The activation of T cells is initiated by the presentation of exogenous or endogenous antigens, by antigen presenting cells through the major histocompatibility complex, which binds to a special receptor on T cells. This recognition triggers a cascade of intracellular signaling that leads to an increase in integrin expression, cytoskeletal modifications, and transcription factors production involved in the liberation of cytokines and inflammatory mediators. One of the most important inducers in cell activation is the enzymatic complex with tyrosine kinase action. The kinases which belong to the SRC (SFK) LCK and FYN family have been involved in a large number of important processes in the activation and modulation of the T cells response, as well as in the development of autoimmune diseases. Regulating the kinases signaling, as well as the adapter proteins involved in T cell activation, is essential for maintaining an activation threshold, as well as the modulation of cell response. The phosphorylation of the positive regulation sites of these proteins is important to allow an active configuration of the protein and thereby its maximum capacity as kinase. The phosphorylation of negative regulation sites leads to a closed configuration of the protein that reduces its kinase function, and thereby inhibits its own function. The alteration in signaling by the modification of certain cytoplasmic proteins in some cases is associated with the development of autoimmune diseases, such as systemic lupus erythematosus. Under physiological conditions the T cell receptor complex regroups with protein complexes that interact harmonically to generate an internal signal. The altered signaling events are partly responsible for an anomalous expression of cytokines, including the interleukin-6 (IL-6), IL-10, IL-2, IFN, and CD40 linking, these modifications affect the cells ability to over-stimulate T and B cells, resulting in an increased production of autoantibodies and the triggering of the autoimmune disease.
La activación de los linfocitos T se inicia a través de la presentación de antígenos endógenos o exógenos por células presentadoras de antígenos a través del complejo mayor de histocompatibilidad, el cual se une a un receptor especializado presente en los linfocitos T. Este reconocimiento desencadena una cascada de señalización intracelular que conlleva a un aumento en la expresión de integrinas, modificaciones del citoesqueleto y producción de factores de transcripción involucrados en la liberación de citocinas y mediadores inflamatorios. Uno de los inductores más importantes en la activación celular es el complejo enzimático con acción tirosina cinasa. Las cinasas que pertenecen a la familia SRC (SFK), FYN y LCK están involucradas en un gran número de procesos importantes en la activación, modulación de la respuesta linfocitaria y el desarrollo de enfermedades autoinmunes. La regulación de la señalización de las cinasas, así como de proteínas adaptadoras involucradas en la activación del linfocito T, son fundamentales para mantener el umbral de activación y modulación de la respuesta del linfocito. La fosforilación de sitios de regulación positiva de estas proteínas es importante para permitir una configuración activa de la proteína y de esta forma su máxima capacidad como cinasa. La fosforilación de los sitios de regulación negativa conlleva a una configuración cerrada de la proteína de tal forma que reduce su función de cinasa e inhibe su función. Las alteraciones en la señalización por modificación de algunas proteínas citoplasmáticas se asocian en algunos casos al desarrollo de enfermedades autoinmunes, como el lupus eritematoso sistémico. En condiciones fisiológicas, el complejo receptor de linfocitos T se reagrupa con complejos proteicos que interactúan armónicamente para generar una señal interna. Los eventos de señalización alterados son en parte los responsables de una expresión anómala de citocinas, entre ellas la interleucina-6 (IL-6), IL-10, IL-2, IFN y CD40 ligando; estas modificaciones alteran la capacidad de los linfocitos T para sobre estimular a los linfocitos B, traduciéndose en un aumento en la producción de autoanticuerpos y en el desencadenamiento de la enfermedad autoinmune.
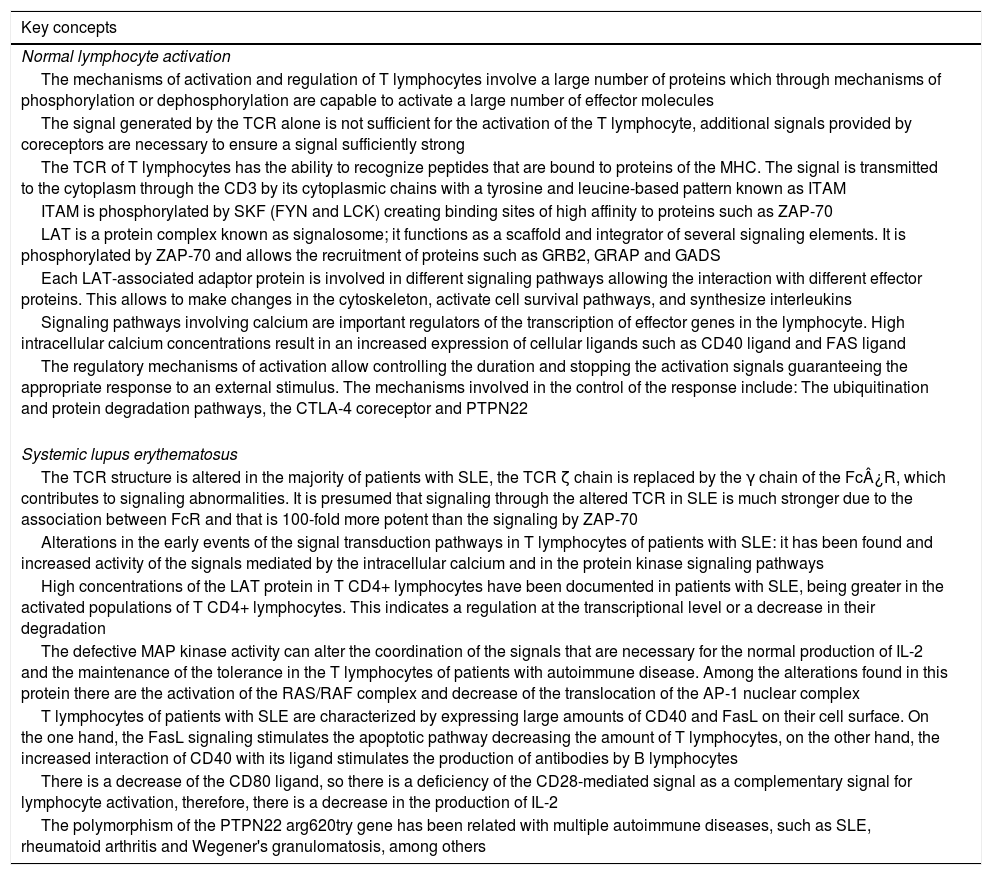
The mechanisms of activation and regulation of T lymphocytes involve a cascade of internal signaling events in which a large number of proteins play a relevant role, inducing in some cases phosphorylation and activation of tyrosine kinases, which leads to cellular activation with release of cytokines and other soluble factors. An alteration in the proteins involved in the signaling events may result in the loss of their effector mechanism, which would mean a change in the activation of the cell. The functional changes induced as a consequence of these alterations generate different behaviors in the T and B lymphocytes, producing, in some cases, overexpression of inducing proteins and an increase in the synthesis of antibodies. This review aims to include the most important proteins and markers involved in internal signaling events in T lymphocytes, as well as modifications in the expression of some of them induced by mutations or by external factors that trigger autoimmune processes such as systemic lupus erythematosus (SLE) (Table 1).
Relationship between the normal lymphocyte activation and systemic lupus erythematosus.
| Key concepts |
|---|
| Normal lymphocyte activation |
| The mechanisms of activation and regulation of T lymphocytes involve a large number of proteins which through mechanisms of phosphorylation or dephosphorylation are capable to activate a large number of effector molecules |
| The signal generated by the TCR alone is not sufficient for the activation of the T lymphocyte, additional signals provided by coreceptors are necessary to ensure a signal sufficiently strong |
| The TCR of T lymphocytes has the ability to recognize peptides that are bound to proteins of the MHC. The signal is transmitted to the cytoplasm through the CD3 by its cytoplasmic chains with a tyrosine and leucine-based pattern known as ITAM |
| ITAM is phosphorylated by SKF (FYN and LCK) creating binding sites of high affinity to proteins such as ZAP-70 |
| LAT is a protein complex known as signalosome; it functions as a scaffold and integrator of several signaling elements. It is phosphorylated by ZAP-70 and allows the recruitment of proteins such as GRB2, GRAP and GADS |
| Each LAT-associated adaptor protein is involved in different signaling pathways allowing the interaction with different effector proteins. This allows to make changes in the cytoskeleton, activate cell survival pathways, and synthesize interleukins |
| Signaling pathways involving calcium are important regulators of the transcription of effector genes in the lymphocyte. High intracellular calcium concentrations result in an increased expression of cellular ligands such as CD40 ligand and FAS ligand |
| The regulatory mechanisms of activation allow controlling the duration and stopping the activation signals guaranteeing the appropriate response to an external stimulus. The mechanisms involved in the control of the response include: The ubiquitination and protein degradation pathways, the CTLA-4 coreceptor and PTPN22 |
| Systemic lupus erythematosus |
| The TCR structure is altered in the majority of patients with SLE, the TCR ζ chain is replaced by the γ chain of the Fc¿R, which contributes to signaling abnormalities. It is presumed that signaling through the altered TCR in SLE is much stronger due to the association between FcR and that is 100-fold more potent than the signaling by ZAP-70 |
| Alterations in the early events of the signal transduction pathways in T lymphocytes of patients with SLE: it has been found and increased activity of the signals mediated by the intracellular calcium and in the protein kinase signaling pathways |
| High concentrations of the LAT protein in T CD4+ lymphocytes have been documented in patients with SLE, being greater in the activated populations of T CD4+ lymphocytes. This indicates a regulation at the transcriptional level or a decrease in their degradation |
| The defective MAP kinase activity can alter the coordination of the signals that are necessary for the normal production of IL-2 and the maintenance of the tolerance in the T lymphocytes of patients with autoimmune disease. Among the alterations found in this protein there are the activation of the RAS/RAF complex and decrease of the translocation of the AP-1 nuclear complex |
| T lymphocytes of patients with SLE are characterized by expressing large amounts of CD40 and FasL on their cell surface. On the one hand, the FasL signaling stimulates the apoptotic pathway decreasing the amount of T lymphocytes, on the other hand, the increased interaction of CD40 with its ligand stimulates the production of antibodies by B lymphocytes |
| There is a decrease of the CD80 ligand, so there is a deficiency of the CD28-mediated signal as a complementary signal for lymphocyte activation, therefore, there is a decrease in the production of IL-2 |
| The polymorphism of the PTPN22 arg620try gene has been related with multiple autoimmune diseases, such as SLE, rheumatoid arthritis and Wegener's granulomatosis, among others |
