Septic shock is a highly lethal and prevalent disease. Progressive circulatory dysfunction leads to tissue hypoperfusion and hypoxia, eventually evolving to multiorgan dysfunction and death. Prompt resuscitation may revert these pathogenic mechanisms, restoring oxygen delivery and organ function. High heterogeneity exists among the determinants of circulatory dysfunction in septic shock, and current algorithms provide a stepwise and standardized approach to conduct resuscitation.
This review provides the pathophysiological and clinical rationale behind ANDROMEDA-SHOCK-2, an ongoing multicenter randomized controlled trial that aims to compare a personalized resuscitation strategy based on clinical phenotyping and peripheral perfusion assessment, versus standard of care, in early septic shock resuscitation.
El shock séptico es una enfermedad altamente letal y prevalente. La disfunción circulatoria progresiva causa hipoperfusión tisular e hipoxia, evolucionando eventualmente a disfunción multiorgánica y muerte. La reanimación temprana puede revertir estos mecanismos patogénicos, restaurando el suministro de oxígeno y la función orgánica. Existe alta heterogeneidad entre los determinantes de la disfunción circulatoria en el shock séptico, los algoritmos actuales aportan un enfoque gradual y estandarizado para la realización de la reanimación.
Esta revisión aporta el fundamento patofisiológico y clínico que se esconde tras ANDROMEDA-SHOCK-2, un ensayo controlado aleatorizado multicéntrico continuado, cuyo objetivo es comparar una estrategia de reanimación personalizada basada en la fenotipificación clínica y la evaluación de la perfusión periférica, en comparación con el estándar de cuidados, en la reanimación temprana del paciente con shock séptico.
Septic shock is a complex syndrome, with an estimated mortality of 40%,1 and high occupancy rates of intensive care units, thus determining a significant health care expenditure.2 It is estimated that 11 million people die each year from sepsis, with an unequal distribution throughout the world.3
Within the pathogenesis of septic shock, progressive hypoperfusion plays a pivotal role, determining an overall inability to provide oxygen to sustain cell metabolism, generating tissue hypoxia, multiple organ dysfunction and, eventually, death.4
The treatment of this syndrome is complex and facing a narrow window of opportunity to reverse the pathogenic mechanisms that determine adverse outcomes.5 Hemodynamic resuscitation can deactivate this vicious cycle, improve oxygen delivery, and restore organ function. However, if resuscitation is not conducted judiciously, it has the potential to cause damage by over-resuscitation,6 perpetuating organ dysfunction, and adding morbidity to critically ill patients.7
Since the 1990s, different strategies have been proposed to optimize the resuscitation process in patients with septic shock. These strategies have evolved in the light of cumulative physiological and clinical evidence. Pioneers in the protocolization of shock management, such as Hayes et al., focused on optimizing cardiac output or other macrohemodynamic variables as a means to restore tissue perfusion.8 Later on, Rivers et al. and Bakker et al. tested protocols targeting perfusion-related variables such as central venous oxygen saturation (ScvO2) or lactate to improve outcomes with promising results.9,10 This latter became the standard target during the last decade. In ANDROMEDA-SHOCK, capillary refill time (CRT) was validated as a novel resuscitation target exhibiting superiority over lactate in several aspects.11
However, all these protocols are based on common, sequential, and structured care for a highly heterogeneous disease. In addition, the relative determinants of circulatory dysfunction (e.g., hypovolemia, vasoplegia or myocardial dysfunction) may vary from patient to patient, determining true hemodynamic phenotypes.12 Therefore, there is a risk of missing the window of opportunity for effective interventions, and on the other hand, subjecting patients to potentially ineffective interventions, such as fluid administration to patients with predominant vasoplegia.
