Hereditary hemochromatosis is a disease responsible for excess blood iron. The hemochromatosis gene has two predominant variants, H63D and C282Y single nucleotide polymorphisms. Our study aims to analyze the diagnostic utility of genotyping the 63 and 282 loci, and examine the geographic distribution of these mutations in Spain.
Methods and materialsGenotyping was performed on 94 healthy control individuals and 324 patients suspected of hereditary hemochromatosis, and also biochemical test to 313 individuals in the patients group.
ResultsThe comparison of allelic frequencies between East and West of Spain, as well as other countries located at a similar longitude, evidenced a west-east distribution gradient of the C282Y allele. In addition, heterogeneous distribution of the H63D mutation in Spain was observed. Patients who carried the 282YY genotype showed significantly higher biochemical parameters (ferritin>300μg/L, Fe>180μg/L, IST>60%, UIBC>355μg/L and CTFH>370μg/dL), which confirmed the correlation between the mutated homozygous genotype and the associated hemochromatosis phenotype.
ConclusionOur results strengthen the importance of executing genetic tests to increase the efficiency of hereditary hemochromatosis diagnosis, which reveal an interesting variability among geographical regions.
La hemocromatosis hereditaria es una enfermedad responsable del exceso de hierro en sangre. El gen de la hemocromatosis tiene dos variantes predominantes, los polimorfismos de un solo nucleótido H63D y C282Y. Nuestro estudio trata de analizar la utilidad diagnóstica del genotipado de los loci 282 y 63, y examinar la distribución geográfica de estas mutaciones en España.
Material y métodosSe realizó genotipado en 94 controles sanos y 324 pacientes con sospecha de hemocromatosis hereditaria y además, test bioquímico a 313 individuos del grupo de pacientes.
ResultadosLa comparación de frecuencias alélicas entre poblaciones del este y el oeste de España, así como de otros países localizados en longitudes similares, evidenció una distribución en gradiente del alelo C282Y. Además se observó una distribución heterogénea de la mutación H63D en España. Los pacientes portadores del genotipo 282YY mostraron parámetros bioquímicos significativamente más elevados (ferritina >300μg/L, Fe > 180μg/L, IST > 60%, UIBC > 355μg/L y CTFH>370μg/dL), confirmando la correlación entre el genotipo homozigoto mutado y el fenotipo característico de hemocromatosis.
ConclusiónNuestros resultados refuerzan la importancia de realizar pruebas genéticas para confirmar el diagnóstico de la hemocromatosis hereditaria, teniendo en cuenta la variabilidad de los datos entre ámbitos geográficos.
Pathogenic mutations on the human hemochromatosis gene (HFE) are responsible for the predominant disease concerning excess blood iron in Caucasians, hereditary hemochromatosis, an autosomal recessive disease (MIM # 235200). Excess blood iron surpasses the capacity of plasmatic transferrin to transport Fe3+ and remains in circulation as independent Fe2+ able to produce free radicals that accumulate and damage vital organs.
Most cases of hereditary hemochromatosis result from c.845G>A (p.Cys282Tyr; C282Y) and c.187C>G (p.His63Asp; H63D) mutations on HFE. C282Y mutation inhibits interaction with β2-microglobulin annulling expression of HFE protein. On the other hand, H63D alteration reduces affinity between transferrin receptor and its ligand.1 Previous studies concluded that 282YY homozygotes and C282Y/H63D heterozygotes correlate with clinical excess iron.2 However, H63D homozygotes as well as C282Y and H63D heterozygotes show weak phenotypic effects and do not confer enough risk o develop hemochromatosis.3
Following migration patterns from the original ancestor of the C282Y and H63D single nucleotide polymorphisms (SNPs),1,4 studies in population genetics have evidenced an allelic distribution gradient from high allelic frequencies in northern Europe to low frequencies in southern Europe5; thus proving HFE haplotypes to be excellent markers for the study of human population diversity and migratory processes.