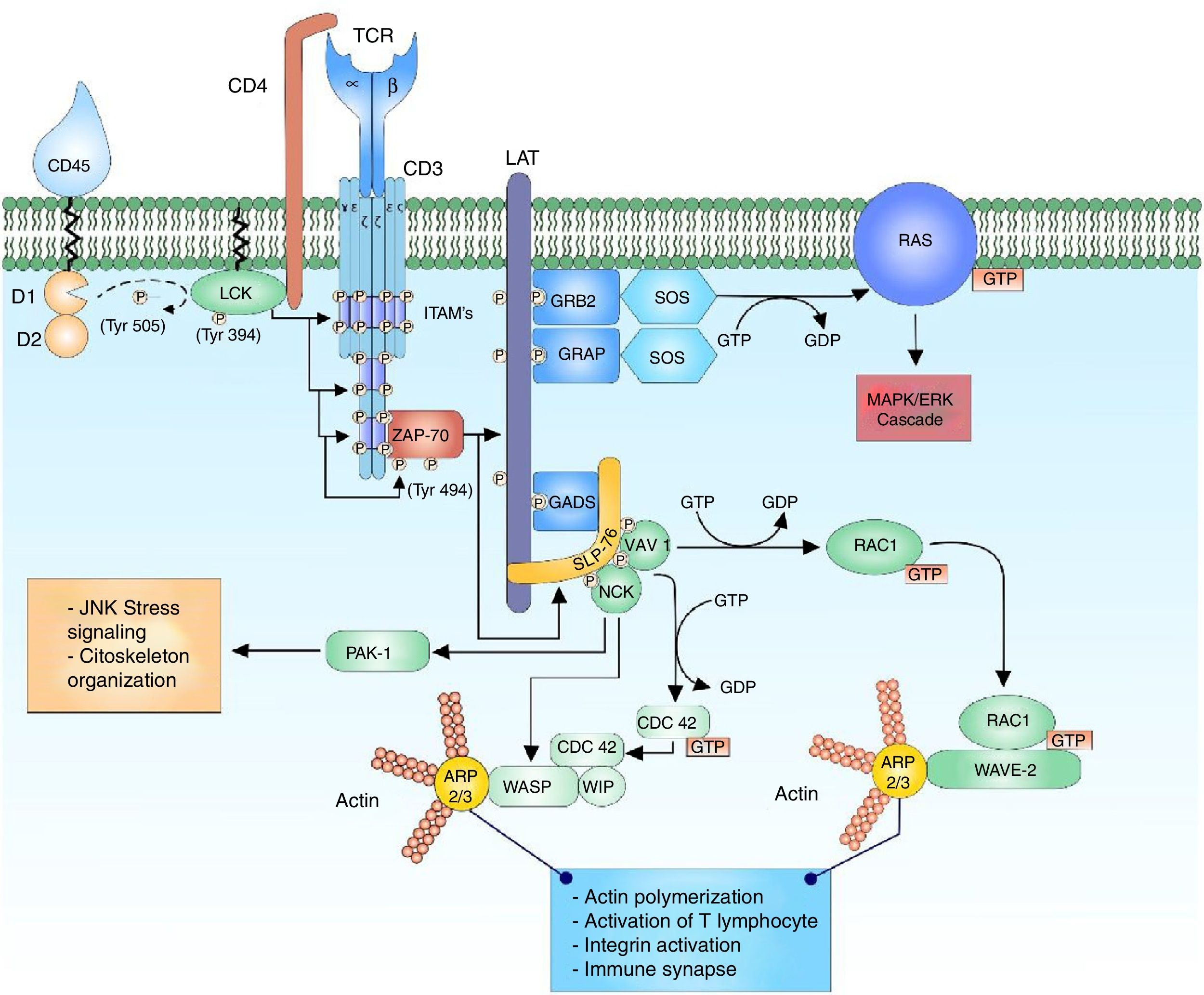
The T-cell receptor (TCR) is a heterodimer composed of an alpha chain and a beta chain that shares structural similarity with immunoglobulins, having a variable domain and a constant domain. Both chains are linked through disulfide bridges at an end close to the cell membrane. The variable domain consists of amino acid sequences encoded by variable (V), diverse (D) and junction (J) gene segments. The D segment is found only in the segments that encode for the receptor beta chain.1,2 This reorganization of segments in the variable region allows the formation of a site for antigen recognition. The TCR of T lymphocytes has the ability to recognize peptides that are bound to the major histocompatibility complex, expressed on the surface of antigen-presenting cells. The alpha and beta chains of the TCR have short cytoplasmic domains, which do not participate in the intracellular signal because they do not present phosphorylation sites, are attached to the chains of the cluster of differentiation 3 (CD3), conformed by the heterodimeric chains CD3 δ¿, γ¿ and the homodimer ζζ of the TCR.1,3–5 The alpha and beta chains of the TCR can transmit the signal after the binding of a peptide linked to the major histocompatibility antigen by means of the CD3 chains when interacting with the cytoplasmic effector molecules1,6 (Fig. 1).
Binding of the TCR and pMHC complex. The binding of the TCR and the MHC-associated peptide generates the first cytoplasmic signal for the lymphocyte activation through the CD3 co-receptor. The LCK kinase of the family of the SRC kinases is bound to the CD4-coreceptor, which facilitates the phosphorylation of the tyrosine residues of the ITAM pattern sequences of the heterodimeric chains CD3 δ¿, γ¿ and the homodimer ζζ of the TCR, allowing the recruitment and activation of ZAP-70. LCK activates ZAP-70 by the phosphorylation of its regulatory domain, allowing its complete function as a kinase.
The binding of the peptide major histocompatibility complex (pMHC) and the TCR originates a primary signal that by itself is not able to generate the activation of the lymphocytes, since it needs other secondary signals.7,8 The binding of co-receptors such as the cluster of differentiation 4 (CD4), the cluster of differentiation 28 (CD28), the lymphocyte function associated antigen (LFA-1), or the cluster of differentiation 2 (CD2) with molecules from antigen presenting cells generates a secondary signal sufficient for the activation and modulation of the response threshold.7–11
The works of Tsokos et al. have evidenced alterations in the early events of the signal transduction pathways in autoreactive T cells of patients with SLE, making emphasis in the calcium-dependent signaling pathway. Experimental designs in which anti-CD3 monoclonal antibodies are used to stimulate T lymphocytes have demonstrated an increase in the activity of protein tyrosine kinases and in the intracellular calcium levels in autoreactive T lymphocytes of patients with SLE, but not in autoreactive T lymphocytes of patients with mixed connective tissue disease (MCTD) or in control T lymphocytes.12
Other groups have reported other types of alterations in the intracellular signaling pathways in T cells of patients with SLE, in the internal signaling based on the antigenic recognition by TCR and in the signal transduction in the T lymphocytes. The expression of the TCR ζ chains is decreased or absent in the majority of T cells of patients with SLE, but not in those who have other autoimmune diseases.13 This abnormality is specific to the disease and independent of the activity and treatment, therefore, it represents a potential intrinsic defect in the pathogenesis of SLE.14,15
ITAM sequences and function in the lymphocyte activationThe binding between TCR and pMHC generates the first cytoplasmic signal for lymphocyte activation through the CD3 co-receptor, by means of its cytoplasmic chains with a tyrosine and leucine-based pattern which forms a consensus sequence of “YxxI/Lx (6–8) YxxI/L” (tyrosine and leucine repeats every 6–8 amino acids).3,5,16,17 This sequence pattern known as immuno receptor tyrosine-based activation motif (ITAM) is phosphorylated by kinases in its tyrosine residues, creating specific binding sites for other enzymes which will allow to transmit and amplify the signal.16,17 The proteins responsible for the phosphorylation of tyrosine residues in the ITAM sequences are part of the kinases of the SRC family (proto-oncogene tyrosine-protein kinase SRC) and the kinases of the SFK family (SRC family kinases).17–19
Activation and regulation of the kinases of the SRC, FYN and LCK familiesThe kinases of the SRC family, FYN proto-oncogene, Src family tyrosine kinase (FYN family) and leukocyte C-terminal Src kinase (LCK family) are involved in a large number of processes related to the activation and modulation of the lymphocyte response. Both FYN and LCK share domains similar to other kinases of the SRC family, such as the homologous regions SRC homology 3 (SRC3 or SH3), homologous regions SRC homology 2 (SRC2 or SH2), tyrosine kinase domains SRC homology 1 (SH1), N-terminal region of addition of saturated fatty acids and a C-terminal domain for negative regulation.20,21 SFKs are positively regulated by the phosphorylation of tyrosine residues in their catabolic site, which promotes a conformational change of the protein and thus its maximum kinase activity, and its negative regulation by the phosphorylation of the tyrosine residues in its carboxy-terminal region predisposes the closed conformation of the protein and a decrease in its kinase activity.19,20 The LCK kinase is attached to the CD4 transmembrane co-receptor by non-covalent bounds, when the interaction pHMC with the TCR occurs, LCK is recruited by its association with CD4.19 The CD4 co-receptor binds the invariable domain of the pHMC facilitating the process of antigenic recognition with the TCR and causing the autophosphorylation or transphosphorylation of LCK in its active region (Y394).20 A very important regulator in the activation and regulation of the kinase activity of LCK is CD45.7 The cluster of differentiation 45 (CD45) is a transmembrane protein with 2 cytoplasmic domains, D1 and D2. Domain 1 has enzymatic activity of phosphatase and domain 2, although its function is not completely known, may be involved in the regulation of the substrates with which D1 interacts.7,22 The domain 1 acts in the dephosphorylation of the inhibitory tyrosine residue (Y505) in the carboxyl-terminal region of LCK allowing complete kinase activity.22
Cytoplasmic signaling of the T-cell receptorThe recruiting of the CD4 co-receptor allows the proximity of LCK kinase to its substrates in the chains of the CD3 co-receptor; and in this way, the phosphorylation of the ITAMs in their tyrosine residues. The ζ subunit of the TCR modulates the signal in the TCR complex by its contribution of 6 ITAM for TCR.3,16 Both FYN and LCK have the possibility to phosphorylate the tyrosine residues of the ITAMs, however, both have different distribution. LCK has greater affinity for the ITAM residues of the CD3 chain and the TCR, while FYN participates in the interaction with cytoskeletal mediators, especially the focal adhesion kinase (FAK).16,17,21,22 Why are there so many ITAMs per TCR complex? (10 per each complex); although the exact answer is not known, it has been proposed that this amount of ITAMs could provide the ability to amplify the signal received by each TCR complex, increasing the sensitivity of the response by the incorporation of 6 specific phosphorylation sites in the ζ chains of the TCR and, in addition, facilitate the cytokine expression.16,18,23–25 The exact mechanism by which the ITAM residues are phosphorylated is not clear either, it is thought that when TCR is inactive, the tyrosine, leucine and isoleucine residues are hidden within the transmembrane region of the T lymphocyte and when the TCR is activated, a structural change of the CD3 chains is generated, allowing the uncovering of the sequences to be phosphorylated by the kinases.3,19
The phosphorylation of 2 tyrosine residues of the ITAM sequences of the CD3 chains acts as binding sites of high affinity to proteins that contain tandem SH2 domains, such as the zeta-chain (TCR) associated protein kinase 70kda (ZAP-70) and spleen tyrosine kinase (SYK) proteins, members of the SYK family kinases.3,5,23,26 ZAP-70 binds to the ITAM tyrosine residues that have been phosphorylated by LCK through its SH2 domains, being close to LCK to be phosphorylated at its tyrosine residue (Y493); this allows its activation as a kinase and thus its autophosphorylation (or transphosphorylation) to develop its maximum activity.18,19
Although the structure and biochemical function of SYK and ZAP-70 are similar, there is a great difference in their expression; this is due to the expression pattern of ZAP-70, which is restricted to T and natural killer lymphocytes, whereas SYK can be found in B lymphocytes, macrophages, monocytes, mast cells and platelets.21 The active ZAP-70 phosphorylates the linker for activation of T cell (LAT) and the lymphocyte cytosolic protein 2 (SH2 domain containing leukocyte protein of 76kDa) (SLP-76), which transmit and diversify the activation signal16 (Fig. 1).
