Lupus nephropathy (LN) is a chronic inflammatory process, characterized by the activation of T cells and high levels of various cytokines, such as MCP-1 at the level of the renal glomerulus and the interstitial tubule. MCP-1 is a chemoattractant of monocytes and lymphocytes, it is responsible for the infiltration of leukocytes in the kidney, which is why MCP-1 levels in urine of patients with LN correlate with the active form of the disease.
ObjectiveThe present study aims to evaluate the expression levels of MCP-1 in patients with LN and to correlate their urinary levels with serum autoimmunity markers.
Material and methodsOur study is of the case-control type, where the groups were made up of 112 patients diagnosed with SLE or LN, and 28 apparently healthy people with no clinical or family history of autoimmune diseases, respectively. MCP-1 expression levels were estimated using qRT-PCR. In addition, clinical parameters and serum levels were evaluated (anti-ds-DNA, anti-nucleosome, anti-C1q antibodies, β2-microglobulin levels, and C3 and C4 complement fraction). Finally, clinical, and molecular data were correlated.
ResultsOur study included 39 patients with active SLE (median 36 years), 32 with active LN (median 32.5 years), 28 with inactive SLE (median 41.5 years), 13 with inactive LN (median 38 years), and 28 control patients (median 28.5 years). The comparison of MCP-1 expression levels between patients with active LN and active SLE did not show statistically significant values (p > 0.05). Likewise, a statistically significant correlation was observed between the expression levels of MCP-1 with the levels of anti-C1q (r = 0.255 p < 0.025); however, no correlation was found with the other markers.
ConclusionThe use of MCP-1 expression levels in the Bolivian population would not be a useful biomarker to evaluate Lupus Nephropathy. However, the anti-C1q biomarker is suggested as a serological marker for monitoring the disease.
La nefropatía lúpica (NL) es un proceso inflamatorio crónico, caracterizado por la activación de células T y niveles elevados de diversas citoquinas, como la MCP-1 a nivel del glomérulo renal y el túbulo intersticial. La MCP-1 es un quimioatractante de monocitos y linfocitos, responsable de la infiltración de leucocitos en el riñón, razón por la cual niveles de MCP-1 en orina de pacientes con NL se correlacionan con la forma activa de la enfermedad.
ObjetivoEl presente estudio tiene como objetivo evaluar los niveles de expresión de la MCP-1 en pacientes con NL y correlacionar sus niveles urinarios con marcadores séricos de autoinmunidad.
Materiales y métodosNuestro estudio es tipo caso-control; los grupos estuvieron conformados por 112 pacientes diagnosticados con LES o NL y 28 personas aparentemente sanas, sin antecedentes clínicos, y familiares para enfermedades autoinmunes, respectivamente. Los niveles de expresión de la MCP-1 fueron estimados mediante qRT-PCR. Además, se evaluaron los parámetros clínicos y los niveles séricos (anticuerpos anti-ds-DNA, anti-nucleosoma, anti-C1q, niveles de β2-microglobulina y fracción del complemento C3 y C4). Finalmente, los datos moleculares y clínicos fueron correlacionados.
ResultadosEn nuestro estudio participaron 39 pacientes con LES activo (mediana 36 años), 32 con NL activo (mediana 32,5 años), 28 con LES inactivo (mediana 41,5 años), 13 con NL inactivo (mediana 38 años) y 28 pacientes control (mediana 28,5 años). La comparación de niveles de expresión de la MCP-1 entre pacientes con NL activa y LES activo no presentaron valores estadísticamente significativos (p > 0,05). Asimismo, se observó una correlación estadísticamente significativa entre los niveles de expresión de MCP-1 y los niveles de anti-C1q (r = 0,255 p < 0,025); sin embargo, no se encontró ninguna correlación con los restantes marcadores.
ConclusionesEl uso de los niveles de expresión de la MCP-1 en población boliviana no llegaría a ser un biomarcador útil para evaluar la nefropatía lúpica. Sin embargo, se sugiere al biomarcador anti-C1q como un marcador serológico para el seguimiento de la enfermedad.
