Systemic lupus erythematosus can present with a broad spectrum of symptoms that on some occasions may mask serious complications associated with the same disease. Within these, pancreatitis is an uncommon but high-mortality cause, especially in patients with non-opportune treatment. We report the case of a patient with systemic lupus erythematosus with recent renal and central nervous system involvement that is associated with the onset of pancreatitis and thyroiditis. A satisfactory outcome was obtained with a cyclophosphamide and prednisolone therapeutic regimen.
El lupus eritematoso sistémico se puede presentar con un amplio espectro de síntomas que en algunas ocasiones pueden enmascarar complicaciones graves asociadas a la misma enfermedad. Dentro de estas la pancreatitis es una causa poco común, y sin embargo de alta mortalidad, especialmente en pacientes con un tratamiento no oportuno. Reportamos el caso de una paciente que cursa con lupus eritematoso sistémico con compromiso renal y de sistema nervioso central, de reciente aparición, que se asocia a la aparición de pancreatitis y tiroiditis, presentando evolución satisfactoria con esquema terapéutico de ciclofosfamida y prednisolona.
Systemic lupus erythematosus (SLE) is an autoimmune disease with a wide variety of clinical symptoms and signs, which is associated with a large number of immunological and laboratory findings. Despite the survival of these patients has increased markedly in the last 6 decades, to more than 90% at 5 years,1 the burden of the disease and the morbidity are still high. We review the case of a patient with SLE and nephritis who presented symptoms of thyroiditis and pancreatitis simultaneously.
Clinical caseIt is a 18-year-old female patient, of mixed race, with a diagnosis of SLE, with a family history reporting that her mother was diagnosed with SLE (without personal antecedents, especially of thyroid or pancreatic disease), with an onset of symptoms 3 months before consulting given by polyarthralgias of large and small joints, photosensitivity and hair loss, but without cicatricial alopecia. Management with chloroquine and acetaminophen was started in another institution, but she did not return to follow-up by rheumatology or by any other medical service. The patient consulted to the emergency department for the presence of edema in the lower limbs, progressive dyspnea, asthenia, adynamia and weight loss of approximately 10kg in the past 4 months. In addition, it was observed anasarca, asymmetrical edema of lower limbs, tachypnea, tachycardia, with desaturation in the pulse oximetry without supplementary oxygen (86%), but without the presence of abnormal sounds on cardiopulmonary auscultation and without neurological deterioration. It was decided to hospitalize her because left femoral, and right posterior tibial and femoropopliteal venous thrombosis was evidenced in the Doppler of the lower extremities, and for this reason, anticoagulation with warfarin was started. The blood chemistry showed leukopenia of 3160cells/mm3 (reference value [Vr] 4500–10,000cells), lymphopenia of 110cells/mm3 (Vr. 1200–3400cells), anemia with hemoglobin of 8.4g/dl (Vr. 12–16g/dl), without thrombocytopenia or hemolysis, creatinine of 0.54mg/dl (Vr. 0.6–1.2mg/dl), 24h urine proteins of 3.5g in nephrotic range (Vr. lower than 150mg/24h) and an uroanalysis with the presence of waxy casts, leukocytes of 14–15/hpf (Vr. 0–5/hpf), erythrocytes of 4–5/hpf (Vr. 1–3 erythrocytes/hpf). Other laboratory tests obtained from the patient were: TSH (thyroid stimulating hormone) suppressed in 0.03U/ml (Vr. 0.45–4.5U/ml), free T4 increased in 2.54ng/dl (Vr. 0.8–2.0ng/dl), antinuclear antibodies 1:1280 of homogeneous pattern, anti-double stranded DNA antibodies 1:1280, negative IgG and IgM anti-cardiolipin antibodies, negative B2-glycoprotein IgG and IgM, negative lupus anticoagulant, complement C3 of 65mg/dl (Vr. 90–180mg/dl) and C4 of 9.5mg/dl (Vr. 10–40mg/dl).