Stabilizer of activated T cells signalosomeThe signalosome is a protein complex that is composed of several signaling elements that are associated and regulated by the activity of scaffold proteins.27 LAT is the example of this class of proteins that recruit other proteins to form a large protein complex that facilitates the signaling of T lymphocytes.19 LAT is a transmembrane protein whose expression is limited to hematopoietic cells including T lymphocytes, natural killers, platelets and mast cells. It is composed of a short extracellular domain, a transmembrane domain and a long cytoplasmic domain that contains tyrosine residues.28 LAT does not have enzymatic activity and acts as a molecular adapter for a large number of proteins, it coordinates the recruitment of protein intermediaries and effector enzymes; however, LAT is not essential for the activation of T lymphocytes, but it plays a more important role in the modulation of lymphocyte activation by the activation of proteins that act in the negative regulation of the response.5,28 LAT is rapidly phosphorylated by ZAP-70 after the activation of the TCR, generating multiple high-affinity binding sites for proteins with SH2 domains. The LAT binding adaptor proteins are known as the family of proteins growth factor receptor bound protein 2 (GRB2), of which the GRB2, GRB2 related adapter protein (GRAP) and GRB2-related adaptor downstream of Shc (GADS) proteins are part. These adaptors share a similar structure consisting of two SH3 domains and one SH2 domain. Through their SH2 domains they bind the phosphorylated residues of LAT and allow the binding of other proteins by their exposed SH3 domains.17,18 These proteins bound to LAT amplify the activation signal and, additionally, the diversification of cellular responses after the activation of the T lymphocyte.
Each adaptor protein associated with LAT is involved in different signaling pathways, allowing the interaction with different effector proteins. The adaptor protein LAT, GRB2 is a junction point of multiple proteins thanks to the SH3 domains, such as the son of sevenless (SOS) protein. Once bound to LAT, GRAP recruits SOS, Dynamin and Src-Associated substrate in Mitosis of 68kDa (SAM68).17,18 GADS differs from the other LAT adaptor proteins by its distribution limited to hematopoietic cells and its SH3 domain specific for interaction with the SLP-76 protein.28,29
SLP-76 is a multidomain adaptor protein which is restricted to hematopoietic cells including T lymphocytes, monocytes/macrophages, natural killers, mast cells and platelets.28,30 It consists of a region rich in tyrosine residues which are phosphorylated by ZAP-70, a central region rich in proline and a carboxy-terminal region with a SH2 domain that interacts with a multidomain adaptor protein SLP-76 associated phosphoprotein/FYN binding protein (SLAP/FYB).17 When ZAP-70 phosphorylates SLP-76 in the tyrosine residues it serves as a binding site to proteins with SH2 domains including Vav 1 guanine nucleotide exchange factor (VAV1), non-catalytic region of tyrosine kinase adaptor protein 1 (NCK) and IL2 inducible T cell kinase (ITK).17,28
The comparative studies of ZAP-70, LAT and SLP-76 proteins conducted by Januchowski et al. in 2007, in patients with SLE and disease free individuals, show a significant increase in the concentration of the LAT protein in T CD4+ lymphocytes of patients with SLE, compared with the control group, being even higher the concentrations in the activated populations of T CD4+ lymphocytes compared with the naïve cells. This indicates an upregulation at the transcriptional level of the protein or a decrease in the proteolitic degradation of the protein.31
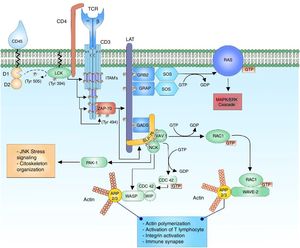
Regulation of the actin cytoskeleton after the activation of the T lymphocyteThe activation of the T lymphocyte requires the regulation of the organization of the actin cytoskeleton to facilitate the immune synapse, allowing the communication with the antigen presenting cells and facilitating different events that involve: differentiation into different cell lineages, migration through the tissues, cellular adherence, cellular reorientation and secretion of cell mediators such as cytokines.32
The reorganization of the cytoskeleton is accompanied by an important number of internal signaling events. The multidomain adaptor protein complex SLP-76 is phosphorylated by ZAP-70 allowing the interaction of proteins specialized in cytoskeleton arrangements such as VAV1 and NCK. VAV1 is a protein specialized in the guanine nucleotide exchange belonging to the Rho GTPase family. The activation of the SLP-76 by ZAP-70 allows the binding of VAV1 to SLP-76 in its phosphorylated domains and this through its DH domain allows the nucleotide exchange from GDP to GTP to the Ras-related C3 botulinum toxin substrate 1 (RAC1) and cell division control protein 42 (CDC42) proteins, activating them and regulating the cytoskeletal changes that will take place in the immune synapse.33–38 The phosphorylated domains of SLP-76 interact with NCK facilitating the recruiting of the Wiskott-Aldrich syndrome protein (WASP) and the p21-activated kinase (PAK1) protein for the cytoskeleton arrangements.33,39 PAK1 is member of the family of kinases Ser/Thr of the RAC1 and CDC42 proteins, and plays an important role in the organization of the cytoskeleton and the activation of the stress signaling pathways, such as the c-Jun N-terminal kinase (JNK) signaling cascade.33
The active configuration of CDC42 modulates the WASP protein and together with WAS/WASL-interacting protein family member 1 (WIP) forms the WIP-WASP complex, which is fundamental for the regulation of actin-related proteins 2/3 (ARP 2/3).36,40 The active configuration of RAC1 activates at the same time the WASP-family verprolin-homologous protein (WAVE2) forming the RAC1-WAVE2 complex that activates the ARP2/3 complex. The ARP2/3 complex interacts with the actin monomers for their polymerization and thus facilitates the organization of the cytoskeleton for the immune synapse33,34,36 (Fig. 1).
WASP is recognized for the Wiskott-Aldrich syndrome, an X chromosome linked inherited immunodeficiency which results from the mutation of the WASP gene, specifically a mutation in the WH1 domain, a region which is required for the protein stabilization through the association with WIP; in the absence of this interaction, WASP is degraded.41
The structure of the TCR is altered in the majority of patients with SLE, the TCR ζ chain is replaced with the γ chain of the Fc¿R, which contributes to signaling abnormalities.42 The signaling through the altered TCR in SLE is presumed to be much stronger due to the association between FcR γ, which is 100-fold more potent than the signaling by ZAP-70.43 The studies conducted by Tsokos showed different molecular associations between the interaction of SYK with FcR γ triggers of the TCR signaling in patients with SLE, finding: (1) increased expression of SYK but not of ZAP-70; (2) greater association of SYK with the actin cytoskeleton compared with ZAP-70; (3) inhibition of SYK normalizing the actin polymerization kinetics, and (4) differences between SYK and ZAP-70, in their patterns of association with key signaling molecules, which generate different signaling profiles induced by the TCR.43
Effector signaling of the T lymphocyteThe binding of the TCR with the peptide bound with the MHC initiates a series of signaling events that prepare the lymphocyte for the cell differentiation, proliferation and effector function. The signaling pathways that lead to the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), the activator protein 1 (AP-1) factor and the nuclear factor of activated T-cells (NFAT), are crucial for the expression of effector molecules required for the lymphocyte function.