Lupus nephropathy (LN) is one of the most severe clinical manifestations of systemic lupus erythematosus (SLE),1 and is observed in approximately 50% of patients.2 Its pathogenesis is a complex process that involves the deposition of immune complexes in the glomerulus, complement activation, macrophage activation, cell proliferation and the production of proinflammatory cytokines and chemokines, which are then interrelated through multiple mechanisms to cause glomerular and tubular damage, tubulointerstitial inflammation and fibrosis.3
LN continues to be one of the most severe manifestations of SLE. Conventionally used laboratory parameters such as elevated creatinine, proteinuria, anti-ds-DNA antibodies, and low complement levels are not enough sensitive or specific to detect disease activity.4 Significant kidney damage may occur before impaired renal function becomes clinically evident.2
MCP-1 is a potent chemoattractant of monocytes, T cells and natural killer cells, responsible for inducing tubular or glomerular kidney damage. It is known that renal parenchymal cells, particularly mesangial cells and tubular epithelial cells, produce MCP-1 in response to the stimulus produced by IL-1, TNF-α, IFN-γ and circulating IgG immune complexes.5 Previous research have demonstrated that MCP-1 levels in the urine of patients with LN correlate with disease activity. It is proposed to measure the levels of MCP-1 excretion in urine, which would be an excellent indicator of its local production and secretion, and therefore could be considered a biomarker that reflects inflammatory activity in the kidney.2,6–8
The identification of reliable biomarkers will help to assess LN activity,9,10 identify patients at risk for kidney damage and facilitate early diagnosis. Therefore, the present study aims to evaluate the expression levels of the chemokine MCP-1 in patients with LN.
Materials and methodsStudy populationParticipants were selected by applying the criteria of the American College of Rheumatology,11–14 according to which patients with SLE or LN must meet at least 4 requirements to be included in the case group. For the selection of controls, apparently healthy people with no clinical or family history of autoimmune diseases or kidney disease were selected.
The present study included 112 patients with SLE or LN, who were recruited in the Institute of Diagnostic Laboratory Services and Health Research (Instituto de Servicios de Laboratorios de Diagnóstico e Investigación en Salud) (Seladis). As a control group, 28 healthy individuals were selected.
Study designThe present study is of case-control type. The case group was subdivided into 4 subgroups based on the disease activity, which were: patients with active SLE without antecedents of LN; patients with active LN, patients with inactive SLE without a history of LN, and patients with inactive LN. The disease activity was evaluated by analysis of clinical data, levels of anti-ds-DNA, anti-nucleosome, anti-C1q, β2-microglobulin, and complement C3 and C4. The qRT-PCR test was used in the first urine sample of the morning to evaluate the expression levels of the chemokine MCP-1; these samples were submitted by patients from the different study groups, including the participants from the control group. The urinary levels of MCP-1 were associated with serum values of the aforementioned serological markers.
qRT-PCRTotal RNA was isolated using the Trizol reagent; cDNA synthesis was performed from 30 μg of total RNA extracted, using the commercial kit Super Script III Reverse Transcriptase (Invitrogen, USA.), according to the protocol specified by the manufacturer. Real-time PCR reactions were developed in the Step One Plus thermal cycler (Applied Biosystems). For the PCR reaction, specific primers were used based on the sequences of the MCP-1 human gene 5′-AACACTCACTCCACAACCCAAG-3′ (forward), 5′-TGTGGTTCAAGAGGAAAAGCAAT-3′ (reverse); as endogenous control, primers of the human β-actin gene 5′-GCTCCTCCTGAGCGCAAG-3′ (forward) and 5′- CATCTGCTGGAAGGTGGACA-3′ (reverse) were used.