The present article was prompted by a previous study on HFE polymorphisms performed in Extremadura (western region of Spain) that reported allelic frequencies of 0.196 for H63D and 0.046 for C282Y.3 Our study intended to analyze the frequency of C282Y and H63D alleles in Valencia (eastern region of Spain). Since Valencia is located at a similar latitude as Extremadura, our purpose was to compare our results with those by Rodríguez-López,3 and evaluate a putative West-East allelic distribution. Our second objective was to verify the utility of testing for C282Y and H63D SNPs in patients under study for probable hereditary excess iron in Valencia through their association with biochemical parameters.6
Patients and methodsPatientsThe group of patients comprised 324 eastern Spanish individuals (228 males and 96 females, age 54.43±17.28) under suspicion of suffering hemochromatosis or liver pathology. Due to the diagnosis protocol consented with the Hematology Service; no individuals had received treatment before the genetic testing. They were referred to the Laboratory of Molecular Genetics of the General University Hospital of Valencia (Spain) to be evaluated for suspected serum iron overload after excluding causes of exogenous origin. The control group included 94 random healthy subjects from the same area that were unrelated and gender-matched to our patients group (63 males and 31 females, age 45.48±15.8), they were included as reference of the genetic background of the population.
All individuals analyzed in this study pertained to our public health area, situated in a 200km range from the 0° meridian. Keeping in mind the Valencian ancestors of individuals included in the study, we considered a representative population of the genetic background of our area. Individuals whose last names had an obvious non-local origin were not included in the series. All participants gave written informed consent.
Biochemical analysisBiochemical parameters evidenced altered iron metabolism when: transferrin saturation index, TSI>60% for men and >50% for women; serum ferritin>300μg/L for men and >200μg/L for women; serum transferrin>50%; total iron binding capacity, TIBC>450μg/dL; unsaturated iron binding capacity, UIBC>470μg/dL. Ferritin, iron, and transferrin serum concentrations were measured by standard photometric methods using an automated clinical chemistry inmunoanalyzer (AU5400, Olympus, Chicago, IL, USA). TIBC was calculated as: TIBC=transferrin×1.25, TSI as: TSI=serum iron/TIBC×100 and UIBC as: UIBC=TIBC−serum iron. We obtained results of these biochemical parameters from 313 patients.
HFE genotypingBlood samples were extracted from each participant. Genomic DNA was isolated using a QIAamp DNA Blood Kit method (Quiagen Inc., Chatsworth, UK). PCR reactions and genotypes of the rs1799945 (H63D) and rs1800562 (C282Y) SNPs were performed by reverse dot blot commercial kit (InnoLipa HFE; Innogenetics, Zwijnaarde, Belgium) in 146 patients. Allelic discrimination Taqman probes ID C_1085600-_10 for H63D and ID C_1085595-_10 for C282Y were used in 178 patients and all controls. An Applied Biosystems TaqMan 7900 system (Applied Biosystems, Foster City, CA, USA) was used for this procedure. Detailed conditions are available on request.
Statistical analysisChi-square or Fisher's exact tests were utilized to evaluate departure from Hardy–Weinberg equilibrium of the SNPs markers and to compare allelic and genotypic frequencies in cases and controls. The effect of haplotypes was calculated through linear regression modeling assuming an additive mode of inheritance using SNPstats.7 The distribution of the quantitative variables (i.e. biochemical parameters) across the different genotypes was compared by Student's T/ANOVA or Mann–Whitney/Kruskal–Wallis tests, as appropriate (according to Kolmogorov–Smirnov test results). Following the format of an association study to compare between cases (patients referred due to biochemical alterations) and controls, odds ratio (OR) with 95% confidence intervals was used to associate between the studied SNPs and the risk to develop the condition.
In all instances, results were considered significant at the two-sided p of 0.05 level. Statistical analyses were conducted using SPSS software version 15.0 for windows (SPSS; IBM, Chicago, IL, USA).