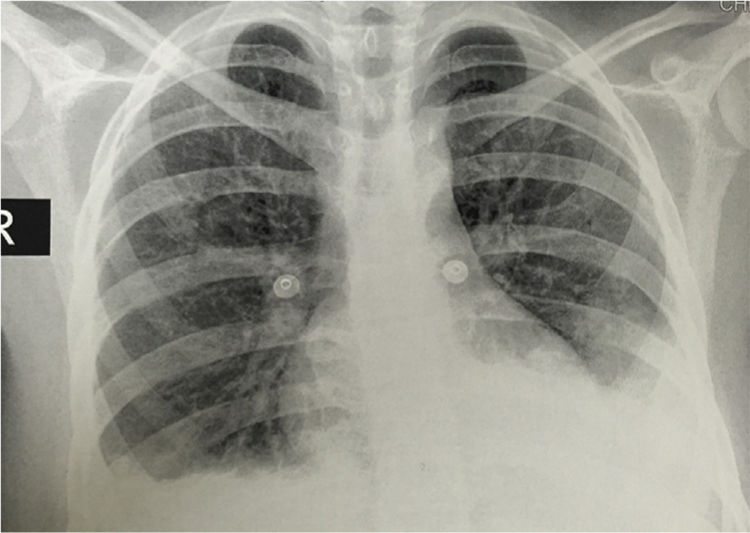
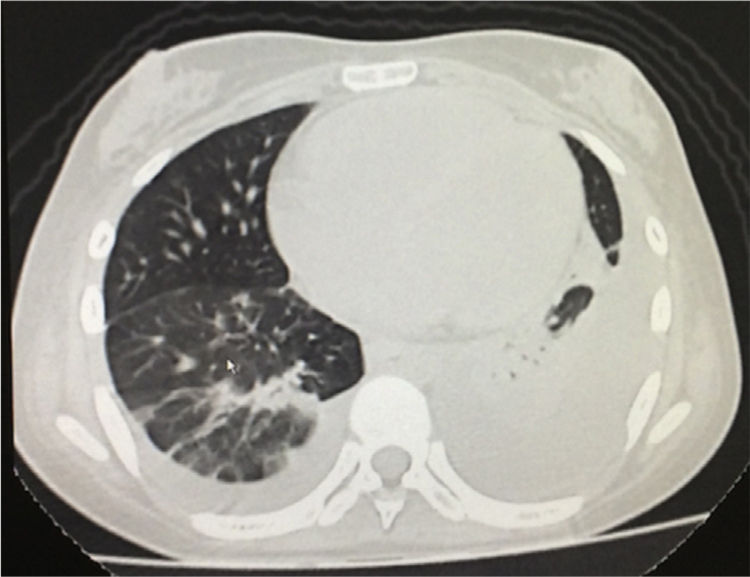
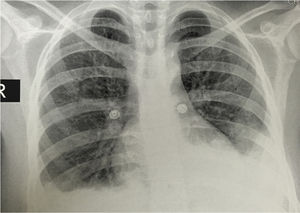
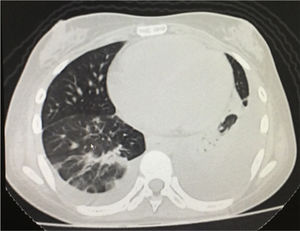
The simple chest X-ray showed the presence of pleural effusions and an image suggestive of left basal consolidation, despite the fact that the patient did not have cough, sputum or fever (Fig. 1). Left pleural effusion with ipsilateral compressive atelectasis and parenchymal bands in the right base were evidenced in the simple chest CT scan (Fig. 2). The echocardiogram reported a preserved ejection fraction with an elevated pulmonary artery systolic pressure of 42mmHg (Vr. PASP lower than 36mmHg). The Systemic Disease Activity Index (SLEDAI) was calculated in 21 points, considering it a severe exacerbation of the SLE. Treatment was started with prednisolone at 30mg/day, with which the patient presented improvement of the dyspnea and of the oxygen saturation.
During the second week of hospitalization the patient presented a clinical picture of sudden and diffuse abdominal pain associated with nausea, a report of amylase of 817U/l (27–131U/l) and a hepatobiliary ultrasound with diffuse increase of hepatic echogenicity, free fluid in the peritoneal cavity and no alterations in the biliary tract. It was performed a lipid profile, which was reported as: total cholesterol of 146 mg/dl (Vr. less than 200mg/dl), HDL of 37 mg/dl (Vr. higher than 50mg/dl for women), LDL of 77mg/dl (Vr. less than 160mg/dl, variable value according to cardiovascular risk factors) and triglycerides of 153mg/dl (Vr. less than 150mg/dl). Abdominal tomography was not performed because it was considered to be of high risk of nephrotoxicity due to the required contrast media. With the diagnosis of acute pancreatitis, she was treated symptomatically with meperidine and metoclopramide, with which the abdominal clinical picture resolved in the following 7 days.