The PCR mix was designed for a final volume of 20 μl, complying with the manufacturer's specifications: 4 μl of cDNA (30 μg/ml), 10 μl of SYBR Green Supermix (iTaqTM optimized mix), 0.4 μl of direct specific primer (2 μM), 0.4 μl of reverse specific primer (2 μM) and nuclease-free water.
Finally, the PCR mix with the cDNA samples, was taken to the thermal cycler, where the sample was amplified as follows: a first initial denaturation step of 10 min at 95 °C, followed by 40 cycles (15 s to 95 °C of denaturation, 60 s at 60 °C of alignment and extension).
Relative quantification of gene expressionFor the relative quantification of the amplified product, the threshold cycle (Ct) value, registered in the Applied Biosystems software, based on the model Delta Delta Ct (ΔΔCt), developed by PE Applied Biosystems (Perkin Elmer, Forster City, CA, USA.) was used. The values obtained for each target gene were normalized with the levels of Ct obtained from a calibrator gene (control patients). The calibrator gene is considered an internal control for inter-analysis variations. The formula 2–ΔΔCt15 was used for the calculation:
The interpretation of the expression level of the gene under study is based on the value of the expression normalized to a constant value of 1, which means that a value equal to 1 corresponds to an identical expression between the target gene and the calibrator, values lower than 1 correspond to a lower expression of the target gene compared to the calibrator and values higher than 1 correspond to a greater expression of the target gene compared to the calibrator.
Immunoenzymatic assaysThe following laboratory tests were performed on all patients under study: anti-ds-DNA antibodies (Trinity, USA, Cod. 2327670), β2-microglobulin (ORGENTEC, Germany, Cod. ORG 5BM), anti-nucleosome antibodies (ORGENTEC, Germany, Cod. ORG 528) and anti-C1q antibodies (ORGENTEC, Germany, Cod. ORG 549). All immunoserology assays were performed using the instructions of the manufacturers of the commercial kits.
Statistical analysisThe data were analyzed with the statistical package PSPP 1.6.2 (GNU General Public License), while quantitative variables were described using median and 25th and 75th percentiles, and categorical data were presented as frequency and percentage; for nonparametric tests, the analysis was performed using the Mann-Whitney U test. In the case of independent samples (urinary biomarker levels) between study groups, the Kruskal-Wallis test was used. The Spearman Rho test was used to correlate urinary biomarkers with other variables (serological markers). The differences were considered significant with a p-value < 0.05.
The gene expression was assessed by the 2–ΔΔCt method, which provides information about the expression level of a gene in relative values. The values obtained represent the number of times the target gene is expressed with respect to the expression level of the calibrator gene.
Ethical considerationsThe protocol of this study was reviewed and approved by the Research Ethics Committee of the Universidad Mayor de San Andrés, La Paz, Bolivia, through the CEI-UMSA 0416 endorsement of 2016. All participants were informed verbally and in writing about the objective and scope of this study; the benefits and implications of their participation were also explained. The informed consent was signed by all participants without any pressure mechanism.
ResultsAmong the initial results that were detected in our study, we observed that the study population was made up of people whose ages were within the range of 17–76 years, with different age ranges between the different groups that made up this research, a fact that is shown in Table 1; for this variable, no significant differences were observed between the different groups. Likewise, since LN and SLE are diseases more common in women, our majority population was of female sex, a data that is also visible in Table 1.
Comparison of demographic characteristics, clinical findings, and laboratory data between control-case patients.
