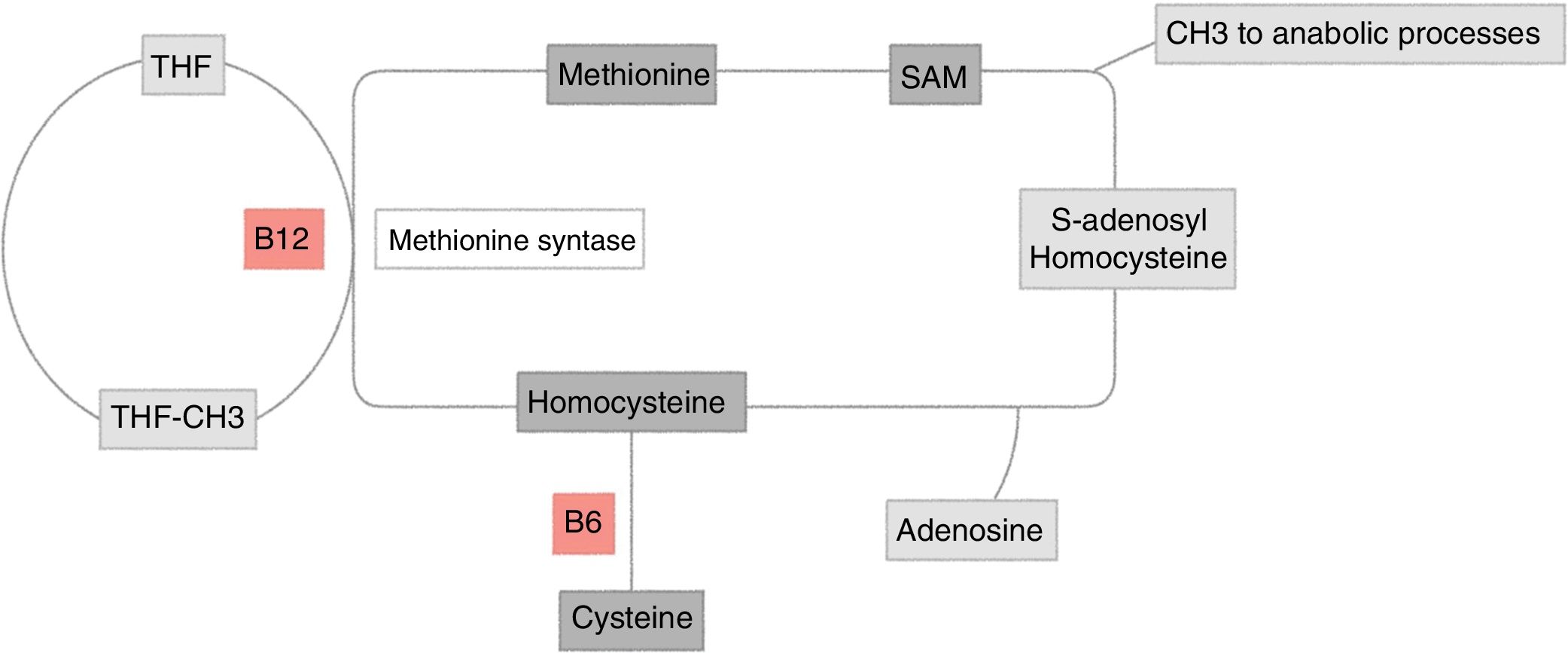
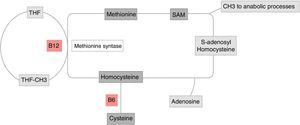
Homocysteine metabolismHomocysteine is a sulphur containing amino acid, derived from the metabolism of methionine. It may be cleared through two pathways (Fig. 1):
Homocysteine metabolism. Methionine re-methylation pathway: essential amino acid obtained through food intake. Its reaction is catalyzed by methionine synthetase, which is vitamin B12 dependent and where vitamin B9 is the donor of the methyl group. This reaction is regulated by S-adenosylmethionine (SAM) as allosteric inhibitor. The second pathway of homocysteine transsulfuration is regulated by the activation of SAM and depends on vitamin B6 to obtain cysteine as the final result.