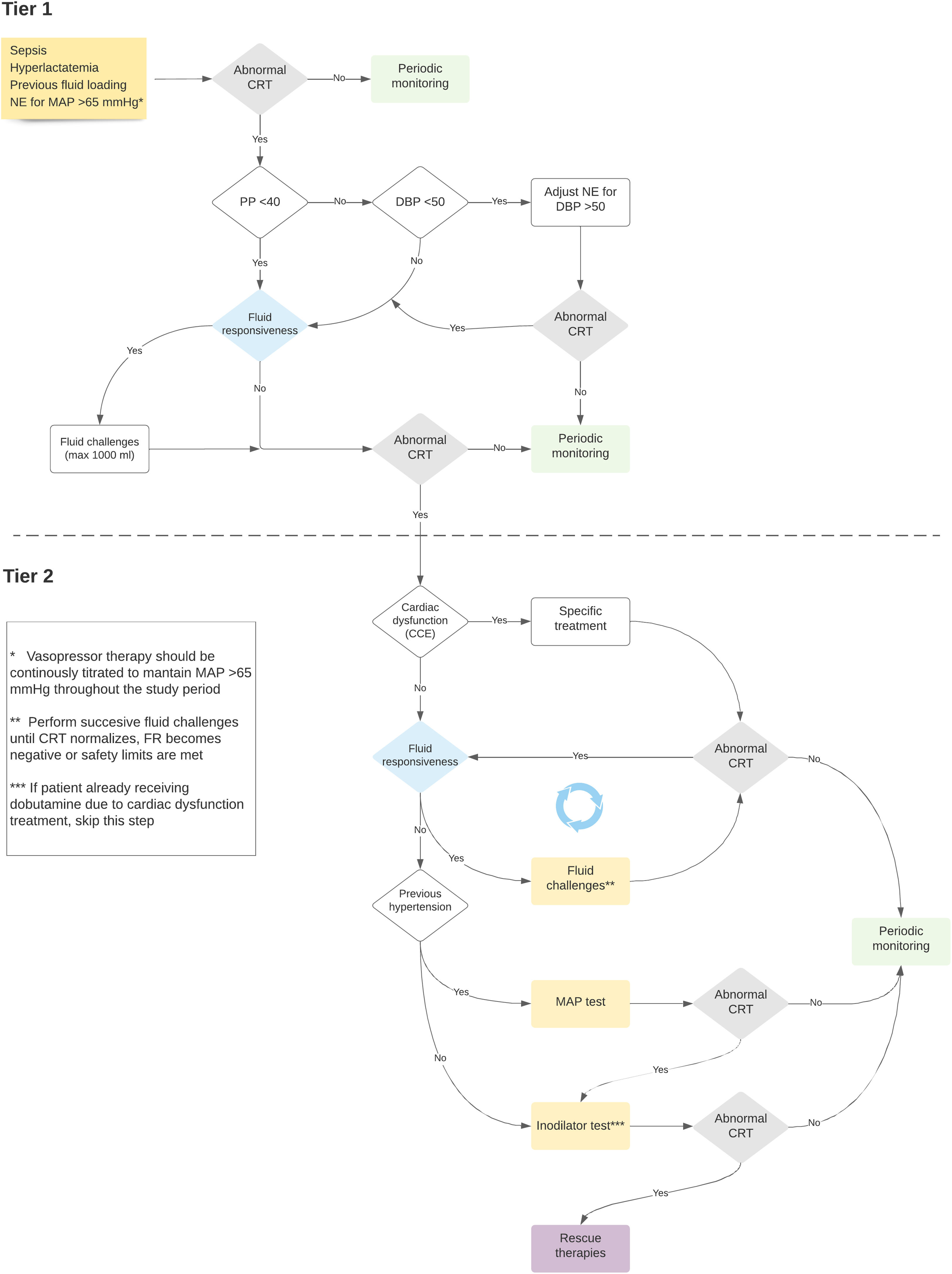
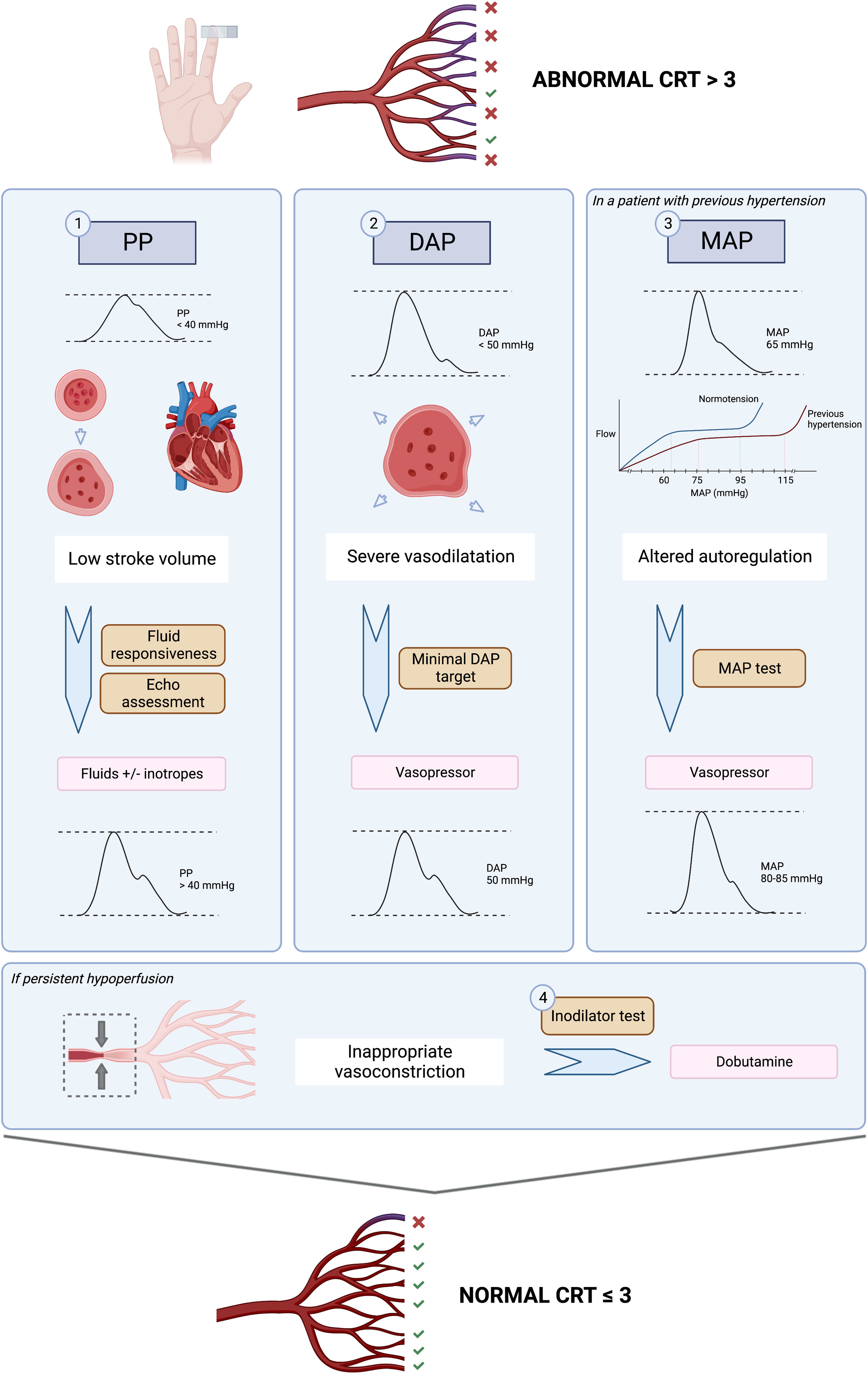
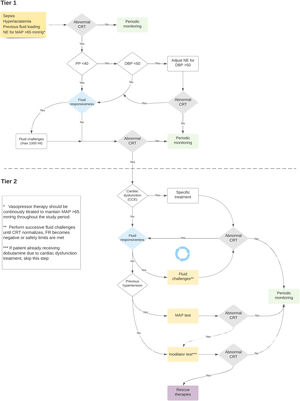
The ANDROMEDA-SHOCK-2 (A2) study is a randomized clinical trial that aims to compare a resuscitation strategy based on hemodynamic phenotypes and normalization of peripheral perfusion versus standard of care in patients with septic shock.13 The proposed strategy is a multidimensional effort to try to personalize early septic shock resuscitation by using simple bedside available tools with a strong physiological background (Figs. 1 and 2, Table 1). The aim of this review is to explore the physiological and clinical rationale for the management algorithm proposed in A2
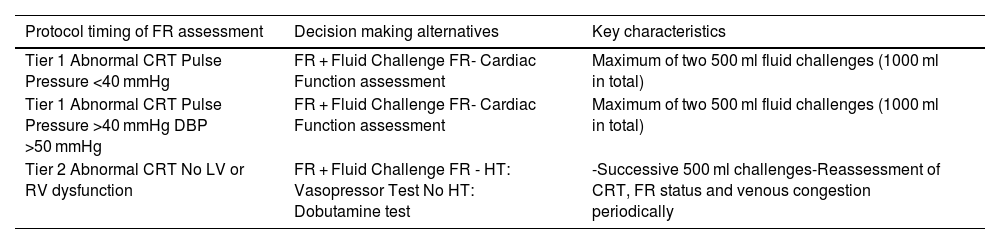
Timing of fluid responsiveness assessment and fluid challenges throughout the study protocol.