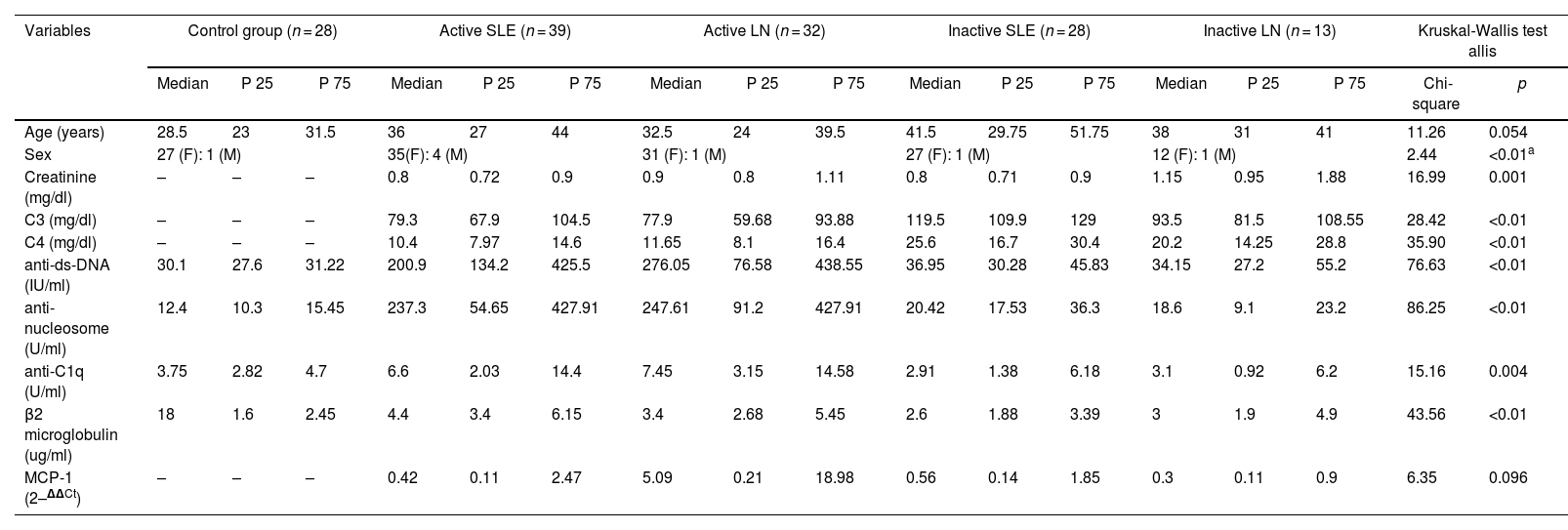
| Variables | Control group (n = 28) | Active SLE (n = 39) | Active LN (n = 32) | Inactive SLE (n = 28) | Inactive LN (n = 13) | Kruskal-Wallis test allis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | P 25 | P 75 | Median | P 25 | P 75 | Median | P 25 | P 75 | Median | P 25 | P 75 | Median | P 25 | P 75 | Chi-square | p | |
| Age (years) | 28.5 | 23 | 31.5 | 36 | 27 | 44 | 32.5 | 24 | 39.5 | 41.5 | 29.75 | 51.75 | 38 | 31 | 41 | 11.26 | 0.054 |
| Sex | 27 (F): 1 (M) | 35(F): 4 (M) | 31 (F): 1 (M) | 27 (F): 1 (M) | 12 (F): 1 (M) | 2.44 | <0.01a | ||||||||||
| Creatinine (mg/dl) | – | – | – | 0.8 | 0.72 | 0.9 | 0.9 | 0.8 | 1.11 | 0.8 | 0.71 | 0.9 | 1.15 | 0.95 | 1.88 | 16.99 | 0.001 |
| C3 (mg/dl) | – | – | – | 79.3 | 67.9 | 104.5 | 77.9 | 59.68 | 93.88 | 119.5 | 109.9 | 129 | 93.5 | 81.5 | 108.55 | 28.42 | <0.01 |
| C4 (mg/dl) | – | – | – | 10.4 | 7.97 | 14.6 | 11.65 | 8.1 | 16.4 | 25.6 | 16.7 | 30.4 | 20.2 | 14.25 | 28.8 | 35.90 | <0.01 |
| anti-ds-DNA (IU/ml) | 30.1 | 27.6 | 31.22 | 200.9 | 134.2 | 425.5 | 276.05 | 76.58 | 438.55 | 36.95 | 30.28 | 45.83 | 34.15 | 27.2 | 55.2 | 76.63 | <0.01 |