It was received the report of the renal biopsy taken at admission to the hospital which informed the presence of lupus nephropathy type IV G, according to the classification of the International Society of Nephrology and Renal Pathology Society 2004 with an index of activity of 4 points and of chronicity of 5 points, the presence of tubular atrophy was not reported. A therapeutic scheme for lupus nephritis was started with cyclophosphamide (scheme of the National Institutes of Health of the United States) and intravenous boluses of methylprednisolone 500mg for 3 consecutive days, followed by oral prednisolone at 1mg/kg day, in addition to oral methimazole 15mg/day, oral propranolol 20mg/day due to the associated diagnosis of thyroiditis, evidenced in the thyroid function tests. The patient presented an adequate clinical evolution with progressive improvement of the edemas, without deterioration of the renal function, resolution of the abdominal pain with improvement of the SLEDAI scale and normalization of the cardiac frequency, and therefore it was decided to discharge her 4 weeks after admission. Complementary thyroid function tests were taken on an outpatient basis, evidencing a thyroid scan with presence of homogeneous goiter with increased uptake, without focal defects and with a retention index of 12.5 (Vr. 2.5–4.5). After the substitution of methimazole for 8 weeks, it was observed the normalization of the thyroid function tests with TSH in 2.34IU/ml and free T4 of 1.09ng/dl.
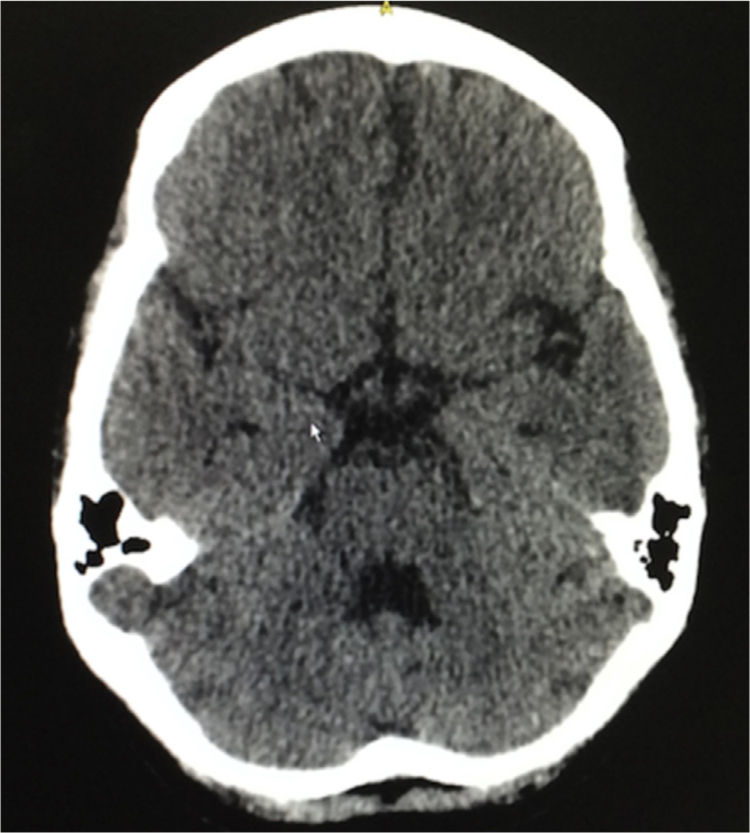
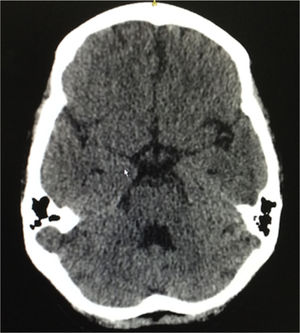
Eight weeks after the hospital discharge, the patient presented 2 tonic-clonic convulsive episodes, and for this reason she consulted again to the emergency department, being performed a lumbar puncture that ruled out neuroinfection, a simple brain tomography that evidenced slight cortical atrophic and small vessels microangiopathic changes, in addition to a slight dilation of the ventricular system (Fig. 3). The following laboratory results were documented: complement C3: 77mg/dl, complement C4: 18mg/dl, serum creatinine: 0.62mg/dl, hemoglobin: 10.1g/dl, leukocytes: 3650 cells and lymphocytes: 820 cells. The SLEDAI calculated in this new hospitalization was 23. A new scheme of intravenous methylprednisolone of 250mg/day for 3 consecutive days was administered, and subsequently it was continued the scheme of prednisolone at 1mg/kg per day that she had been receiving; and it was decided not to give anticonvulsant management. The patient had a satisfactory evolution, without new convulsive episodes, with clinical normalization of lupus activity and without deterioration of the renal function, and was discharged 10 days after admission. She continues currently under treatment with prednisolone in progressive decrease of the dose and with monthly cyclophosphamide, without presenting new neurological events or episodes of abdominal pain.