THF: tetrahydrofolate.
Original figure developed by Maldonado.
The methionine remethylation pathway: this reaction is catalyzed in the liver by methionine synthetase, which requires vitamin B9, donor of the methyl-tetrahydrofolate (MTHF) group, the active and circulating form of folic acid, which depends on vitamin B12 as cofactor. There is an alternate pathway to this reaction through betaine, which gives out a methyl group for the homocysteine methylation, a reaction catalyzed by betaine homocysteine methyltransferase.5
Transsulfuration pathway: homocysteine is transformed into cystathionine and then into cysteine by the cystathionine b-synthetase enzyme, which requires pyridoxal-5′-phosfate, one of the most active forms of vitamin B6.5
These two pathways are coordinated by S-adenosylmethionine (SAM), acting as an allosteric inhibitor of the methylenetetrahydrofolate reductase reaction and as activator of the cystathionine beta-synthetase5,6 (Fig. 1).
Folate (vitamin B9), and vitamins B12 (cobalamin), B6 (pyridoxine) and B2 (riboflavin) play a key role in the metabolism of homocysteine7,8; consequently, plasma homocysteine increases with low levels of vitamin B.
Therefore, homocysteine may be used as a functional biomarker – though unspecific – of the levels of vitamin B.
The definition of hyperhomocysteinemia varies among the different studies7; however, a consensus has been reached defining it as a medical condition with plasma levels above 15 μmol/l8. The normal homocysteine plasma concentration is classified as follows:
- •
Normal: 0−15 μmol/l measured with high-performance liquid chromatography, or 5−12 μmol/l by immunohistochemistry.
- •
Moderate: 16−30 μmol/l.
- •
Intermediate: 31−100 μmol/l.
- •
Severe hyperhomocysteinemia or syndrome: ≥ 100 μmol/l.
There are two types of hyperhomocysteinemia:
- •
Common causes: environmental factors, nutritional deficiencies (folate, vitamin B6 and B12), thyroid dysfunction, cancer, psoriasis, diabetes mellitus, alcohol abuse, drugs, coffee and elevated creatinine levels.7–9
- •
Genetic causes: Methylenetetrahydrofolate Reductase Polymorphism (MTHFR), an enzyme that regulates homocysteine metabolism6,10,11 or cystathionine b-synthetase deficit, a rare entity associated with intellectual disability, atherosclerotic cerebral infarction, and osteoporosis.6
It has been shown that plasma homocysteine levels >15 μmol/l may double the risk of developing dementia, Alzheimer’s disease, chronic non-communicable diseases, and cardiocerebrovascular risk.10,11 The relationship of homocysteine with cardiovascular risk has been well studied over the last few years, showing that elevated homocysteine levels results in endothelial damage, reduces the flexibility of the blood vessels, and alters homeostasis, thus generating a risk factor for acute myocardial infarction and cerebrovascular accidents.