| anti-nucleosome (U/ml) | 12.4 | 10.3 | 15.45 | 237.3 | 54.65 | 427.91 | 247.61 | 91.2 | 427.91 | 20.42 | 17.53 | 36.3 | 18.6 | 9.1 | 23.2 | 86.25 | <0.01 |
| anti-C1q (U/ml) | 3.75 | 2.82 | 4.7 | 6.6 | 2.03 | 14.4 | 7.45 | 3.15 | 14.58 | 2.91 | 1.38 | 6.18 | 3.1 | 0.92 | 6.2 | 15.16 | 0.004 |
| β2 microglobulin (ug/ml) | 18 | 1.6 | 2.45 | 4.4 | 3.4 | 6.15 | 3.4 | 2.68 | 5.45 | 2.6 | 1.88 | 3.39 | 3 | 1.9 | 4.9 | 43.56 | <0.01 |
| MCP-1 (2–ΔΔCt) | – | – | – | 0.42 | 0.11 | 2.47 | 5.09 | 0.21 | 18.98 | 0.56 | 0.14 | 1.85 | 0.3 | 0.11 | 0.9 | 6.35 | 0.096 |
As for the results for the markers associated with LN and SLE, all participants of the control group gave non-reactive results to the tests of anti-ds-DNA antibodies, anti-nucleosome antibodies, anti-C1q antibodies and β2-microglobulin, a situation that was predictable, confirming that these were people who did not have any type of autoimmune disease (Table 1). In contrast, the patients in the case group presented results with significant differences, with respect to the control group, in the following serological tests: serum levels of anti-ds-DNA, anti-nucleosome antibodies, levels of β2-microglobulin and levels of C3 and C4 complement (p < 