DiscussionThe association of pancreatitis and thyroiditis in the patient with SLE has not been reported in the literature, possibly due in part to the lack of suspicion of pancreatitis as a differential diagnosis in patients with active SLE who present abdominal pain. It is also unusual the report of a thyroid involvement in this group of patients. Non-considering the symptoms of hyperthyroidism in patients with active systemic inflammatory response is very likely to be the cause of the under-reporting of thyroid disease in this group. The result of this is that it is not possible to identify in the literature the clinical profile of the patients with this combination of findings or the clinical profiles that have been described in each of the entities individually.
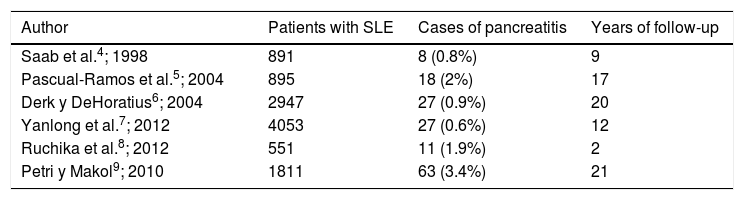
Regarding the pancreatic commitment described in SLE, it is considered uncommon (Table 1) and in different series it has been observed an approximate incidence of 0.4–11 per 1000 patients/year.2 The etiology has not been clarified yet due to its low prevalence and the lack of anatomopathological studies or animal models. However, there are physiopathological hypotheses that have been considered, being the vascular the most plausible, which suggests different mechanisms, such as the compromise by necrotizing vasculitis, thrombotic vasculopathy secondary to antiphospholipid syndrome, intimal proliferation and thickening; deposits of immune complexes and activation of the complement in the vascular wall.3
Cases of pancreatitis in patients with systemic lupus erythematous according to different cohorts.
| Author | Patients with SLE | Cases of pancreatitis | Years of follow-up |
|---|---|---|---|
| Saab et al.4; 1998 | 891 | 8 (0.8%) | 9 |
| Pascual-Ramos et al.5; 2004 | 895 | 18 (2%) | 17 |
| Derk y DeHoratius6; 2004 | 2947 | 27 (0.9%) | 20 |
| Yanlong et al.7; 2012 | 4053 | 27 (0.6%) | 12 |
| Ruchika et al.8; 2012 | 551 | 11 (1.9%) | 2 |
| Petri y Makol9; 2010 | 1811 | 63 (3.4%) | 21 |
An idiopathic etiology was considered in up to 41% of the cases in the study of Pascual-Ramos,5 identifying other possible etiologies such as the mechanical-obstructive observed in 34% and the toxic-metabolic in 24% of the cases according to the same study.5 In 20% of the cases is reported the initial presentation of SLE and another 60% in the 2 following years,5 with a mean of 5 years.6 Pancreatitis is associated with a significant disease activity, however, in 10% of the cases associated with SLE it did not present any other clinical or laboratory manifestation.4 The remaining 90% presented significant lupus activity with an important cutaneous, articular and renal involvement.4 The risk factors identified for the appearance of pancreatitis in SLE are: pleuritis, psychosis, anemia, hypertriglyceridemia and there is no relationship with antiphospholipid, anti-DNA, anti-Ro or anti-La antibodies.9
The treatment-related risk factors were fully analyzed in 77 cases by Breuer et al.,10 observing that corticosteroids and azathioprine are rarely identified as a cause of pancreatitis and without being able to demonstrate clearly causality,10 and for this reason, in most cases the treatment was continued. Pascual-Ramos et al. compared the frequency of use of steroids in patients with lupus and idiopathic pancreatitis, without identifying a risk association.5
The most frequently reported clinical manifestations were: abdominal pain (80%), nausea or vomiting (60%), fever (50%) and diarrhea (9%).10 In the great majority of cases the diagnosis was based on the clinical findings coupled with an elevation of pancreatic enzymes, either amylase or lipase, although it has been described that up to 30% of asymptomatic patients can have these tests positive. Antinuclear antibodies are present in 98% of cases, anti-DNA antibodies in 73% and low complement levels in 75%.11 The treatment of the SLE-associated pancreatitis almost always contemplates glucocorticoids, occasionally the addition of azathioprine and in more severe cases cyclophosphamide, plasmapheresis, or gamma globulin. Breuer considered that the complications (respiratory failure, pleural effusion, infection, shock and pancreatic pseudo-cyst) can appear if there is no timely treatment in up to 57%, which drastically increases mortality up to 45%, which is significantly higher compared with the mortality of 3% in the cases without the presence of complications.10 Pascual-Ramos et al. reported a general mortality of 22%,5 while Derk et al., reported 18%.6