| Protocol timing of FR assessment | Decision making alternatives | Key characteristics |
|---|---|---|
| Tier 1 Abnormal CRT Pulse Pressure <40 mmHg | FR + Fluid Challenge FR- Cardiac Function assessment | Maximum of two 500 ml fluid challenges (1000 ml in total) |
| Tier 1 Abnormal CRT Pulse Pressure >40 mmHg DBP >50 mmHg | FR + Fluid Challenge FR- Cardiac Function assessment | Maximum of two 500 ml fluid challenges (1000 ml in total) |
| Tier 2 Abnormal CRT No LV or RV dysfunction | FR + Fluid Challenge FR - HT: Vasopressor Test No HT: Dobutamine test | -Successive 500 ml challenges-Reassessment of CRT, FR status and venous congestion periodically |
FR: fluid responsiveness; CRT: capillary refill time; DBP: diastolic blood pressure; LV: left ventricle; RV: right ventricle; HT: hypertension.
The skin territory lacks auto-regulatory flow control, and therefore, a fall in systemic blood flow or perfusion pressure, and subsequent sympathetic activation, may impair skin perfusion in shock states.14 This can be dynamically evaluated by peripheral perfusion assessment.15 Observational studies have shown that abnormal peripheral perfusion after initial or advanced resuscitation is associated with increased morbidity and mortality in septic shock.16,17 Indeed, the excellent prognosis associated with CRT recovery, its simplicity and fast kinetics of recovery,18 its availability in resource-limited settings, as well as its capacity to change in parallel with hepatosplanchnic perfusion,19 constitute strong reasons to consider CRT as the target for fluid resuscitation in septic shock patients. Furthermore, there is recent evidence of the correlation between CRT and sublingual microcirculatory indexes,20 and its rapid response to a passive leg raising maneuver or fluid loading.21
CRT-targeted resuscitation was associated with lower mortality (34.9% vs. 43.4%), beneficial effects on organ dysfunction, and less intensity of treatment in the ANDROMEDA-SHOCK trial,11 a fact that was confirmed by a subsequent Bayesian analysis.22 Some post-hoc analyses of the ANDROMEDA-SHOCK trial further confirm the relevance of CRT monitoring. First, patients with normal CRT at baseline exhibited a significantly lower 28-day mortality than those with abnormal CRT (27% vs. 43%, p = 0.001).23 Thus, it appears that baseline CRT could define severity phenotypes in septic shock, while at least 25% of patients with normal CRT could eventually avoid exposure to over-resuscitation. Second, among patients presenting a normal CRT at 2 h, those originally allocated to the lactate-guided arm continued resuscitation per protocol if lactate was still abnormal as compared to the CRT-guided arm in whom resuscitation was stopped. The former received significantly more resuscitative interventions such as fluid boluses, and vasoactive drugs, and exhibited a significantly higher mortality (40 vs. 23%, p = 0.009).24
The worldwide impact and immediate application of CRT-targeted resuscitation makes further research an urgent task. The key novelty of A2 is to combine a CRT-targeted strategy with a clinical hemodynamic phenotyping that may aid to personalize initial resuscitation with potential additional fluid-sparing effects.13
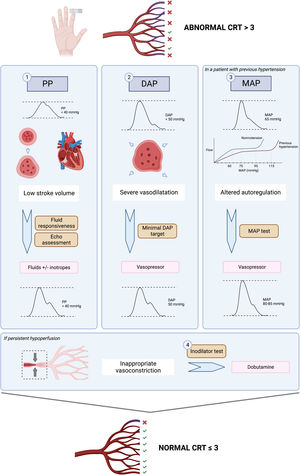
Pulse pressurePulse pressure (PP) refers to the difference between systolic and diastolic blood pressure. According to a 3-compartment Windkessel model, PP will be determined by stroke volume, total arterial compliance and aortic characteristic impedance.25 Since early 1900’s, different researchers have tried to study the correlation between arterial PP and stroke volume, demonstrating that PP can adequately track stroke volume.26,27 In fact, mathematical derivations of this model provided the basis for cardiac output monitors based on pulse-contour analysis.28 Of note, other pathophysiological conditions that produce abnormal pulse wave contours and alter pulse pressure include aortic stenosis, patent ductus arteriosus and aortic regurgitation.29
Thus, PP becomes a straightforward window to assess the heart function and its’ interactions with the vascular system, without the need of advanced cardiac output monitoring, which is not always readily available at the bedside.30 PP normally increases with age due to changes in aortic impedance, but broadly speaking, a PP < 40 mmHg is clearly low and reflects a decrease in stroke volume that could be explained either by a decreased preload or a severe cardiac dysfunction.