0.001). Another result that was also observed is the existence of significant differences in serum creatinine levels and anti-C1q levels (p < 0.05) between patients who had active disease and those who were in remission (Table 1).
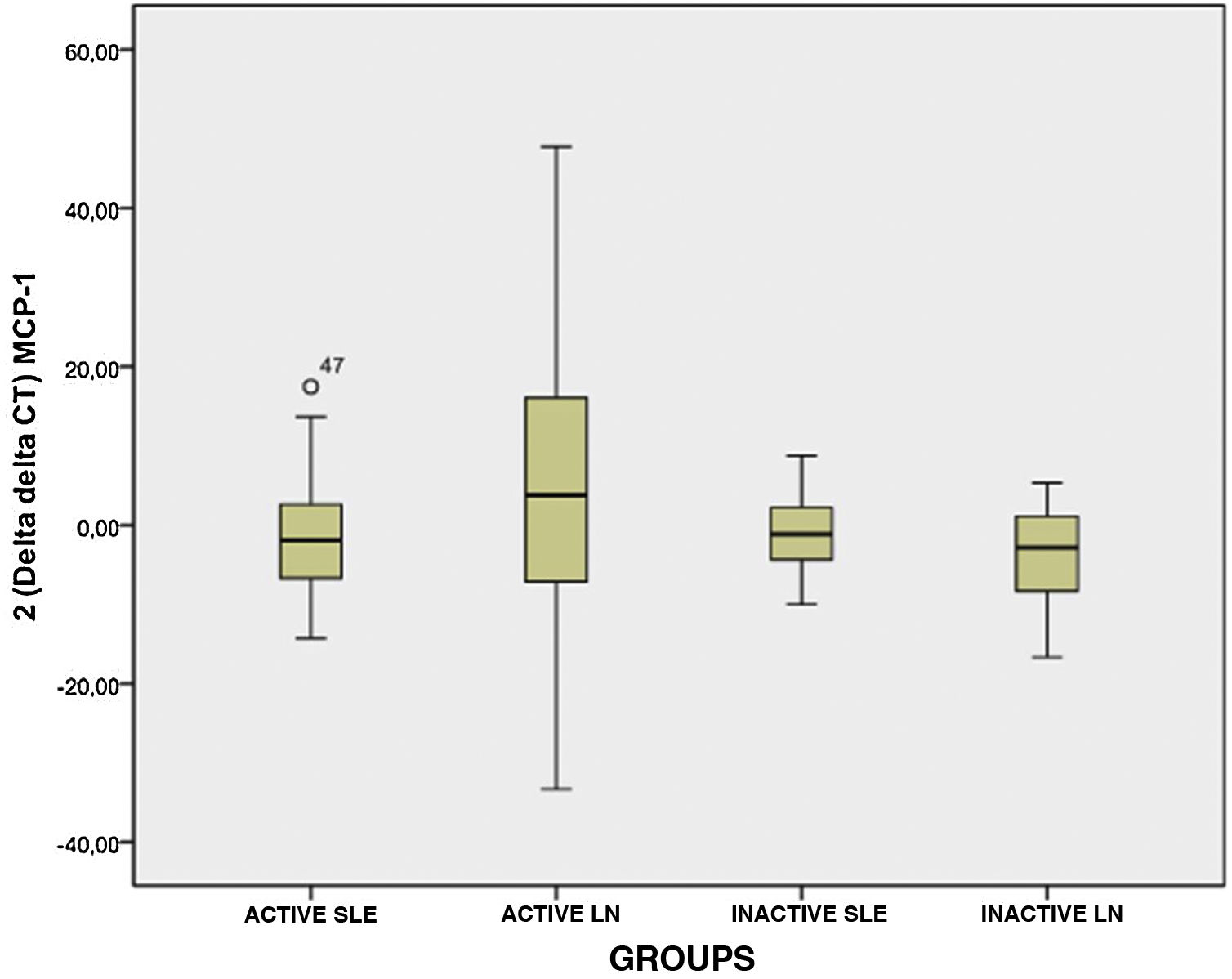
Fig. 1 shows the results of the comparison made for the expression levels of MCP-1 between the different subgroups that made up the case group; it is observed that there is no significant difference between these subgroups (p > 0.05), as well as that the expression levels for subgroup II (active NL) presented a wide range, and also between subgroups I, II and IV; as shown in Fig. 1, it is seen that these presented similar expression levels.
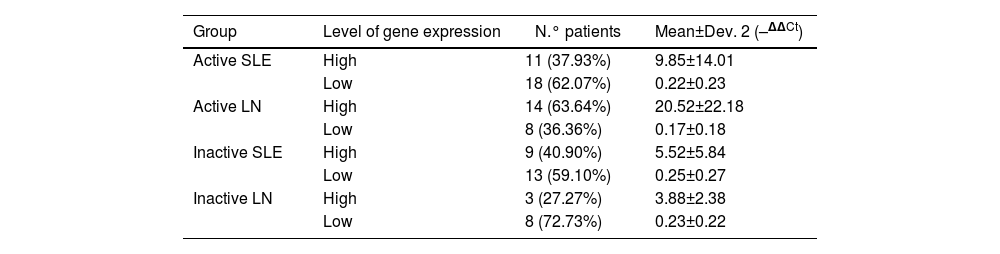
The values of the expression levels of the chemokine MCP-1 in the different subgroups that make up the group of cases are presented in Table 2. The patients who present active LN (subgroup II) show that they have high levels of expression of this marker, which turn out to be 20.52±22.18 times higher than the levels of the control group; these levels of expression are increased in 63.64% of the cases that make up this subgroup.