Homocysteine and bone metabolismin vitro studies have shown that hyperhomocysteinemia may modulate bone remodeling, mainly by increasing osteoclast activity and differentiation,12–14 inducing bone marrow apoptosis of the mesenchymal stem cells,15–17 osteocytes18 and osteoblasts,19 and to a lesser extent, inhibiting osteoblast differentiation20; these effects are suggested to be the consequence of an intracellular increase of reactive oxygen species that contribute to bone resorption.13,14,21 Moreover, hyperhomocysteinemia has been associated with disorders in bone irrigation, directly affecting the extracellular matrix; likewise, hyperhomocysteinemia binds directly to the extracellular matrix, disorganizing collagen cross-linking, with a negative impact on bone strength.14,22–24
Herrmann et al. analyzed the effect of elevated homocysteine levels on osteoclast activity, using as a reference 4 homocysteine levels (0, 10, 50 and 100 μmol/l) after 20 days of culture of osteoclasts. The authors observed an increase in the tartrate resistant acid phosphatase (TRAP) function by about 20% in the 10 μmol/l group, 15% in the 50 μmol/l group and 42% in the 100 μmol/l group, in addition to increased resorptive activity at homocysteine levels between 50−100 μmol/l.13
Hyperhomocysteinemia and bone mineral densityThe data about the relationship between plasma homocysteine levels and BMD are sparse. In a study with Croatian women between 45 and 65 years old, no significant correlation was found between homocysteine, folate or vitamin B12 and BMD of the skeletal sites measured5; these data were replicated by other authors.25–28 Cagnacci et al., observed in their study that the homocysteine levels were not associated with BMD and only folate was independently associated with BMD (r = 0.254, p < 0.011). However, when BMD was stratified by serum folate quartiles, a progressive BMD increase was documented, from the lowest to the highest quartile (1.025 ± 0.03 g/cm2 vs. 1.15 ± 0.03 g/cm2, p < 0.01); and also, the higher the folate level, the higher the level of vitamin B12, and the lower the level of homocysteinemia.26
Several studies have identified a significant and negative correlation between homocysteinemia and BMD. Gram Gjesdal et al. concluded that hyperhomocysteinemia and low folate levels are significantly associated with a decline in BMD in women (p < 0.001) but not in men, suggesting that these may be modifiable risk factors for osteoporosis.29 Ouzzif et al. also showed that homocysteinemia levels were significantly higher in the osteoporotic group (p = 0.017) and that they were inversely related to BMD in the lumbar spine and total hip.30 These data have been validated in other populations,31–36 confirming hyperhomocysteinemia as an independent risk factor for osteoporosis.
Hyperhomocysteinemia and risk of fracturesHyperhomocysteinemia has been suggested as a treatable risk factor for osteoporotic fractures. In 2 cohort studies, the relative risk of homocysteine levels adjusted for age and BMI and hip fracture were calculated, reporting that homocysteinemia is associated with a 1.9 increased fracture risk (95% confidence interval [95% CI]: 1.4–2.6).25,37 Another prospective trial showed a positive association between plasma homocysteine and the risk of fracture in both genders, with a 2.42 risk for women (95% CI: 1.43–4.09) and 1.37 for males (95% CI: 0.63–2.98); in addition to a negative association between serum folate and the risk of fracture only in females.38 Moreover, Kuroda et al. in 2013 analyzed the risk factors for severe vertebral fracture, observing by multiple regression that the levels of homocysteine are a significant risk (OR = 1.27, 95% CI 1.04–1.58, p = 0.021) for moderate to severe fractures, when comparing grade 0 fractures against grade 3 fractures.27
The constant association of hyperhomocysteinemia with the risk of fracture, which is not present with BMD, may indicate the effect of homocysteine on bone structure and quality, hence suggesting the basis for the impact of plasma homocysteine on the risk of fracture. Few studies have dwelled on the relationship of homocysteine levels and bone turnover markers. The Amsterdam longitudinal aging study observed a significant association between elevated serum homocysteine levels (>15 μmol/l) and low vitamin B12 levels (<200 pg/mL), with elevated concentrations of bone turnover such as urinary deoxypyridinoline (DPD) and serum osteocalcin (OC) in elderly women, and that the relative risk for fractures in these patients is 3.8 (95% CI: 1.2–11.6).39 The results by Herrmann et al. showed a significant correlation between homocysteine and DPD (p = 0.022), but not between homocysteine and OC, or osteoprotegerin (OPG) in peri and postmenopausal women.40