In the ANDROMEDA-SHOCK-2 study protocol, pulse pressure becomes the first pivotal hemodynamic assessment to further categorize patients with abnormal CRT into broad phenotypes according to its values.13 This simple hemodynamic variable determines subsequent divergent resuscitation tracks. Thus, if a patient presents low PP, fluid responsiveness is tested (see below) and up to 1000 ml may be administered as fluid challenges during first tier interventions. On the contrary, a normal PP together with a low diastolic blood pressure (DBP) represent a predominant vasoplegia and in this setting, a minimal DBP may be targeted by increasing vasopressors (see below).
Diastolic blood pressureDBP in healthy individuals is mainly determined by vascular tone and heart rate and remains almost constant from the ascending aorta to the peripheral vessels. Thus, low diastolic blood pressure in the vessels reflects systemic vasodilatation as long as the aortic valve is competent. In elderly patients, lower diastolic blood pressure is associated with increased rate of cardiovascular events.31 Diastolic blood pressure, in addition to being a marker of arterial tone, represents the inflow pressure for coronary perfusion, especially left ventricular subendocardium.32 A low DBP (below 50 mmHg) can impair myocardial perfusion.33 In a cohort of patients with coronary artery disease, low DBP was associated with higher rates of cardiovascular events and mortality.
In patients with septic shock, DBP of less than 50 mmHg is an early predictor of in-hospital mortality and could be considered as a therapeutic target.34 Therefore, the assessment of vascular tone through the severity of diastolic hypotension has important implications for therapeutic decisions and the early administration of vasoactive agents to promptly restore tissue perfusion, while avoiding ineffective fluid administration. On the other hand, in patients with septic shock in which there is also a large proportion of patients with coronary artery disease, low DBP increases the risk of myocardial ischemia, which can be associated with decreased stroke volume and greater impairment of tissue perfusion and microcirculation.35 This becomes especially true in the case of tachycardia (as it usually occurs in septic shock), in which a higher-than-normal DBP should result as the decreased diastolic time prevents lower DBP values.34,36 Thus, if tachycardia is present and DBP is low, vasoplegia must be a relevant determinant, and could be a signal for adjusting vasopressor therapy.33,37
ANDROMEDA-SHOCK-2 will be the first trial testing if correction of a low DBP septic shock patients (vasoplegic phenotype) through enhanced vasopressor administration, is associated with recovery of tissue perfusion.
Systematic assessment of fluid responsivenessFluid responsiveness (FR) can be generally understood as the capacity of the heart to increase cardiac output after a fluid bolus.38 This will only happen if the heart is located in the steep part of the Frank Starling curve (preload dependency). Different bedside methods of fluid responsiveness assessment have emerged in the literature, which either reversibly increase preload either through cardiopulmonary interaction (i.e. pulse pressure variation) or induce a mobilization of 300 ml of blood from the unstressed to the stressed volume compartment (i.e., passive leg raising).39