Normalized relative quantification of the expression of the MCP-1 gene assessed by RT-qPCR in urine samples.
| Group | Level of gene expression | N.° patients | Mean±Dev. 2 (–ΔΔCt) |
|---|---|---|---|
| Active SLE | High | 11 (37.93%) | 9.85±14.01 |
| Low | 18 (62.07%) | 0.22±0.23 | |
| Active LN | High | 14 (63.64%) | 20.52±22.18 |
| Low | 8 (36.36%) | 0.17±0.18 | |
| Inactive SLE | High | 9 (40.90%) | 5.52±5.84 |
| Low | 13 (59.10%) | 0.25±0.27 | |
| Inactive LN | High | 3 (27.27%) | 3.88±2.38 |
| Low | 8 (72.73%) | 0.23±0.22 |
An elevated level of expression of the chemokine MCP-1 was also observed in those patients who were part of subgroup I (active SLE), with an expression level 9.85±14.01 times higher than the levels of the control group, present in 37.93% (Table 2).
In the case of patients with inactive diseases, MCP-1 chemokine expression values lower than those presented by patients with active diseases were observed, these values being 5.52±5.84 and 3.88±2.38 for subgroups III (inactive SLE) and IV (inactive LN), respectively; these levels of expression were present in a minority percentage of the subgroup (Table 2); the majority percentage was the low level of expression, with values of 0.25±0.27 (59.1%) and 0.23±0.22 (72.73%) for subgroups III (inactive SLE) and IV (inactive LN), respectively.
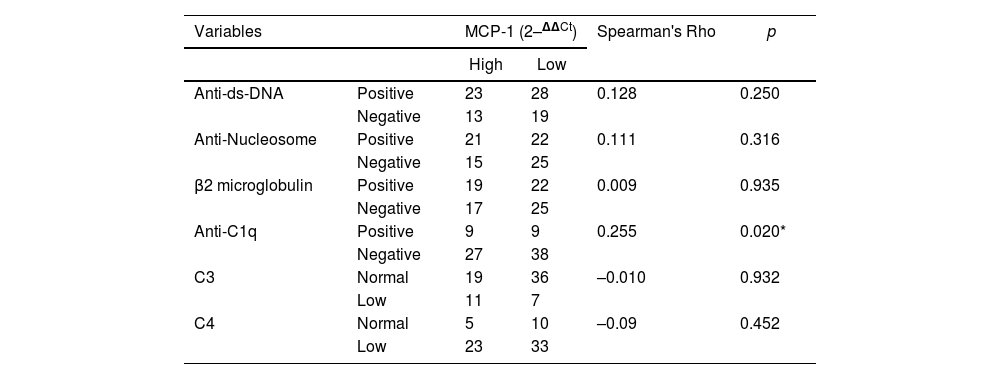
Table 3 shows the correlation between the expression levels of the chemokine MCP-1 and the values of the markers: anti-ds-DNA, anti-nucleosome antibodies, anti-C1q, C3, C4 and β2-microglobulin; Spearman's Rho test was performed for this correlation. A striking result observed in our study was that no significant response was evident in the correlation of the expression of the chemokine MCP-1 and the markers anti-ds-DNA, anti-nucleosome antibodies, C3, C4 and β2-microglobulin, which in all cases presented a p > 0.05. In contrast, there was an interesting finding of positive correlation between urinary MCP-1 levels and the serological marker anti-C1q antibodies (p < 0.05), suggesting that this marker can be used to assess disease activity.
Summary of the correlation analysis of urinary expression levels of MCP-1 chemokine with the serological markers evaluated.
| Variables | MCP-1 (2–ΔΔCt) | Spearman's Rho | p | ||
|---|---|---|---|---|---|
| High | Low | ||||
| Anti-ds-DNA | Positive | 23 | 28 | 0.128 | 0.250 |
| Negative | 13 | 19 | |||
| Anti-Nucleosome | Positive | 21 | 22 | 0.111 | 0.316 |
| Negative | 15 | 25 | |||
| β2 microglobulin | Positive | 19 | 22 | 0.009 | 0.935 |
| Negative | 17 | 25 | |||
| Anti-C1q | Positive | 9 | 9 | 0.255 | 0.020* |
| Negative | 27 | 38 | |||
| C3 | Normal | 19 | 36 | –0.010 | 0.932 |
| Low | 11 | 7 | |||
| C4 | Normal | 5 | 10 | –0.09 | 0.452 |
| Low | 23 | 33 | |||
The control of LN activity is essential to prevent an irreversible loss of kidney function, and a prompt and timely treatment is required. In clinical practice, no laboratory test is sensitive or specific enough to detect disease activity early.16 Consequently, the identification of non-invasive biomarkers, easy to measure and highly accurate, would be useful in monitoring the disease.