An interesting study conducted by Gerdhem et al. analyzed the relationship between homocysteine levels and bone turnover markers, BMD and risk of fracture. Their results evidenced that women in the highest quartile of homocysteinemia had higher CTX levels (p < 0.001), DPD corrected for urinary creatinine, OC and parathormone (PTH). With regards to BMD, an inversely proportional relationship was observed, which was no longer significant when adjusting the results for known risk factors. Finally, the results of other trials25,27,37,38 on the risk of fracture due to hyperhomocysteinemia were not replicated.41 A similar trial was able to relate hyperhomocysteinemia with osteoporotic fractures, as well as its positive correlation with the CTX levels, without identifying an association with BMD.42 Similar data were obtained by Álvarez-Sánchez et al., showing that homocysteinemia has an independent and positive relationship with CTX (B = 0.22; 95% CI: 0.09−0.34; p = 0.001), PTH and PINP (B = 0.24; 95% CI: 0.09−0.39; p = 0.002).43
In 2017 Vijayan and Gupta induced hyperhomocysteinemia in mice to observe the pathogenesis in the cortical bone, showing that homocysteine affected the mineral density of the tissues and led to lacunar mineralization. The effect is mediated by osteocytes through the abnormal expression of mineralization genes such as DMP1 and SOST which induce apoptosis, affecting the bone’s long term biomechanical stability.44
Kuroda et al. analyzed the risk of fractures in Japanese patients with vitamin B, D and K deficiency, and showed that notwithstanding the fact that the Japanese have the longest life expectancy in the world, it is believed that diet plays a key role in the risk of fractures. However, it is believed that there are several confounding factors, including osteoporosis treatment. Consequently, these authors made adjustments for the potential confounding factors and showed that the number of deficiencies was significantly associated with the risk of fracture (risk ration 1.25; 95% CI: 1.04–1.50; p = 0.018).45
Hyperhomocysteinemia treatment: does it reduce the risk of fractures?The hyperhomocysteinemia treatment data for the reduction of the risk of fracture are limited. The largest study on this topic is the Women’s Antioxidant and Folic Acid Cardiovascular Study, conducted by Bassk et al., which studied the effects of folic acid supplementation (2.5 mg/day), vitamin B6 (50 mg/day) and vitamin B12 (1 mg/day), and the reduction of the risk of fracture in women with pre-existing cardiovascular disease or with 3 or more coronary risk factors. The patients were followed for 7.3 years and the authors failed to find a significant effect between supplementation and reduced risk of fracture (risk ratio = 1.08; 95% CI: 0.88–1.34).46
Stone et al. conducted an ancillary trial based on the Women’s Antioxidant and Folic Acid Cardiovascular Study, showing that there were no significant effects of vitamins B6 and B12 on the risk of fracture between women with elevated baseline homocysteine plasma levels or low levels of vitamins B12 or B6, or folate. Furthermore, treatment with vitamins B6 and B12 had no impact on changes in bone turnover markers. No evidence was found that daily vitamin B supplementation reduces the risk of fracture or the rates of bone metabolism in middle-aged and elderly women with high risk or fracture, or the rates of bone metabolism in middle-aged and elderly women, with a high risk of cardiovascular disease.47
López et al., in a meta-analysis, analyzed the data of a polyp prevention study with folic acid and acetylsalicylic acid (AFPPS) and an updated meta-analysis of randomized controlled trials (RCT). The objective of the study was to show the possible association between homocysteine-lowering vitamin B and the risk of fracture. The AFPPS trial was conducted between 1994 and 2004 in 9 US clinical centers and 1021 participants were randomized to a daily dose of 1 mg folic acid (n = 516) or placebo (n = 505).48 The end point was any type of fracture. The risk of hip fracture was also analyzed. The AFPPS trial did not find any statistically significant association between folic acid therapy and the risk of any type of fractures (risk ratio [RR] = 0.95; 95% CI: 0.61–1.48) or hip fracture (RR = 0.98; 95% CI: 0.25–3.89). The meta-analysis included 6 RCTs with a total of 36,527 participants. For the interventions including folic acid or vitamin B12, the combined RR for treatment was 0.97 (95% CI: 0.87–1.09) for any type of fractures (n = 1199) and 1.00 (95% CI: 0.81–1.23) for hip fractures (n = 335). In conclusion, no association was found between homocysteine-lowering treatment with vitamin B (folic acid and vitamin B12) and the risk of fracture.49
Vitamin BIt has been found that patients with pernicious anemia have a significant increase in the risk of fractures and osteoporosis (p < 0.05).50 The group of vitamin B is an important factor for the homocysteine metabolism and vitamin B12 and folic acid supplementation has proven to be effective in normalizing homocysteine levels. Deficiencies in any of these vitamins will lead to increased homocysteine.51,52 It is also believed that they may have an impact on osteoblastic activity and bone formation.1,50 For all of these reasons, several observational and interventional studies have examined the relationship between the group of vitamin B BMD, risk of fracture and bone resorption markers.