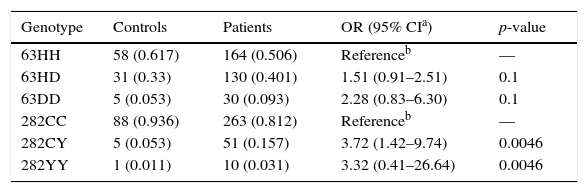
ResultsGenotypic analysis in patients and controlsThe results detailed in Table 1 obtained by univariate analysis show that both the 63DD and 282YY homozygous genotypes were more frequent among patients. However, only in the case of 63DD did the difference reach statistical significance [OR=3.32 (0.41–26.64) p<0.01 respectively]. Furthermore, the 282CY heterozygote was also found to be significantly more frequent in patients than in controls [OR=3.72 (1.42–9.74), p<0.01].
Distribution of HFE variants in 324 patients with suspicion of surplus iron and 94 control subjects.
| Genotype | Controls | Patients | OR (95% CIa) | p-value |
|---|---|---|---|---|
| 63HH | 58 (0.617) | 164 (0.506) | Referenceb | — |
| 63HD | 31 (0.33) | 130 (0.401) | 1.51 (0.91–2.51) | 0.1 |
| 63DD | 5 (0.053) | 30 (0.093) | 2.28 (0.83–6.30) | 0.1 |
| 282CC | 88 (0.936) | 263 (0.812) | Referenceb | — |
| 282CY | 5 (0.053) | 51 (0.157) | 3.72 (1.42–9.74) | 0.0046 |
| 282YY | 1 (0.011) | 10 (0.031) | 3.32 (0.41–26.64) | 0.0046 |
There was no departure from Hardy-Weinberg equilibrium in the genotype frequencies of the control population (p>0.05 for both SNPs). A complete linkage disequilibrium (D′=0.997; r′=0.20) was observed between the studied polymorphisms.
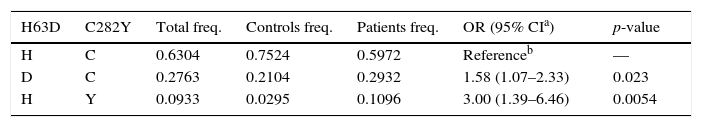
The estimation of haplotype frequencies is shown in Table 2. The wild type HC haplotype was present in 59.72% of patients and 75.24% of controls. The DC heterozygous haplotype was found in 29.32% of patients and 21.04% of controls, whilst the other heterozygous haplotype, HY, was identified in 10.96% of patients and 2.95% of controls. The double mutant haplotype, DY, was not found in our series as a result of the complete linkage disequilibrium between SNPs.
The statistical differential study on the distribution of the haplotype diversity among our group of patients and controls (Table 2) showed significant p-values (p-value=0.023 for heterozygous DC haplotype and p-value=0.0054 for the homozygous HY haplotype) for both haplotypes, confirming the correlation between C282Y-H63D SNPs and the development of the associated biochemical alterations.
Association of genotypes and haplotypes with biochemical parameters of iron statusEvaluation of biochemical parameters in our group of 324 patients found 11 patients with no biochemical data, and 60 individuals who had all measured values of iron metabolism in normal range. At least one altered biochemical parameter was observed in 253 patients.
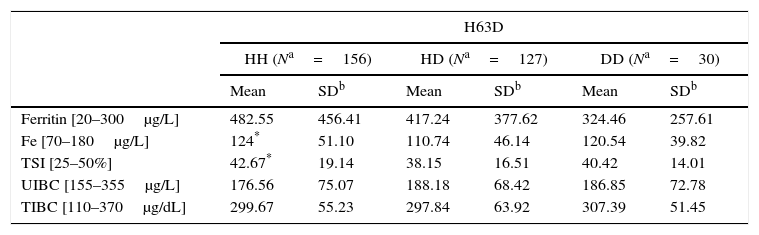
The correlation analysis between biochemical parameters and the different genotype distribution in our group of 313 patients (we did not have biochemical results from 11 patients) showed significant statistical differences in serum iron levels and TSI, between 63HH wild type homozygotes and 63HD heterozygotes (124% vs. 110.74% and 42.67% vs. 38.15%, respectively). Since no differences were found according to the mutant 63DD genotype, we did not consider these results to be clinically relevant (Table 3).