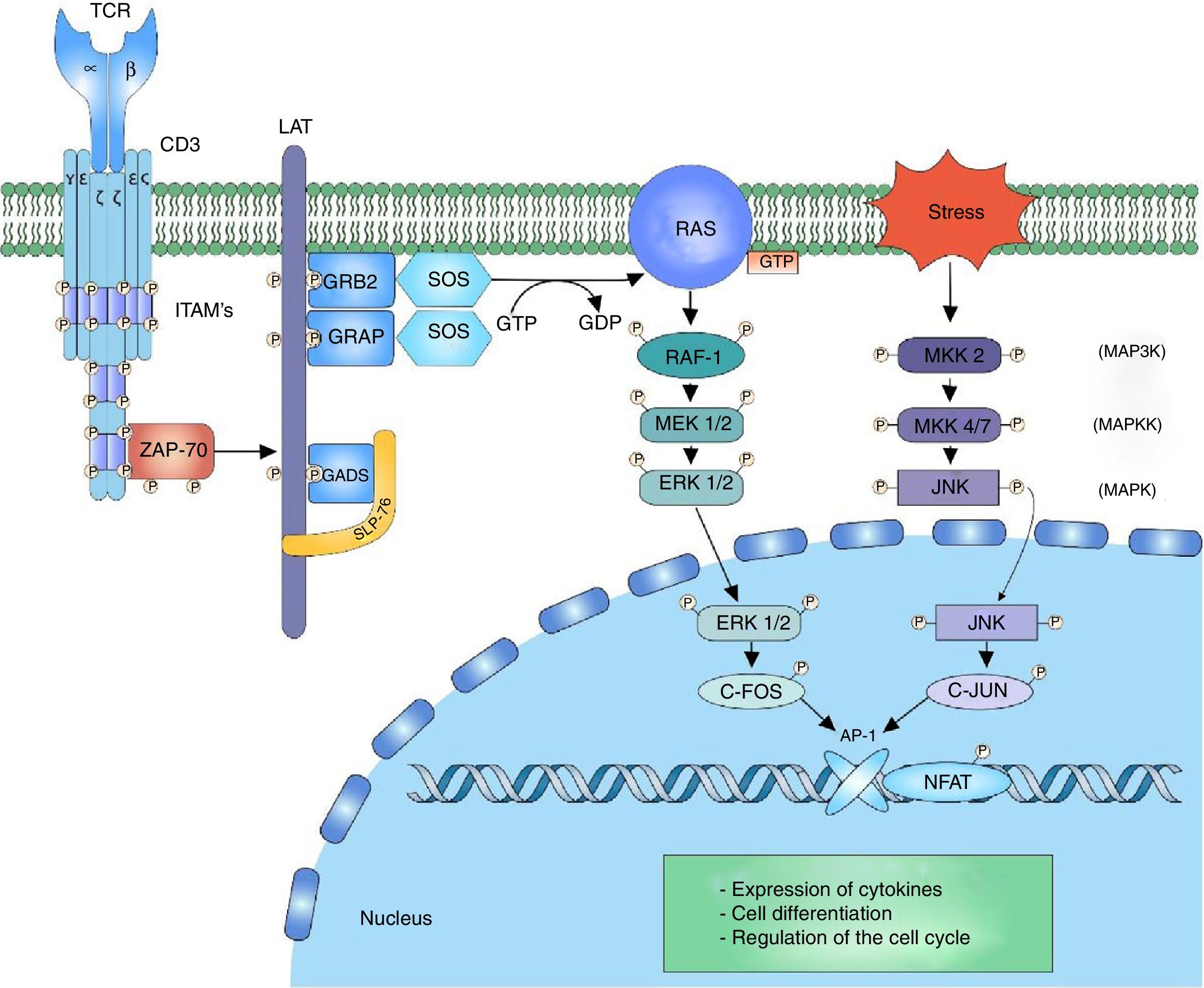
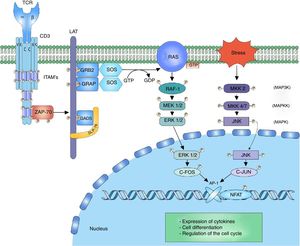
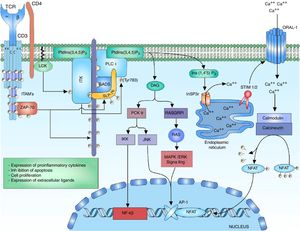
ERK-c-Fos signalingThe mitogen activated protein kinase (MAPK) cascade is a signaling way for cellular processes such as differentiation, cell growth, proliferation, mobility and response to stress. The signaling is developed thanks to a nucleus of 3 kinases (MAP3K, MAPKK and MAPK) which allow the activation of effector components (MAP kinase activated protein kinase (MAPK-APK).44 The cascade is propagated thanks to the sequential activation of the kinases allowing the phosphorylation of regulatory components. The signaling of extracellular-signal-regulated kinases (ERK1/2) begins after the phosphorylation of LAT and the binding of GRB2 and GADS proteins, which act as adaptors for the recruitment to the membrane of the SOS nucleotide exchange factor for the activation of RAS.7,45,46 RAS bound to the GTP recruits and activates the first RAF-1 kinase (MAP3K), which activates MEK1/2 (MAPKK) allowing the transmission of the signaling to the ERK1/2 (MAPK) kinases. The signaling continues in the MAPK-APK complexes or in other substrates that are found both in the cytoplasm and in the nucleus; however, the phosphorylation of ERK1/2 of the c-Fos nuclear factor is important to prevent its degradation44 (Fig. 2).
MAP kinase cascade: the MAP kinase cascade begins after the phosphorylation of the LAT signalosome by ZAP-70 for the recruitment of the adaptor proteins to LAT, GRB2 and GRAP. GRB2 and GRAP, which recruit the SOS nucleotide exchange factor, which exchanges GDP for GTP to the RAS protein, a G protein. RAS allows the activation of the first kinase of the MAP kinase RAF-1 signaling. RAF-1 phosphorylates MEK1/2 activating it and thus allowing the transmission of the signaling to the ERK1/2 kinases. The activated ERK1/2 phosphorylates the c-fos nuclear factor, preventing its degradation and allowing its c-jun interaction.
The JNK cascade, also called stress-activated protein kinase cascade (SAPKs),44 involves different signaling pathways responsible for the cell response to stress stimuli. The stress stimulus is perceived by the cell and the signaling is transmitted to the CDC42 and RAC1 GTPases, which will activate the MAPK signaling, either directly on the MEKK2 (MAP3K) kinases or indirectly through the MAP4K kinases, which in turn activate the MAP3K kinases.47 The activation of MAP3K transmits the signal through the phosphorylation to MKK4/7 (MAPKK), activating the JNK1/2/3 (MAPK) kinases. The function of the three isoforms of JNK is the phosphorylation of c-Jun inhibiting its degradation and allowing the interaction with other transcription factors.47 The activation of the c-Fos factor along with the activation of the c-Jun factor form the AP-1 factor, a heterodimeric factor that interacts with NFAT for the synthesis of interleukin (IL)-2 and is involved in the cell survival48 (Fig. 2).
The studies on alterations in the signaling pathways in T lymphocytes of patients with SLE have shown defects in the activation of the MAP kinase cascade in response to the signaling given by the TCR/CD3 complex. The defects are related with activation of the RAS/RAF complex and decrease of the translocation of the AP-1 nuclear complex; likewise, it has been demonstrated a decrease in the binding of SOS to GRB2 in T lymphocytes of patients with SLE.49. The defective MAP kinase activity can alter the coordination of the signals that are necessary for the normal production of IL-2 and the maintenance of the tolerance in the T lymphocytes of patients with autoimmune disease.
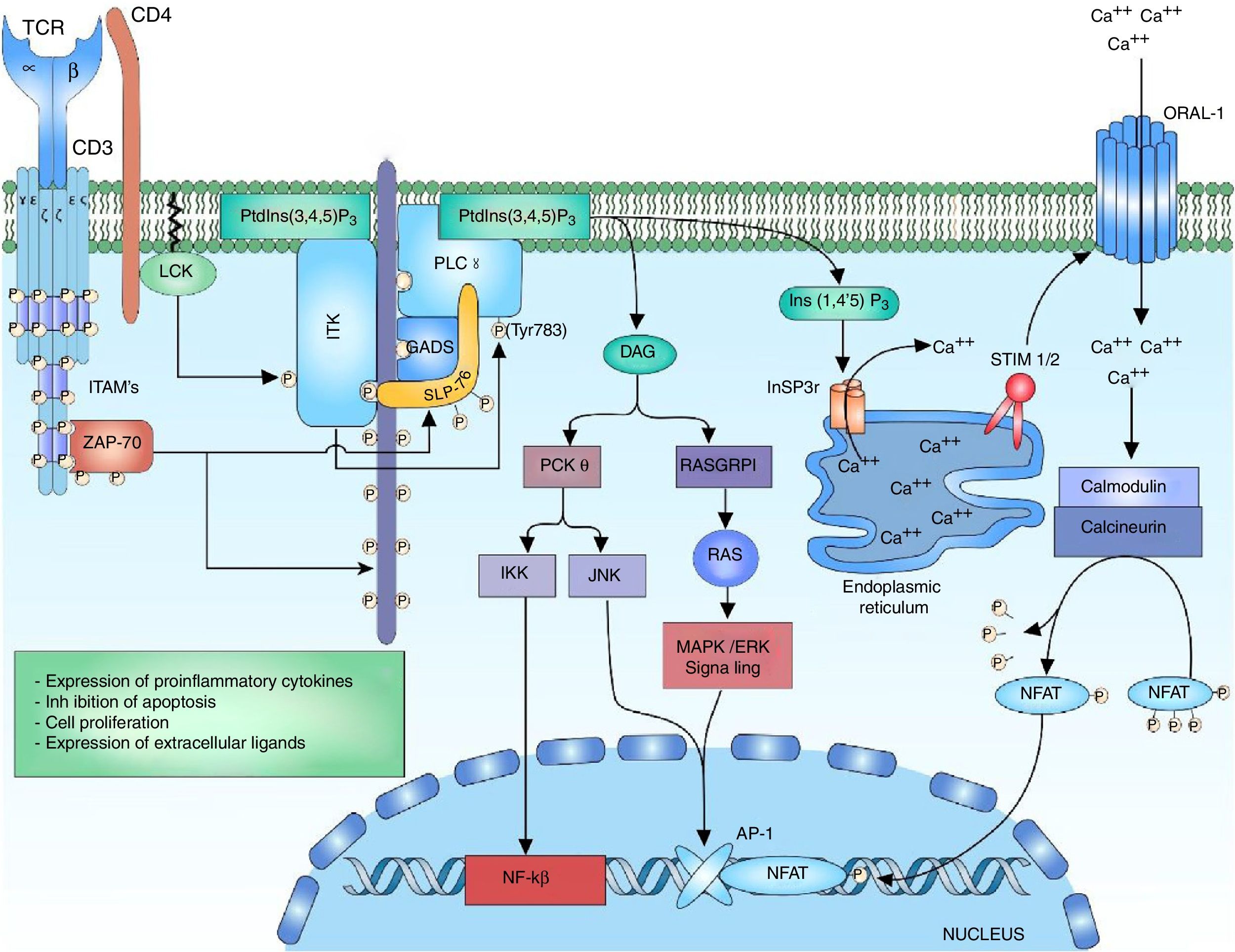
Signaling of phospholipase C and the nuclear factor of activated T cellsThe ITK enzyme is part of the tecprotein tyrosine kinase (TEC) family, the ITK domains include the SH2 and SH3 domains for the proteic interactions and the pleckstrin-homology (PH) binding domain to PtdIns (3,4,5) P3 (phosphatidylinositol (3,4,5)-trisphosphate), which is necessary for its activation.36,50 The activation of ITK requires several related steps, the first one is the recruiting and the binding of the ITK protein to the cell membrane through its PH domain to the PtdIns (3,4,5) P3. PtdIns (3,4,5) P3 is the product of the phosphorylation of PtdIns (4,5) P2 (phosphatidylinositol 4,5-bisphosphate) by the phosphatidylinositol 3-kinase (PI3K), the phosphatase and tensin homologue (PTEN) and the SH2-domain-containing inositol-5-phospatase (SHIP) regulate the breakage of the phosphate of the PtdIns (3,4,5) P3 to form PtdIns (4,5) P2, thus regulating the lymphocyte signal.50–54 The second step is the presence of a kinase of the SRC family for the phosphorylation of the ITK-PtdIns (3,4,5) complex for its activation. Within the SRC family that interacts with ITK is found the LCK kinase, which is necessary for its activation.55 The third step consists in the interaction of ITK with proteins involved in the signaling of TCR such s the LAT-GADS-SLP-76 complex.52,55 The activation of the ITK enzyme allows the amplification and diversification of the signal through the phosphorylation and activation of the enzyme phosphoinositol phospholipase C gamma 1 (PLCγ1). PLCγ1 regulates the metabolism of the inositol phospholipids through the hydrolysis of the PtdIns (4,5) P2 for the formation of inositol 1,4,5-triphosphate (InsP3) and diacylglycerol (DAG). There are two forms of the protein, PLCγ1 and PLCγ2, predominating the form PLCγ1 in T lymphocytes56,57 (Fig. 3).