A study conducted in patients with celiac disease, showed that vitamin B12 is a significant predictor for BMD in males, but not in females, since vitamin B12 was only significantly correlated with the BMD of the femur neck (p = 0.011) and total hip (p = 0.049).53 In 2004, Stone et al. observed that in their study there was not a significant trend in BMD changes through the serum vitamin B12 quintiles. However, they showed that the participants with serum vitamin B12 levels <280 pg/mL experienced an annual drop of 1.6% (95% CI: –2.4% to –0.8%) in total hip BMD; a more accelerated decline as compared to the 0.2% participants with levels >280 pg/mL (–0.5% to 0.2%) (p = 0.003).54
Three trials conducted between 2009 and 2013 found a positive correlation between the levels of serum vitamin B12 and BMD of the femoral neck and the lumbosacral spine, but failed to identify the same correlation with folate levels35,36,55; these results have been ratified by 3 different trials conducted in different populations.54,56–58 Other studies showed the negative association of the methylmalonic acid, a functional indicator of the level of vitamin B12 with BMD in different populations (p < 0.01).58–60 Two studies reported a positive correlation between serum folate and BMD of the spine and femur, but not with vitamin B12 (p < 0.02).26,34 A higher average BMD loss of the femoral neck was however observed in the participants with low concentrations of pyridoxine (p < 0.01),61 and a meta-analysis failed to identify any correlation between any of the vitamins B and BMD.51 Although the mechanisms whereby vitamin B12 participates in osteoporosis has not been fully elucidated, several in vitro studies have suggested that the osteoclast activity is stimulated by a cobalamin deficiency.21,52
Yazdanpanah et al. investigated the influence of a dietary intake of cobalamin, folate, pyridoxine and riboflavin on BMD and the risk of fracture, observing that riboflavin and pyridoxine were strong predictors for BMD of the femur neck (p < 0.002), and that only pyridoxine, as a continuous variable, was inversely associated to the risk of non-vertebral (p = 0.005) and fragility fractures (p = 0.0004).62 Later on, this same author in a paper on genetic characterization of the MTHFR homozygous suggested that riboflavin may modify the risk of fractures in homozygous for the T allele of MTHFR 677 T, since the patients in the lower quartile of riboflavin have a 1.8 increased (95% CI: 1.1–2.9, p = 0.01) risk of osteoporotic fractures and 2.6 (95% CI: 1.3–5.1, p = 0.01) higher risk of fragility fractures, as compared to the patients with the 677-CC genotype (p = 0.0002).63
A prospective trial published in 2013 that also assessed the dietary intake of vitamins B and the association with hip fracture over a 13.8-year follow-up, identified an inverse correlation between the intake of pyridoxine and the risk of hip fracture in women, but not in men (p = 0.002), whilst no relationship could be found with the intake of other types of vitamin B. Compared against the women in the lower quartile of pyridoxine intake (0.37-0.61 mg/1000 kcal/day), the women in the higher quartile (0.78–1.76 mg/1000 kcal/day) have a 22% lower risk of hip fracture (HR: 0.78; 95% CI: 0.66−0.93).64 Other studies have suggested that the presence of low serum levels of vitamin B12 have a 2-fold higher relative risk of fractures in women, and if it is associated with hyperhomocysteinemia, the relative risk is 3-fold higher for both men and women.39,61 McLean et al. also associated the risk of hip fracture to pyridoxine deficiency (p < 0.05).61 In contrast, Gjesdal et al. observed that women in the lowest folate category had a higher risk of hip fracture, with an adjusted HR of 2.40 for lower (<2.9 ng/mL) vs. higher (>6.6 ng/mL) folate concentrations, while no relationship was found between the risk of fracture and vitamin B12.38