Association of SNPs with biochemical parameters.
| H63D | ||||||
|---|---|---|---|---|---|---|
| HH (Na=156) | HD (Na=127) | DD (Na=30) | ||||
| Mean | SDb | Mean | SDb | Mean | SDb | |
| Ferritin [20–300μg/L] | 482.55 | 456.41 | 417.24 | 377.62 | 324.46 | 257.61 |
| Fe [70–180μg/L] | 124* | 51.10 | 110.74 | 46.14 | 120.54 | 39.82 |
| TSI [25–50%] | 42.67* | 19.14 | 38.15 | 16.51 | 40.42 | 14.01 |
| UIBC [155–355μg/L] | 176.56 | 75.07 | 188.18 | 68.42 | 186.85 | 72.78 |
| TIBC [110–370μg/dL] | 299.67 | 55.23 | 297.84 | 63.92 | 307.39 | 51.45 |
| C282Y | ||||||
|---|---|---|---|---|---|---|
| CC (Na=253) | CY (Na=50) | YY (Na=10) | ||||
| Mean | SDb | Mean | SDb | Mean | SDb | |
| Ferritin [20–300μg/L] | 456.05 | 414.89 | 365.81 | 376.61 | 431.90 | 501.29 |
| Fe [70–180μg/L] | 116.16 | 47.05 | 121.04 | 49.72 | 157* | 62.82 |
| TSI [25–50%] | 39.90 | 17.17 | 41.37 | 19.55 | 54.49* | 18.48 |
| UIBC [155–355μg/L] | 183.49* | 69.93 | 186.59* | 82.77 | 132.01 | 60.12 |
| TIBC [110–370μg/dL] | 298.70 | 58.12 | 307.21 | 61.41 | 287 | 53.84 |
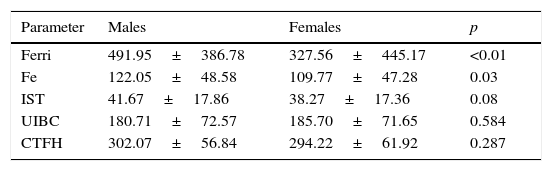
In contrast to the results of H63D, the comparison between 282CC wild type homozygotes and 282CY heterozygotes as well as 282YY homozygotes, determined significant higher values of serum iron (116.16 vs. 121.04 and 157; p<0.05) and TSI (39.90 vs. 41.37 and 54.49; p<0.05) (Table 3). The values of iron, ferritin and IST parameters were significantly lower among female patients (Table 4).
Differences observed in the analytical parameters of ferric metabolism, between male and female patients.
| Parameter | Males | Females | p |
|---|---|---|---|
| Ferri | 491.95±386.78 | 327.56±445.17 | <0.01 |
| Fe | 122.05±48.58 | 109.77±47.28 | 0.03 |
| IST | 41.67±17.86 | 38.27±17.36 | 0.08 |
| UIBC | 180.71±72.57 | 185.70±71.65 | 0.584 |
| CTFH | 302.07±56.84 | 294.22±61.92 | 0.287 |
The utility of testing for C282Y and H63D SNPs to diagnose hemochromatosis was assessed in the 282YY homozygote, the genotype displaying pathologically altered biochemical parameters. Among the 11 homozygous carriers, one was asymptomatic (9.1%) but the other ten individuals were diagnosed of hemochromatosis (90.9%). Among the 10 affected patients, one presented normal biochemical parameters while nine subjects exhibited deleterious biochemical parameters. In the group of nine 282YY individuals with altered biochemical status, one revealed low transferrin levels; and eight showed high TSI (>45%), high serum ferritin (>400μg/L) and/or low UIBC (<155μg/dL).