Activation of the TCR complex: after the activation of the TCR, the phosphorylation of the LAT residues allows the recruitment of the anchoring protein to LAT GADS; this one is phosphorylated by ZAP-70 allowing the binding to SLP-76, which in the same way is phosphorylated by ZAP-70. The phosphorylation of the residues of the multidomain protein SLP-76 allows the binding of ITK kinase. For the activation of ITK kinase is necessary the anchor of ITK to the cell membrane by means of its PH domain to the PtdIns (3,4,5) P3, the phosphorylation of the ITK tyrosine residues by the LCK kinase of the family of SRC kinases for the activation. Interaction of ITK with other proteins such as SLP-76, GADS and LAT. The active ITK phosphorylates the enzyme PLCγ1, which regulates the metabolism of inositol phospholipids through the hydrolysis of the PtdIns (4,5) P2 forming DAG and InsP3.
The diacylglycerol activates the protein kinase C theta (PKCθ), which is a serine/kinase member of the subfamily of the calcium-independent PKCs, whose expression is limited to T lymphocytes, muscle cells and platelets.58 It has been demonstrated in studies in vitro with Jurkat T cells that LCK phosphorylates PKC-θ in its C2 domain, which is a tyrosine domain (Y90); however, it has not been demonstrated that this phosphorylation is involved in changes of the enzyme activity or in its regulation, but mutations in tyrosine 90 (change from tyrosine to phenylalanine) have demonstrated alterations in the activation of NFAT and AP-1.59 Once PLCy1 is activated and there is a release of DAG, the DAG binds to the C1 domain of PKC-θ, changing its conformation to a open status of its active site and allowing the phosphorylation of its substrates.58
PKC-θ is involved in the activation of 2 transcription factors that are important for the activation of the IL-2 gene, which are AP-1 and NFκB. The activation of the AP-1 transcription factor by PKC-θ is achieved through the interaction with Ste20/SPS1 related proline and alanine rich kinase (SPAK), a kinase of the Ste-20 family involved in the MAP kinase signaling.60 The activation of NFκB can be induced by the phosphorylation of CARD11/CARMA (caspase recruitment domain-containing protein 11) by PKC-θ, releasing the IκB kinase (IKK) complex that interacts in the degradation mediated by ubiquitination of the inhibitor of kappa B (IκB), thus releasing NFκB for its translocation to the nucleus.61 The translocation of NFκB to the nucleus allows the transcription of genes involved in the expression of proinflammatory cytokines, expression of cytotoxic molecules, inhibitor of apoptosis proteins and the expression of genes involved in cell proliferation.
DAG acts in the stimulation of the MAPK/ERK pathway, and along with the JNK activates the AP-1 factor. The calcium and the DAG positively regulate the translocation of the RAS guanyl releasing protein 1 (RASGRP1) of the membrane of the Golgi apparatus.19,62 RASGRP1 changes GDP to GTP for the activation of the MAPK/ERK signaling by RAS62,63 (Fig. 3). The regulation of calcium is mediated by channels located both in the endoplasmic reticulum and in the cell membrane. The activation of the TCR rapidly increases the concentrations of InsP3 engaging the InsP3R receptor. Ins (1,4,5) P3 receptor is a glycoprotein bound to the membrane of the endoplasmic reticulum which acts as a calcium channel that releases the calcium stored in the reticulum to the cytoplasm, increasing intracellular concentrations.51,64,65 However, the calcium released from the endoplasmic reticulum is not enough to stimulate the calcium-dependent proteins, and therefore it is necessary its release by other pathways such as the activation of transmembrane channels. The loss of calcium within the endoplasmic reticulum is sensed by STIM1 or STIM2 (stromal interaction molecule) proteins. The STIM proteins are transmembrane proteins located in the endoplasmic reticulum which act as sensors of the calcium levels maintaining the homeostasis within the cell.66 The N-terminal portion of the STIM proteins is located in the lumen of the reticulum and possesses specialized domains to sense small changes in the concentration of calcium within the reticulum. Once the low calcium concentrations are detected, is generated a change from a basal dimeric state into an oligomeric state, which migrates to the plasma membrane and is able to trigger the entry of calcium into the cell through transmembrane calcium channels ORAI (calcium release-activated calcium modulator 1) by domains located in the C-terminal cytoplasmic region.66,67
Calcium within the cell acts as a second messenger for the activation of NFAT. High concentrations of calcium within the cell are crucial for the activation of the intracellular calcium sensor, calmodulin.26 Normally, without the presence of calcium, the protein remains in an inactive or “closed” state and when there is an increase in calcium concentrations, the calcium binds to calmodulin and undergoes a conformational change to an “open” state, allowing its binding to the enzyme calcineurin.68 Calcineurin is a calcium-calmodulin-dependent serine/phosphatase protein that dephosphorylates multiple regions of NFAT, including its DNA recognition domain, thereby allowing the translocation of the transcription factor to the nucleus7,69,70 (Fig. 3).
It has been demonstrated the role of NFAT in the transcription of a large number of cytokines involved in the effector response of the T lymphocyte, such as IL-2, IL-4, IL-10, IFN-gamma, granulocyte–monocyte colony growth stimulating factor (GM-CSF), and TNF. It has also been demonstrated the role that NFAT plays in the expression of IL-5 as in the expression of CD40L and CD95L in T lymphocytes of patients with SLE.70
The calcium as a second messenger of multiple signaling pathways is closely regulated to ensure an optimal cell response to a stimulus; alterations in its concentration involve abnormal signals that have implications in the cellular response. High concentrations of calcium result in an increased expression of cellular ligands, such as the CD40 ligand (CD40L) and the Fas ligand (FasL).12 Studies conducted by Tsokos et al. have demonstrated that T lymphocytes of patients with SLE express high amounts of FasL on their surface, compared with the control groups, explaining the high apoptosis rates that are evidenced in lymphocytes of patients with autoimmune disease.71 It was also evidenced an increased expression of the CD40L on the cell surface of T lymphocytes and an increased expression of CD40 on the surface of B lymphocytes in patients with SLE, thus increasing the CD40–CD40L interaction, which leads to an increased stimulation of B lymphocytes and production of antibodies with important immunological consequences.72
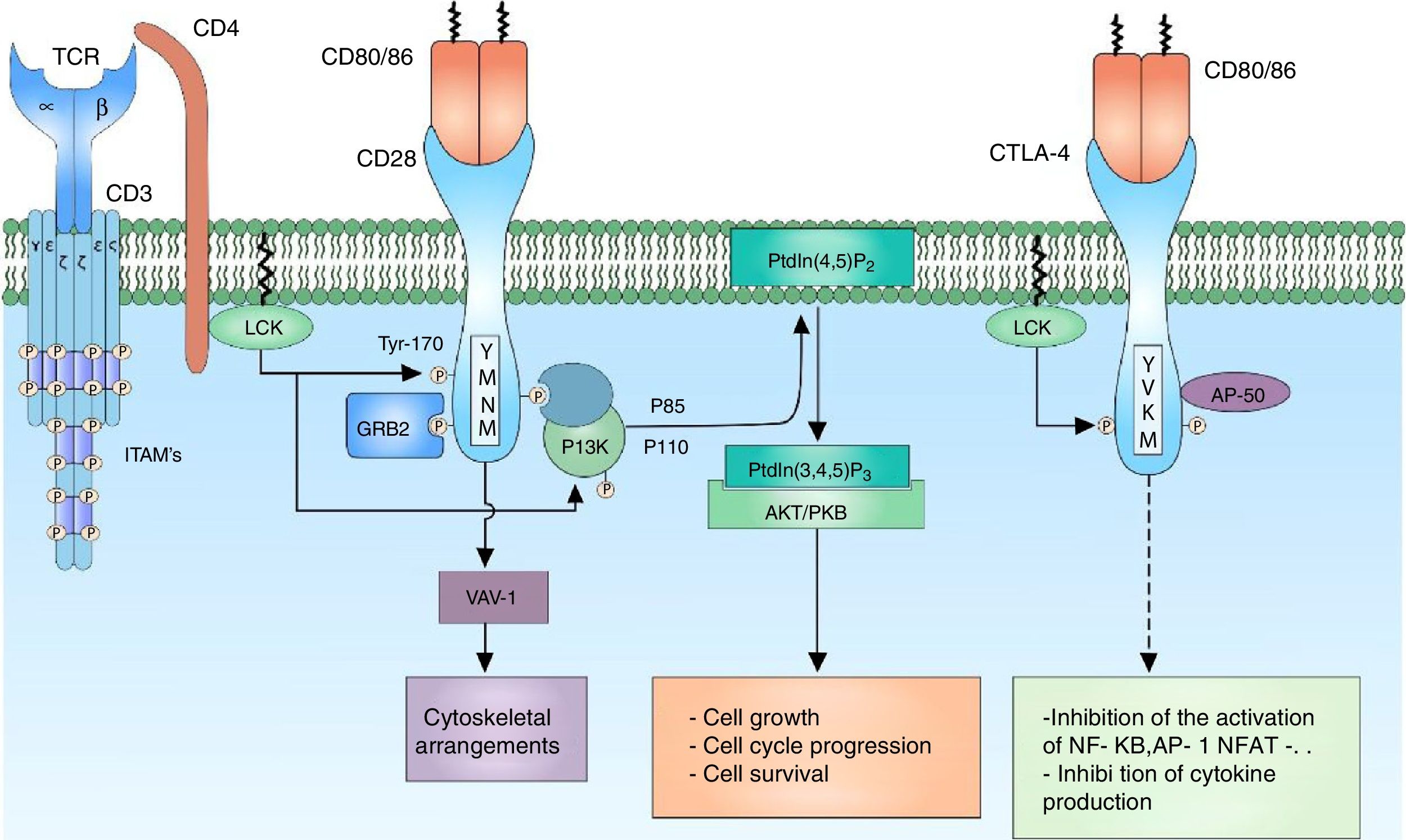
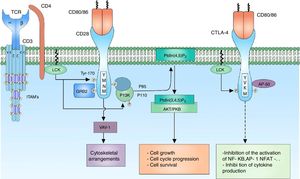
CD28 co-receptor signalingThe signal generated by the activation of the TCR by itself fails to trigger a complete activation of the lymphocyte; the presence of co-receptors that provide additional signals by means of signaling pathways in common with the main signal is necessary to guarantee a signal sufficiently strong for the activation of the T lymphocyte. The CD28 co-receptor has great importance in the cell activation for its participation in the generation of co-stimulator signals. It is a transmembrane glycoprotein that is mainly expressed both in activated and resting T lymphocytes.9 It binds to the CD80 (B7-1) ligands and CD86 (B7-2) co-receptors of the antigen presenting cells, therefore, it is involved in proliferation processes, avoids anergy processes, facilitates the expression of cytokines and mediates cell survival73 (Fig. 4).