Effective fluid administration during shock states increases mean systemic filling pressure, increasing venous return and eventually, cardiac output. Unfortunately, only 40–50 % of patients admitted to the ICU are fluid responsive.40 Thus, pursuing fluid administration without testing for fluid responsiveness, might place a considerable number of patients at risk of venous congestion, without reaching the intended goal of increasing cardiac output. Despite its’ sound physiological background and being recommended by current guidelines, FR tests are not routinely used. In the FENICE study, 43% of clinicians did not use any test to predict FR, and only 20% used dynamic variables.41
As previously mentioned, different FR tests have been proposed in the literature, with varying degrees of specificity and sensitivity, and applicability in particular clinical contexts.42 Probably, the most challenging scenario is that of patients under spontaneous breathing, in which cardiopulmonary interactions are highly unpredictable, precluding the use of tests such as pulse pressure variation or stroke volume variation.43
Two recent randomized controlled trials have integrated the systematic assessment of fluid responsiveness into their resuscitation algorithm and provide insights into their clinical value. The FRESH trial compared standard of care vs guiding fluid administration through dynamic assessment of fluid responsiveness (passive leg raising maneuver), targeting a lower fluid balance at 72 h.44 In this trial, 42% of patients in the intervention group presented a FR + status at study inclusion. Only 12% of patients maintained a persistent FR + status during the study period, and 18% had a persistent FR- status during the first 72 h. The intervention group received less fluids (-1.37 lt [−2.53 to −0.21], p = 0.021) and had fewer requirements of both renal replacement therapy and invasive mechanical ventilation.44
The ANDROMEDA-SHOCK trial integrated systematic assessment of fluid responsiveness into their resuscitation algorithm with a pragmatic approach of test selection according to the clinical context.45 A post-hoc analysis of this RCT exploring this topic demonstrated that in 82% of patients FR assessment was feasible, and of this group, 70% had a positive FR status at study inclusion. Like the FRESH trial, FR + status was evanescent throughout the resuscitation period. Patients with a FR- status received 1500 ml less than the FR + group but reached their predefined resuscitation endpoints in a similar proportion. This provides evidence of the safety of withholding fluids in a FR- context.40
In A2, systematic assessment of fluid responsiveness through multiple dynamic tests will be pursued in the intervention group. In this sense, fluid administration must respond to a hierarchical trigger for resuscitation, namely, a signal of tissue hypoperfusion that might be corrected through increasing cardiac output. The systematic evaluation of the FR is the cornerstone on which the optimization with fluids in sepsis is established. FR should be a mandatory test before each fluid load and whenever the patient's hemodynamic profile changes, either due to the evolution of sepsis, or because of the therapies used. This approach will make it possible to individualize fluid therapy safely, avoiding fluid overload in septic shock (Table 2).
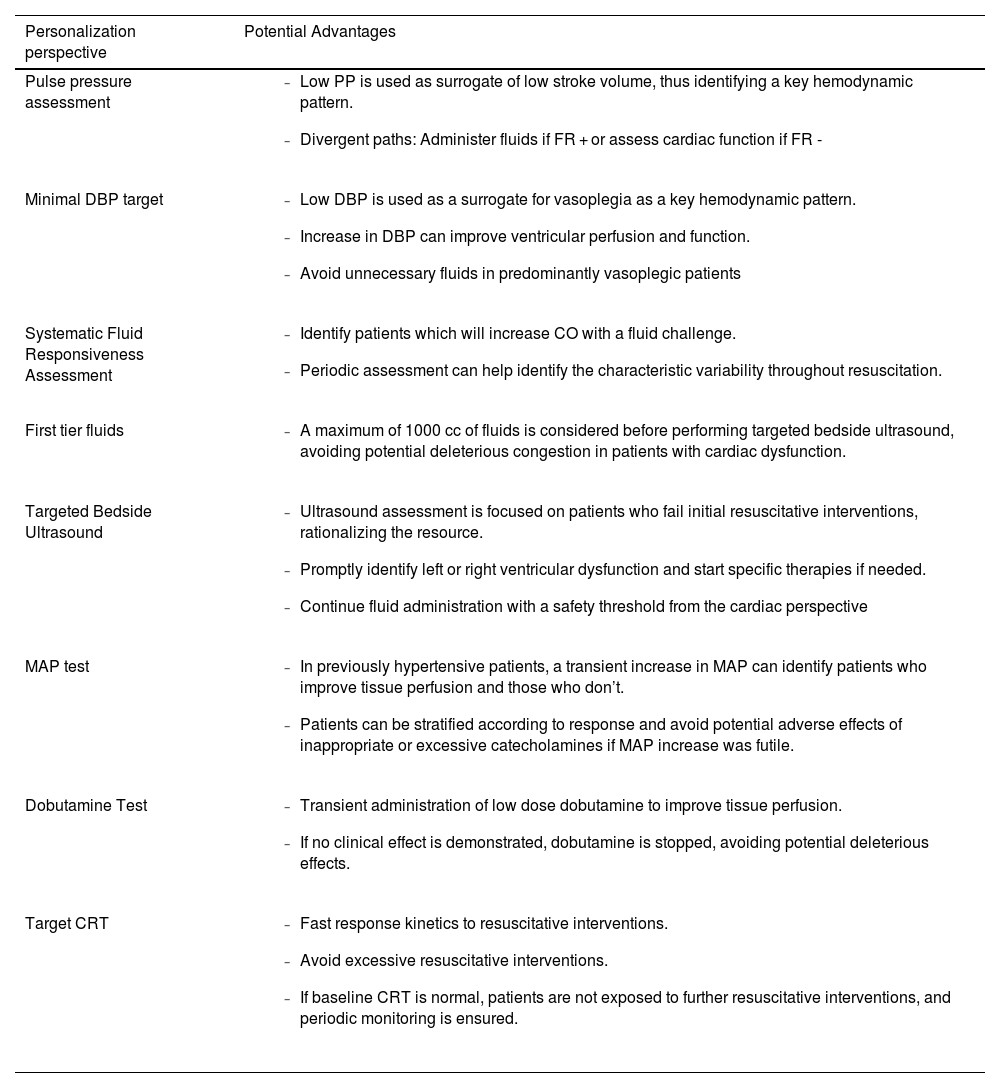
Perspectives on hemodynamic personalization and potential advantages present on the ANDROMEDA-SHOCK-2 trial.