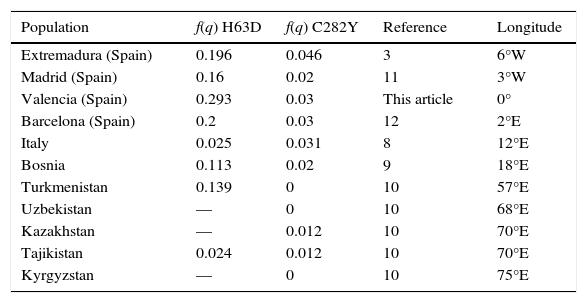
Distribution gradient of allelic frequenciesThe assessment of the general Valencian population (control group) distinguished higher allelic frequencies for C282Y in western Spain (0.046)3 in contrast with eastern Spain (0.03 (q) MAF), displaying a tendency that confirmed a west-east gradient in Spain. When compared with eastern countries located at a similar latitude (Table 5), decreasing frequencies of C282Y allele manifest a west-east gradient; frequency of 0.031 was determined for Italy,8 0.020 for Bosnia,9 and 0.012 for Kazakhstan and Tajikistan.10 No individuals carrying C282Y mutations were found in farther eastern countries.
Frequency of C282Y and H63D variants in different longitudes.
| Population | f(q) H63D | f(q) C282Y | Reference | Longitude |
|---|---|---|---|---|
| Extremadura (Spain) | 0.196 | 0.046 | 3 | 6°W |
| Madrid (Spain) | 0.16 | 0.02 | 11 | 3°W |
| Valencia (Spain) | 0.293 | 0.03 | This article | 0° |
| Barcelona (Spain) | 0.2 | 0.03 | 12 | 2°E |
| Italy | 0.025 | 0.031 | 8 | 12°E |
| Bosnia | 0.113 | 0.02 | 9 | 18°E |
| Turkmenistan | 0.139 | 0 | 10 | 57°E |
| Uzbekistan | — | 0 | 10 | 68°E |
| Kazakhstan | — | 0.012 | 10 | 70°E |
| Tajikistan | 0.024 | 0.012 | 10 | 70°E |
| Kyrgyzstan | — | 0 | 10 | 75°E |
Frequency of H63D SNP in Valencia (0.293 (q) MAF) was compared to other areas of the Iberian Peninsula; 0.196 was estimated in Extremadura,3 0.16 in Madrid,11 0.2 in Barcelona12 and 0.270 in Murcia,13 revealing a more heterogeneous distribution of the H63D in Spain than in Northern Europe. However, when Spanish H63D allelic frequencies were compared with eastern countries, no evident distribution gradient was recognized; frequency of 0.025 was determined in Italy,8 0.113 in Bosnia,9 0.139 in Turkmenistan and 0.024 in Tajikistan.10
DiscussionThe present study demonstrated the adequacy of genotyping C282Y risk SNP to diagnose hemochromatosis on patients suspected of suffering hereditary excess blood iron, since 10 out of 11 subjects carrying the homozygote 282YY genotype were diagnosed with hereditary hemochromatosis. Carriers of both C282Y and 282YY genotypes were more frequently found among hereditary hemochromatosis suspected patients than in the healthy control group. In addition, the 282YY homozygotes exhibited an evident biochemical impact, with high serum ferritin, TSI and serum iron, when comparing them with the CC282 homozygote and C282Y heterozygote (Table 3). The series corroborates that altered biochemical parameters among women reach lower levels than among males, in proportion to what is described in physiological ranges. That is consistent with that the proportion of females among probands diagnosed on the basis of clinical symptoms is around 20% percent lower than in males. This sex difference has been attributed to the lower degree of iron overload in women because of menstruation, pregnancy, and lactation.14
Celtic origin for C282Y mutation in Ireland (longitude 8°W) is widely accepted due to its geographic distribution and high frequency in Northern Europe,15 cause of the detailed distribution gradient of the C282Y change from higher frequencies in the north to lower frequencies in Southern Europe.16 Our data also revealed a decreasing gradient from west to east along Spanish geography, from longitude 6°W to 0°, which is according to minimal allele frequencies flanking our longitudes, and concurs with the distribution of Celtic settlements in Celtiberia.17 A west-east gradient decreasing from Ireland through Northern Europe was also described by Olsson.18 Therefore, C282Y mutation spread from north to south of Europe and from west to east toward Asia (Table 5).