CD28 co-receptor: the CD28 co-receptor has great importance in cellular activation because of its participation in the generation of a costimulatory signal. The binding of the CD28 coreceptor with its CD80/86 ligands and the phosphorylation of its cytoplasmic residues allow the activation of the receptor and thus the recruiting of the PI3K and GRB2 proteins. PI3K regulates the inositol lipid metabolism through the phosphorylation of the PtdIns (4,5) P2 to PtdIns (3,4,5) P3, which in turn is involved in the activation of the AKT/PKB-mediated signaling.
The amino acids that comprise the cytoplasmic tail of the CD28 co-receptor possess intrinsic enzymatic activity. This cytoplasmic tail has 4 tyrosines that are key for intracellular signaling and are phosphorylated by FYN or by LCK. The phosphorylation of the tyrosine residue 170 (Y170) which is located in the highly conserved sequence pattern TYR-MET-ASN-MET (YMNM) allows the recruitment of proteins with SH2 domains such as PI3K and GRB273–75 (Fig. 4).
PI3K is part of the family of enzymes that regulate the biological function through the generation of lipids as second messengers. It is divided into different classes according to their function; the PI3K class I predominates mainly in the lymphocytes. In turn, the PI3K are divided into 2 groups, according to their activation, being the group IA activated by receptors associated with tyrosine kinases, cytokine receptors and coreceptors.74 PI3K IA consists of 2 subunits, the P85 subunit, which is the regulatory subunit, binds the pattern sequence YMNM of the CD28 co-receptor for its activation; the P110 subunit, which is the catabolic complex, acts in the phosphorylation of the PtdIns (4,5) P2 to PtdIns (3,4,5) P3.74,76 This lipid acts in molecules containing PH domains, such as AKT/PKB and ITK. AKT/PKB (protein kinase B) is related in the regulation of numerous signaling pathways that promote the cell growth, the progression in the cell cycle and the survival77,78 (Fig. 4).
It has been demonstrated that without the second costimulatory signal, the T lymphocytes fail in the production of IL-2 and a state of anergy is established.79,80 Although the signaling mediated by CD28 in T lymphocytes of patients with lupus is intact, the CD80 ligand is decreased leading to a deficiency in the CD28-mediated signaling and, therefore, in the decreased production of IL-2.81
Lipid raftsLipid rafts are microdomains in the cell membrane composed of lipids different than those that are normally part of the cell membrane, among these lipids there are the glycosphingolipids, sphingomyelin, cholesterol, among others. These lipid rafts create an environment that allows to accumulate or secrete different proteins in a specific region.82
Sphingolipids have a high boiling point, which allows them to form arrays separated from the layer of phospholipids which have a lower boiling point; the cholesterol is preferably mixed within sphingolipids to stabilize the structure and fluidity of the membrane. Therefore, the lipid rafts can act as platforms that move freely in the cell membrane.83
The complexity of the environment of the lipid rafts allows a temporospatial regulation of the T lymphocyte signal. Several proteins, such as LAT, CD4 and LCK, are found in the lipid rafts thanks to lipid-lipid or lipid-protein interactions. The addition of saturated groups to the proteins with intracellular, extracellular or transmembrane domains increases the affinity of these proteins to the organized environment of the lipid rafts. LCK is modified with the presence of groups of saturated lipids and its presence in the lipid rafts is required for the transduction of the signal through the activation of the TCR.84 LAT, which is also modified by the addition of these groups of saturated lipids, once the TCR is activated, there is a recruiting of proteins characteristic of the signaling to the lipid rafts which facilitates the signal transduction.83,84
Regulation of lymphocyte activationCo-receptor CTLA-4Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a co-receptor which plays a fundamental role in the negative regulation of the activation signal of the T lymphocyte; it is a glycoprotein that shares great similarity with CD28 and, like this one, possesses extracellular domains similar to those of immunoglobulins. The expression of the CTLA-4 receptor is restricted to T lymphocytes, either CD4 or CD8; however, it is not expressed in naïve T lymphocytes, but is expressed in the membrane once the T lymphocyte is activated.85,86
The CTLA-4 binding ligands are CD80/86, located in the membrane of the antigen presenting cell. It has been demonstrated that the binding of CTLA-4 to its CD80/86 ligands is much more akin than the binding of CD28 thereof.85 The reason by which both CTLA-4 and CD28 share the same ligands indicates the presence of a costimulatory signal necessary for the activation of the T lymphocyte generated by the binding of CD28 and its ligand, and in contrast, a regulatory signal that modulates the activation of the lymphocyte generated by the binding of CTLA-4 to its ligand; in this way, it maintains a balance between the activation signal and the modulating signal. CTLA-4 inhibits the lymphocyte activation through the reduction in the production of IL-2 and the decrease in the expression of the IL-2 receptor, stopping the cell cycle in G1 phase.87
CTLA-4 is located in the cytoplasm, in cytoplasmic vesicles and its expression on the cell surface is given by mechanisms regulated by clathrin, thus limiting the binding of CD80/86 ligands, preventing the premature termination of the immune response. CTLA-4 has a cytoplasmic tail without intrinsic enzyme activity composed by characteristic pattern sequences YVKM and 2 tyrosine residues involved in the activity and regulation of this protein (Y201, Y218). In its non-phosphorylated form, the Y201VKM pattern of CTLA-4 is associated with the AP50 subunit of the clathrin-associated protein complex, which determines the cytoplasmic state of the protein. Multiple proteins with kinase activity act on the critical tyrosine residue of CTLA-4 (Y201), among them, LCK and FYN are the most important.88,89 The phosphorylation of this residue causes a translocation of the cytoplasmic vesicles to the cell membrane allowing the interaction of CTLA-4 with its ligand and thus modulates the TCR signaling inhibiting the binding of AP-1 and NFAT to the nucleus and suppressing the ERK and JNK signaling pathways. CTLA-4 acts through phosphatases that act directly on specific substrates, however, how CTL-4 signaling exerts its inhibitory effect has been quite controversial and even contradictory.90
Ubiquitination and degradationUbiquitination is the process by which the cells can discriminate proteins which will be degraded; this takes place thanks to the labeling of the protein with ubiquitin, which allows its degradation. Ubiquitin is a peptide of 76 amino acids; it binds to proteins through 3 enzymes, E1, E2 and E3 by the process of ubiquitination. The first step of ubiquitination consists in the formation of a thioester bond with the glycine residue of the C-terminal of ubiquitin and the sulfhydryl group (or thiol) of the cysteine of E1 in its active center. The second step consists in the transfer of ubiquitin from an E1 enzyme to an E2 conjugating enzyme (Ubc). The final step consists in the binding of E2-ubiquitin to an E3 ligase; which catalyzes the formation of an isopeptide bond between the glycine of the C-terminal of the ubiquitin and the lisine of the specific substrate.91 E3 enzymes have the function of recognition of the specific protein which will be led to ubiquitination, as well as the ability to interact with E2 enzymes. Polyubiquitinated proteins labeled in their lysine 48 are substrates for protein degradation by the 26S proteasome.91,92
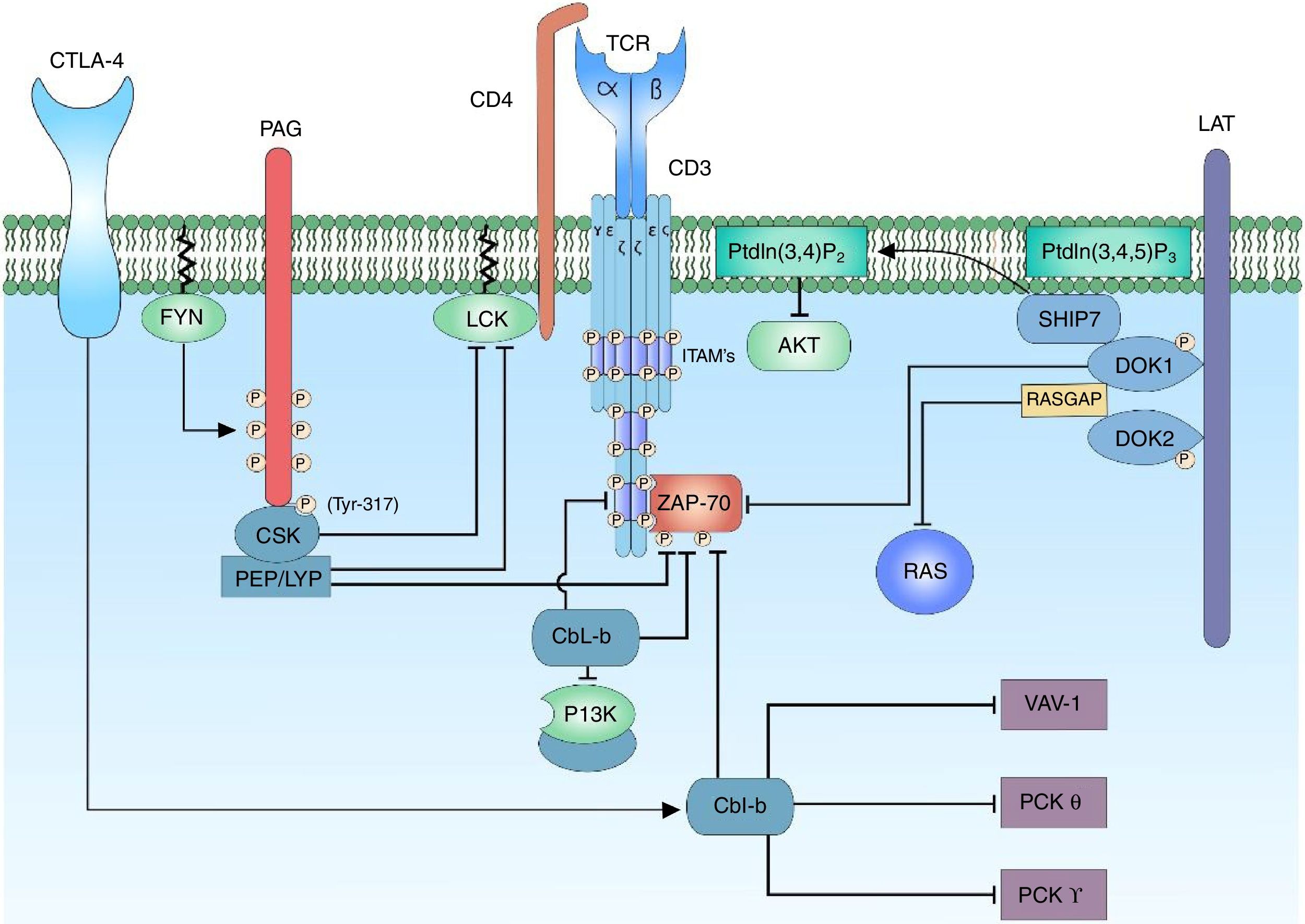