Chronic autoimmune thyroiditis (Hashimoto's disease), as well as immunogenic hyperthyroidism (Graves's disease) are frequently associated with many other autoimmune diseases, such as Sjögren's syndrome, autoimmune hemolytic anemia, megaloblastic pernicious anemia, immune thrombocytopenic purpura, rheumatoid arthritis, diabetes mellitus, Addison's disease, celiac disease and other connective tissue disorders such as SLE and systemic sclerosis.12 A great variety of studies have assessed the association and the prevalence of the clinical and immunological aspects of hyperthyroidism in patients with SLE.13–22 The exact prevalence of hyperthyroidism coexisting with SLE is not determined in the medical literature, but it has been estimated in up to 8.9%. In patients with SLE, the studies reveal a higher risk of hypothyroidism (including subclinical), but without evidencing a significant increase in the risk of hyperthyroidism.12,17 The presence of antinuclear antibodies in 94% of cases, anti-DNA in 100% and hypocomplementemia in 66% of cases stands out as immunological findings. The most frequent clinical findings of lupus activity are joint, renal and cutaneous involvement.20
In the present case, the magnitude and extent of the systemic involvement were evidenced by the presence of pancreatitis and thyroiditis associated with other manifestations such as renal, articular, neurological, serous, hematologic, complement consumption and production of anti-DNA antibodies, which is consistent with that was reported in the literature regarding the association of pancreatitis and SLE. The presence of this picture is remarkable in a scenario of risk factors for the presentation of the disease and the severity thereof such as gender, age, family history and race, thus assuming their interaction with the clinical phenotype of the patient. Likewise, calls attention the presence of thyroid disease, which despite not having been characterized immunoserologically (anti-TPO, TSH receptor, antimicrosomal), puts into consideration the coexistence or autoimmune thyroid disease, and therefore the possibility of syndromic diagnosis of polyautoimmunity and in the case of demonstrating the antiphospholipid syndrome in the follow-up, the diagnosis of multiple autoimmunity syndrome. On the latter point, it is necessary the result of the second set of the antiphospholipid profile (IgG and IgM anticardiolipin, B2 glycoprotein iand lupus anticoagulant) to rule out or confirm the diagnosis of antiphospholipid syndrome, not considering necessary to perform other types of serologies (IgA or tests of total or differentiated antiphospholipids) because they do not contribute significantly to the diagnosis,23 being essential the diagnostic clarification in the therapeutic plan of anticoagulation and in the prognosis. The pancreatitis reported here presented a benign course, without identifying the presence of associated risk factors such as hypertriglyceridemia, alcohol consumption or biliary lithiasis, and without documenting a clinical spectrum suggestive of another autoimmune pancreatic disease (autoimmune pancreatitis), and as reported in the literature, a high activity of the base disease was observed, with an adequate initial response to management with oral corticosteroids.
It is important to highlight the adequate clinical response to the management with immunosupressants, corticosteroids, antithyroid drugs and beta blockers along with the supportive treatment for the manifestations of the pancreatic and thyroid clinical picture, without registering subsequent recurrences.
ConclusionThe diagnosis of pancreatitis and thyroiditis in the scenario of lupus is not common, and even less the association of each other; likewise, the interaction in their natural history and prognosis with their synchronous presentation is unknown. The case described, like what is reported in the literature, indicates a clinical and paraclinical profile of high lupus activity, so it can lead to under-reporting due to the overlapping of the symptoms of these conditions with other manifestations of the lupus, being necessary to suspect this complications in scenarios of high activity in order to be able to detect and treat them in a timely manner.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestNone.
Please cite this article as: Abella J, Medina YF, Rondón F, Quintana G, Morales R, Díaz E, et al. Tiroiditis y pancreatitis como presentación simultánea en una paciente con nefritis lúpica. Reporte de caso. Rev Colomb Reumatol. 2018;25:136–140.