In addition to the proposed North African origin for the H63D polymorphism, it has been described that genetic diversity in Southern Europe is higher than in other regions of the continent due to gene flow from North Africa.19 Population genetic studies corroborated this hypothesis using identical by descent segments as a calculation method of recombination degree, showing a significant longitudinal gradient where the highest sharing is in the Iberian Peninsula.20
Eastern Mediterranean populations share more identical by descent segments with the Near East, where low or inexistent frequencies of H63D have been found, than with western North Africa. In similar manner, identical by descent segments confirm that North African ancestry is highest in southwestern Europe and decreases in northern regions.21 These and other conclusions corroborated that the intermingling of gene pools between western North Africa and southwestern Iberia during the Moorish occupation (711–1492 CE), could be responsible for the high H63D frequencies observed in Spain.
Although there are cases of H63D/C282Y heterozygotes, these variants segregate separately. The theory of independent gene flow of H63D and C282Y haplotypes and no homologous recombination between 63 and 282 codon positions is reinforced by the known absence of the 282YY/63DD haplotype. Subsequent to the aforementioned genetic movements, C282Y and H63D variants were also taken to the Americas by Spaniards during the 16th century.22
We could not confirm the results of previous studies3 stating that the H63D SNP contributes to the modification of biochemical parameters, considering we did not obtain statistically significant results from D63D nor the double heterozygote (H63D/C282Y). However, although serum ferritin has been used as main screening test in routine clinical management to suspect hereditary hemochromatosis, cutoffs appropriate for age and sex may be used. It is essential to differentiate the elevation of the serum ferritin concentration in other liver disorders such as alcoholic liver disease, chronic viral hepatitis, and nonalcoholic steatohepatitis. Serum ferritin is also an acute-phase reactant, and levels can be elevated during infection or chronic inflammation or when the subject has a histiocytic neoplasm. Our series present too increased levels of iron, but especially of ferritin, among normal homozygous H63H individuals; this deviation is noted even in normal homozygous individuals for both variants. It seems that this shows an indication of the genetic diagnosis of hemochromatosis, in order to establish a differential diagnosis between this set of hepatic pathologies.
The overall results in ferritin levels for all genotypes (Table 3) suggest that the series could include a large group of individuals affected with hepatitis, whose genetic study indication could have been not adequate; in consequence, we recognize a miscalculation around the obtained false negative association regarding the ferritin levels.
Considering the mentioned studies and results, we conclude that the particular distribution of the C282Y and H63D variants, and their correlation with the manifestation of hemochromatosis disease, demonstrate the importance of studying the regional frequencies of these alleles to conduct a specialized hereditary hemochromatosis diagnosis.
The analytical validity of the transferrin saturation test can be evaluated by its sensitivity, specificity, and predictive value for the genotype, which depend in turn on the underlying genetic risk variant frequencies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestNone to declare.
The authors would like to thank the Digestive Service, Hepatology Service and the patients participating in this study. The authors are also grateful to Dr. María Dolores Real and Dr. María Dolores Moltó from the University of Valencia, for facilitating the staying and execution of the final degree projects of our students.