The proteins of the family Casitas B-lineage lymphoma (Cbl) are molecular adaptor proteins; they bind to proteins for their ubiquitination and degradation. In mammals there are 3 genes that encode for the proteins of the Cbl family: c-cbl, cbl-b and cbl-3 (also known as cbl-c).92–95 The structure of all the Cbl proteins is similar, it contains a tirosine kinase binding domain (TKB), a really interesting new gene domain (RING) responsible for the catalytic function of E3 ligases, a region rich in proline (characteristic of the c-Cbl and Cbl-b proteins) and a C-terminal ubiquitin associated (UBA) domain.93,94 In T lymphocytes the c-Cbl and Cbl-b proteins are responsible for the control of the signaling generated by the activation of the TCR through the ubiquitination of active receptors and tyrosine kinase associated receptors. C-Cbl forms a complex with the zeta chain of the TCR and ZAP-70 to promote the ubiquitination and degradation of the zeta chain.92,95,96 It has been reported that c-Cbl can negatively regulate the proteolysis-independent ZAP-70 function.92 C-Cbl also interacts with the SH2 domain of the p85 subunit of the enzyme PI3K and thus negatively regulates the PI3K signal of the costimulator of the T lymphocyte signaling.97 Studies have been focused on the existing relationship between Cbl-b and VAV1, although it has not been demonstrated that Cbl-b is involved in the degradation of VAV1, but rather in the control of the phosphorylation substrates after the activation of the TCR and the CD28 costimulatory signal for the activation of VAV1.34,94,98 The importance of the antagonistic action of Cbl-b toward PKC-θ is due to the modulation of the response threshold of the T lymphocyte, therefore, Cbl-b regulates the PKC-θ through the negative regulation of VAV1 and PI3K signaling, which can control the transcription of IL-2 in the absence of CD28 costimulation.98,99 However, recent studies have demonstrated that PKC-θ is a regulator of the action of Cbl-b; and through the costimulation of the CD28 receptor is possible its destruction100 (Fig. 5).
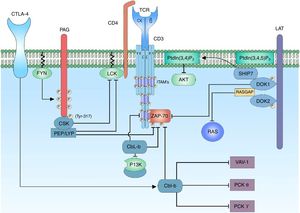
Negative regulation of the activation signal of the TCR complex. The negative regulation of the activation signal of the TCR is important to avoid anergy processes. In the T lymphocytes, the c-Cbl and Cbl-b proteins are responsible for the signaling control through the ubiquitination of active receptors and tyrosine kinase associated receptors. When the CTLA-4 co-receptor is activated, there is activation of the Cbl-b protein for the regulation of the lymphocyte signal; it interacts with the p85 subunit of the enzyme PI3K, thus regulating the signal coming from CD28 coreceptor. Cbl-b interacts with key proteins in the lymphocyte activation such as VAV-1 and PLCy, inhibiting the activation of PKC θ.
The regulation of the activation of the kinases involved in the activation of the T lymphocyte is essential to maintain an activation threshold. The C-terminal SRC kinase (CSK) enzymes are cytoplasmic kinases that phosphorylate regions involved in the negative regulation of the family of SRC kinases. The proximity of CSK to its substrate is important and it is achieved by its interaction with the phosphoprotein associated with glycosphingolipid-enriched microdomain (PAG).101
PAG, also known as Csk-binding protein (CBP) is an ubiquitous transmembrane protein with multiple tyrosine residues which when they are phosphorylated, they act as binding pockets for proteins containing SH2 domains; in addition, it has 2 proline-rich sequences that allow the interaction with proteins containing SH3 domains.101,102 The characteristic of PAG in T lymphocytes, unlike other cells, is it maximum phosphorylation point when the cell is at rest (in cells different from T lymphocytes the binding of membrane receptors with their ligands stimulates the activation of PAG). When the cell is inactive, the phosphorylation of PAG is mediated by the family of SRC kinases, specifically by FYN.101,103 The recruiting of CSK requires the phosphorylation of a specific tyrosine substrate (Y317) of PAG, which allows the binding of CSK to PAG through its SH2 domain; the binding of CSK to PAG not only allows the proximity of CSK to the plasma membrane and, therefore, to its substrate, but it also allows a conformational change of the protein and in this way increases the catalytic activity of the enzyme.104 Once CSK is close to LCK it phosphorylates it in a specific tyrosine substrate (Y505) allowing a conformational change of the protein to a closed state and, therefore, without kinase activity. When the activation of the T lymphocyte occurs, PAG is rapidly dephosphorylated, resulting in the release of CSK from the membrane, and it is sequestered in the cytoplasm by the Ras GTPase-activating protein-binding protein 1 (G3BP); this allows it to be far from the immune synapse.104,105 The enzymes candidates to dephosphorylate PAG are CD45 or tyrosine-protein phosphatase non-receptor type 11 (PTPN 11)104,106 (Fig. 5).
Docking protein 1 (DOK1/2) are cytoplasm molecular adaptors which are related to the negative regulation of the signal of the TCR. DOK1/2 bind to LAT together with phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 1 (SHIP1). SHIP1 hydrolyzes the phospholipid of the PtdIns (3,4,5) P3 to produce PtdIns (3,4) P2 and thus regulate the signal by means of the interference with the recruitment of proteins containing the PH domain such as the AKT or ITK proteins.53,107 DOK1/2 may be associated with the phosphorylated ITAMs of the ζ and ¿ chains, displacing the binding of ZAP-70 to these. It has also been demonstrated that DOK1/2 contain sequences for the SH2 and SH3 domains, so it can be associated to Ras GTPase activating protein (RASGAP).107 RASGAP is an attenuator of the activation of the RAS protein, and therefore it decreases MAPK/ERK signaling.108 The binding of CSK to DOK1/2 through its SH2 domains might be involved in the recruiting for the inhibition LCK107 (Fig. 5).
Negative regulation mediated by PTPN22 phosphatasesTyrosine kinase phosphatases (PTP) play an essential role, both in maintaining the activated phenotype of T lymphocytes, as well as in the reversal of the activated state to a resting state when the immune response ends.106 Each PTP consists of a catalytic domain that has a preference for the specific substrate and non-catalytic domains that are involved in the regulation of phosphatase activity. Among the regulators of the activation signal there is the product of the PTPN22 gene, lymphoid-tyrosine phosphatase (LYP) expressed in humans and its murine orthologue PEP.109 LYP/PEP is a tyrosine phosphatase whose expression is restricted to hematopoietic cells, it contains a phosphatase domain in its N-terminal region, a proline-rich region and a C-terminal region highly conserved within its family of phosphatases called PEST-PEP group, a potent negative regulator of the TCR signal.109 PEP acts by dephosphorylating the positive regulatory residues of the FYN (Y417), LCK (Y394) and ZAP-70 proteins. This negative regulation is possible thanks to its interaction with the SH3 domain of the CSK proteins by proline-rich pattern sequences,110 while PEP dephosphorylates the tyrosines of the positive regulatory regions of the proteins of the SRC family, CSK phosphorylates the negative regulatory regions thereof.
Alterations in the regulator of the lymphocyte signaling PTPN22 have been related to multiple autoimmune diseases, including SLE, rheumatoid arthritis, idiopatic juvenile arthritis, thyroid autoimmune disease, Wegener's granulomatosis, myasthenia gravis, Hashimoto's thyroiditis, Graves’ disease, Addison's disease, systemic sclerosis, among others.109–111 Among the studies of the activation and transduction of the signal through the TCR, it has been documented the polymorphism of the PTPN22 (rs2476601; 1858C→T) gene and its relation with SLE. This polymorphism is characterized by the substitution of the amino acid arginine by a tryptophane in the position 620 of the amino acid sequence. This change occurs in the proline-rich proximal region binding domain to SH3 of PTPN22. This proline-rich portion is an important binding site for the C-terminal domain of CSK, whereby this polymorphism interrupts the interaction between PTPN22 and CSK; in this way, the suppression of T lymphocyte activation does not take place. The net effect of this polymorphism on the interaction between PTPN22 and CSK for the generation of autoimmune disease is a subject of controversy and the proposed mechanisms of action are based on animal models. An explanation of the role of the Trp620 polymorphism in the pathogenesis of the autoimmune disease is the alteration in the balance between the effector T lymphocytes and the regulatory T lymphocytes. It is suggested that the balance between the effector T lymphocytes promoters of autoimmune disease and the protective regulatory T lymphocytes is finally regulated by the expression or activity of PTPN22.111,112
Alterations of the activation signals of T cells in SLEErythematosus lupus is a chronic systemic autoimmune disorder, potentially fatal. The defects can occur in several parts of the immune cascade, resulting in heterogeneous clinical presentations. Multiple signaling pathways implied in the immunopathogenesis of the disease will be described below.