Renal cells (endothelial, mesangial, tubular epithelium, interstitial cells and podocytes) can express chemokines after stimulation.16 Proinflammatory stimuli induce MCP-1 expression, which ultimately leads to tissue injury. MCP-1 plays an important role in the pathogenesis of the progression of kidney disease.6 Recently, several studies related to the evaluation of the clinical activity of MCP-1 were carried out, including the meta-analyses by Lee et al.6 and Wang et al.,17 conducted in populations with different demographic distribution; in both cases, it is suggested that MCP-1 may be a useful biomarker to assess LN activity, being a potential biomarker to differentiate between active and inactive LN, since elevated levels of the chemokine MCP-1 were found in patients with active LN in urine samples, as well as in serum samples.
In 2018, El Shehaby et al.18 and Dong et al.19 evaluated the correlation between the expression levels of the chemokine MCP-1 and various markers, including anti-ds-DNA, antinucleosomes, anti-C1q, creatinine levels, complement levels (C3, C4) and β2 M, and found statistical significance (p < 0.05) in relation to the C3, C4 and anti-ds-DNA markers. This fact differs from our research, since in our case no statistically significant correlation was observed with these markers; however, unlike this study, our participants were outpatients, which made it difficult the control of the variables.
In our study, we compared the expression levels of the chemokine MCP-1 between cases and controls, and found no difference between the groups of active LN (median: 5.09 P25 0.21 and P75 18.98), active SLE (median; 0.42 P25 0.11 and P75 2.47) and the groups inactive LN (median: 0.30 P25 0.11 and P75 0.90) and inactive SLE (median: 0.56 P25 0.14 and P75 1.85) (p = 0.096). In similar studies in Egyptian and Asian populations,5,20 similar results to the present study were observed; however, there are other studies in Caucasian population16,19,21,22 that report differences in MCP-1 levels, which were significantly higher in lupus patients with kidney damage compared to the control group.
Akhter et al.23 suggest anti-C1q as a marker to detect the disease activity of LN, in the present study was found that MCP-1 has a positive correlation with the levels of anti-C1q antibodies (r = 0.255; p = 0.02). These antibodies, strongly related to LN, are found in 30–48%.24 Anti-C1q antibodies in soluble form do not activate the complement cascade; However, their binding to C1q previously attached to the glomerular basement membrane can alter their configuration and, in this way, amplify complement activation. Different researchers observed the presence of anti-C1q antibodies that correlate with NL activity.25–28
In the present study, we determined that there is no correlation between MCP-1 and other serological markers, nor with complement levels (C4, C3), possibly because they are not specific to the pathology; Ferreira et al.29 reported similar results.
An increase in the expression of the chemokine MCP-1 was evident in 63.64% of patients with active LN compared to the other groups; however, the data are not statistically significant. While many studies demonstrated significant levels of MCP-1 in patients with active LN, similar results were not reported in the present study, which possibly corresponds to factors such as the heterogeneity of the study population, or to the fact that the MCP-1 genotype expressed in the study population is different to the other populations.
ConclusionThe results obtained in the present study suggest that the chemokine MCP-1 is not a useful biomarker to evaluate the activity of LN, a fact that may be influenced by the sample size. However, the anti-C1q biomarker is suggested as a serological marker for the follow-up of the disease, and an extension and continuation of the study with a larger sample size is proposed.
FundingThis study was financed with IDH competitive resources from the Universidad Mayor de San Andrés in the 2014–2015 administration.
Conflict of interestThe authors of this article declare that they have no conflict of interest.