A meta-analysis conducted by Van Wijngaarden et al. in 2013, which included most of the articles referenced, observed that a 50 pg/mL increase in vitamin B12 tends to reduce the risk of fracture by 4%, which is within a significant limit (RR = 0.96; 95% CI: 0.92–1.00); in contrast, the relationship between folate and the risk of fracture was heterogenous for the different trials analyzed.51
Several RCTs have been conducted to prove whether the intervention with daily supplementation of vitamin B impacts the risk of fracture. The doses of folic acid used have been between 2–2.5 mg/day, vitamin B6 between 25−50 mg/day, vitamin B12 0.5−1 mg/day, with a follow-up between 3.4–7.3 years, without identifying any effect of vitamin B on the risk of fracture or bone resorption markers65,66; these data have been ratified in the meta-analysis by Chen et al. of the RCTs on the effect of vitamin B supplementation on fractures and bone resorption markers.67
Three RCTs have been conducted between 2006 and 2013 to determine whether vitamin B supplementation affects the levels of bone turnover biomarkers.68–70 The first only compared the doses of folic acid 0.4 mg, 1 mg, 5 mg and placebo for 2 months. The second one supplemented with doses of folic acid of 2.5 mg of folic acid, 0.5 mg of vitamin B12, and 25 mg of B6 for one year. The last study compared the levels of bone turnover biomarkers when supplementing with 1.200 IU of vitamin D3, 0.5 mg of folic acid, 0,5 mg of B12, 50 mg of B6 and 456 mg of calcium carbonate vs. vitamin D3 vs. calcium carbonate alone, for one year. The first two trials did not show any changes in the levels of bone turnover markers such as DPD, CTX, OC and PINP.68,69 In the last study, the levels of biomarkers in both groups decreased, which indicates that the reduction is the result of the influence of vitamin D3 and calcium on PTH.61 These results are consistent with the findings by other authors in their trials, showing that supplementation is only able to reduce the plasma levels of homocysteine.65,67,71
Holstein et al. conducted a study in mice to observe the impact of vitamin B12 and folate deficiency on bone regeneration, following a fracture; the first group received a diet deficient in vitamin B12 and folate, while the control group received a balanced caloric diet. The levels of vitamin B12, B9, homocysteine and osteocalcin were measured after 4 weeks, and the bone callous formation was analyzed, but no differences were found in these measurements between the two groups, or in the tissue composition and callous formation. However, hyperhomocysteinemia was identified in the group with the vitamin B deficient diet.72
ConclusionThere is enough information showing the correlation between hyperhomocysteinemia and the risk of fracture, and its association with a poor BMD and increased bone turnover biomarkers. However, although apparently there is a protective effect of the levels of vitamin B on the risk of fracture, the attempts to show the use of vitamin B supplementation to reduce bone fragility have been in vain, and apparently the only benefit is to moderately reduce the levels of homocysteinemia, but with no clear idea about the subsequent bone benefits.
There are no conclusive studies suggesting a unique relationship between vitamin B and bone health, but probably it has to do with its intervention on homocysteine metabolism and its impact on plasma levels; it has been suggested that the high homocysteine plasma levels may be associated with a higher risk of osteoporosis. The likelihood of delaying the onset and the progression of bone mineral loss when changing risk factors, such as vitamin deficiency and increased homocysteine levels, is not ruled out; these factors should be further investigated, since they may play a role in the comprehensive management of bone health.
Conflict of interestsThe authors have no conflict of interest to disclose.
Please cite this article as: Narváez J, Maldonado G, Intriago M, Cárdenas J, Guerrero R, Luis Neyro J, et al. Rol de la homocisteína y vitamina B en el metabolismo óseo. Rev Colomb Reumatol. 2020;27:278–285.