Alterations in CD3The majority of patients with SLE have defects in the expression of the ζ chains of the TCR, as well as alterations in their phosphorylation. The deficiencies in the phosphorylation of the ζ chains of the TCR after the lymphocyte activation have been observed in more than 78% of patients with lupus. Multiple potential mechanisms have been described to explain the decreased expression.113
Studies conducted by Tsuzaka et al. to investigate the molecular mechanisms of the reduced expression of the ζ chains of the TCR isolated the mRNA of the ζ chains in peripheral T lymphocytes of 8 patients with SLE. On one side, they found that 2 patients presented deletions of exon 7 of the mRNA of the ζ chain; on the other hand, 6 patients had punctual mutations in the binding site of the GTP/GDP in the domain 3 of ITAM that were accompanied by substitutions of amino acids in the domain 3 of the ITAM.114
There are 3 important ITAM domains recognized, that when they are phosphorylated are binding sites for proteins such as ZAP-70, actin, PI3K or Shk. Mutations of one or two tyrosines within the ITAM or when phosphorylation does not occur, or there is only monophosphorylation, lead to alterations in signal transduction. From this observation it is possible to determine that the aberrant mRNA of the TCR is responsible for the decrease in the phosphorylation and dysfunction of the TCR signal.115
It has been demonstrated that the 3′ untranslated region of the TCR ζ chain (3′-UTR – untranslate 3′ region) plays an important role at the posttranscriptional level. 3′-UTR mRNA has cis-regulatory sequences such as adenosine-uridine rich sequences that bind to trans-regulatory proteins and are involved both in the transcript stabilization or destabilization. Analyses in the mRNA of the ζ chains have found alternative splicing with loss of exons, as well as insertions in the transcript.115
In the study conducted by Chowdhury et al., they determined that the TCR ζ chain presented a new alternative splicing with deletion of nucleotides from 672 to 1233 or the exon viii of the ζ chains of the mRNA. This splicing (ζcDNA/AS-3′UTR) of 344pb is expressed predominantly in T lymphocytes of patients with lupus compared with healthy controls. This short sequence of 3′-UTR is less stable than the natural version of the mRNA; in addition, it has a higher level of degradation in T lymphocytes of patients with lupus, indicating that T lymphocytes of patients with lupus have additional factors that promote the degradation of the short chains of the alternative splicing. The results demonstrate that the presence of regulatory elements in the deleted region of the 562pb junction is required for the proper and efficient translation of the mRNA.116 In addition, the production of this chain of alternative splicing represents a molecular mechanism that contributes to the decreased expression of the ζ chains of the TCR in patients with SLE.
The Fc¿ receptor type I γ chain (Fc¿RIγ) is a member of the family of ζ chain proteins, it is also a component of the region of high affinity IgE receptors; it contains a cytoplasmic ITAM that, unlike the ζ chain of CD3, mediates signaling through the SYK protein kinase.117 As previously mentioned, SYK kinase is 100-fold more potent than ZAP-70 and it is preferably recruited by Fc¿RIγ; thus, as there is a decrease in the TCR ζ chains there is a replacement by Fc¿RIγ; this increases the SYK activity. The SYK kinase is a protein capable of phosphorylating ITAM independently of the activity of SFK, unlike ZAP-70, activating multiple signaling pathways through VAV, PLCγ, SLP76 and the PI3K subregulatory unit. In SLE patients, with respect to healthy controls, it has been demonstrated the interaction between the SYK protein with VAV and PLC, producing the increase in the concentrations of intracellular calcium and the increase of the calcium-dependent signaling pathways; another change induced by the SYK-mediated signaling is the polymerization of actin.118
IL-2 is a very important regulatory factor in the response of the T lymphocyte; it is involved in the modulation of the duration and the intensity of the immune response, and also as a T-lymphocyte growth factor that is produced after the stimulation of the TCR. IL-2 has been characterized for being an indispensable factor for the induction of tolerance, generally through two mechanisms: activation of the induced cell death mechanisms, and induction and maintenance of regulatory T cells. As a key element in the modulation between the proliferative immune response and the induction of tolerance, IL-2 must be closely regulated in order to maintain homeostasis in the immune response.119
The search of the mechanisms responsible for the changes in the synthesis of IL-2 has revealed a series of alterations in the site of occupation of the transcription factor at the level of the IL-2 promoter of T lymphocytes, obtained from patients with SLE. The 180 site has demonstrated to be especially important in the deregulation of IL-2 transcription. It comprises a binding site for CREB/CREM. When CREB is phosphorylated, it acts as a positive factor that improves the transcription. On the other hand, when CREM is activated, it displaces pCREB and acts as a transcriptional repressor, avoiding the binding of p300 and CBP. The changes in the CREM levels are associated with the enzyme complex activated calcium/calmodulin dependent protein kinase IV (CaMKIV). The levels of this kinase are higher in T cells derived from patients with SLE.120,121
Th17 lymphocytes, IL-7 and systemic lupus erythematosusThe chronic activation of the inflammatory response in patients with SLE leads to the release of multiple cytokines which will actively contribute to the inflammation and tissue damage. The different subtypes of T lymphocytes have the ability to secrete cytokines that will help in the inflammatory response; in this way, the Th1 lymphocytes are essential to control infections with intracellular microorganisms through the secretion of IFN-γ, which has the ability to activate macrophages, while Th2 lymphocytes produce IL-4, IL-5 and IL-13 which are involved in the control of parasites and allergens through the activation of eosinophils. There is another subtype of lymphocytes, known as Th17, which produce IL-17; this interleukin has multiple inflammatory effects, among these, to induce the secretion of other cytokines, chemokines of multiple cell lineages including epithelial cells and fibroblasts. It promotes the proliferation, maturation and recruiting of neutrophils through multiple growth factors and IL-18, allows the recruiting of inflammatory cells, such as macrophages and other lymphocytes, as well as the release of metalloproteinases that cause destruction of the connective tissue.122
In patients with SLE it has been documented an increase of the cell counts of Th17, as well as of the plasma concentrations of IL-17; however, it has not been possible to relate the levels of IL-17 with the level of lupus activity, indicating that this interleukin is constitutively and stably produced in patients with SLE independently of the disease activity.123 Although the patients with SLE have an increased production of IL-17, the role of this cytokine in the pathogenesis of lupus is not well studied. IL-17 can induce the production of multiple inflammatory mediators of immune and no immune cells that can participate in the activation of inflammatory cells and in tissue damage.
ConclusionsThe activation of the T lymphocyte is a complex process with multiple components which are related each other; this intricate machinery that begins with the recognition between the TCR and a peptide mounted on a major histocompatibility molecule allows the activation of the family of SRC, FYN and LCK kinases, which phosphorylate the tyrosine residues of the ITAM sequences present in the CD3 chains. These phosphorylated domains allow the incorporation of ZAP-70, thus propagating the signal through the phosphorylation of the LAT anchoring protein that diversifies the signal by recruiting new kinases. LAT propagates TCR signaling in 3 important major pathways, the MAP kinase cascade through GRB2 and GRAP, modifications in the cytoskeleton and release of calcium as second mediator by GADS. These different pathways will end up in the activation of multiple promoters that will facilitate the synthesis of cytokines that act as effectors of the lymphocyte response. The regulation of the activation of the T lymphocyte is important to end the activation and avoid states of anergy or autoimmunity. The regulatory components of T lymphocyte activation include the CTLA-4 co-receptor, the ubiquitination and degradation of proteins that are key for activation, the recruitment of SHIP1 by DOK1/2 and the activation of PTPN22. The alteration in the activation and regulation mechanisms can lead to processes of autoimmunity such as SLE, a chronic disorder with heterogeneous clinical manifestations. Key changes have been described, such alterations in the phosphorylation or defects in the expression of the TCR ζ chains, alterations in the expression in the lymphocyte subtypes predominating the Th17 lymphocytes with an increase in IL-17 or changes in key proteins for the signal transduction, such as the replacement of the CD3 ζ chain with Fc¿RIγ that will generate aberrant signals in the lymphocyte activation.
Once the normal activation mechanism of the T lymphocyte, the mechanisms of cell signaling and the regulation of the transmission pathways are known, we can understand the pathogenesis of the autoimmune disease and the heterogeneity of the clinical manifestations that characterizes it. In addition, such knowledge have allowed us to make use of key elements in lymphocyte signaling in search of diagnostic tools with new biomarkers that allow to make a follow-up of the disease, its activity and the research of new therapeutic targets.
Selection of the informationFor the realization of a narrative review article, the search was made through PubMed database, using the terms: “T cell activation”’, “TCR signals”, “Inmune cell signaling in lupus”, “pathogenesis of systemic lupus”, “Signal transduction TCR”, “ITAM AND TCR”, “T cell AND SYK”, “ZAP-70 AND TCR”, “TCR structure”, “Regulation activation TCR”, “TCR AND autoimmunity”, “Lipid rafts AND T cell”. Articles between the years 1945 and 2017, in English and in Spanish, and of review, clinical trial, research, editorials, and opinion types were selected. Those articles that explain the lymphocyte signaling pathway, as well as those studies that were related with alterations in the activation of the T lymphocyte and the pathogenesis of SLE were selected. From the selected articles, the references of those topics in which we wished to deepen were identified; subsequently, the abstract was reviewed and it was determined if it was relevant or not for the review. Articles were also selected using the PubMed option “similar article”.
Conflict of interestThe authors declare they do not have any conflict of interest.
Thanks to Daniela Alejandra Gordillo for making the graphics and for her participation in the elaboration of the manuscript.
Please cite this article as: Nicolás Téllez C, Siachoque JJ, Siachoque SJ, Siachoque JMA, Siachoqu MH. Activación de la célula T, alteraciones en el lupus eritematoso sistémico, una revisión narrativa. Rev Colomb Reumatol. 2018;25:38–54.