| Personalization perspective | Potential Advantages |
|---|---|
| Pulse pressure assessment |
|
| Minimal DBP target |
|
| Systematic Fluid Responsiveness Assessment |
|
| First tier fluids |
|
| Targeted Bedside Ultrasound |
|
| MAP test |
|
| Dobutamine Test |
|
| Target CRT |
|
PP: Pulse pressure; FR: fluid responsive; DBP: diastolic blood pressure; MAP: mean arterial pressure; CO: cardiac output; CRT: capillary refill time.
Traditionally, left ventricular dysfunction has been described as a late phenomenon in the evolution of septic shock, but current evidence does not support this idea and the incidence varies widely in the literature.46,47 The identification of echocardiographic patterns combined with hemodynamic variables in a cohort of patients with early septic shock has allowed the identification of five well-defined phenotypes: hyperdynamic, persistent hypovolemic, left ventricular dysfunction, right ventricular dysfunction and adequate resuscitation.12 These different phenotypes reflect diverse combinations of alterations in venous return, inotropism and ventricular-arterial coupling, and may vary over time and in response to therapeutic maneuvers. Their identification has prognostic and therapeutic implications.48
Indeed, the identification of patients with severe left or right ventricular dysfunction before administrating more fluids or vasoactive combinations may prevent fluid overload and allow a more rational management that may include inotropes, prone position, or PEEP adjustment depending on findings. On the other hand, the knowledge that around 40% of septic shock patients (hyperdynamic and persistent hypovolemic phenotypes) have conditions on which the indiscriminate use of dobutamine may be associated with severe adverse effects, highlights the relevance of properly assessing cardiac function in patients unresponsive to initial septic shock management.12
Echocardiographic assessment of left systolic function is difficult due to the dependence of the loading conditions, heart rate, the effect of resuscitation maneuvers, and the time of evaluation. Left ventricular ejection fraction (LVEF) is a classic and universally used parameter, although its sensitivity is lower than that of other techniques such as global longitudinal deformation by speckle tracking.49 Fractional area change (FAC) measured in the parasternal plane short axis at the level of the papillary muscles presents a good correlation with LVEF and has prognostic value. The velocity-time integral (VTI) measured by pulsed Doppler in the outflow tract of the left ventricle is a good surrogate parameter of the systolic volume since the diameter of the outflow tract is constant. Its normal values are between 16 and 20 cm.50–52 In line with this concept, the A2 study defines severe left ventricular dysfunction by the association of depressed systolic function measured by FAC, and a low stroke volume as assessed by VTI (Figs. 1 and 2). It does not attempt to distinguish sepsis-associated systolic dysfunction from pre-existing systolic dysfunction, but rather to identify those patients who require specific out-of-protocol management.
Right ventricular (RV) systolic function parameters (TAPSE, S', FAC) present the same limitations as those on the left, and the complex anatomy of the right ventricle prevents the calculation of right ventricular ejection fraction with conventional equipment. A recent proposal has been to define right ventricular failure based on its pathophysiological characteristics: presence of RV dilatation associated with elevation of central venous pressure (CVP) as a surrogate parameter of elevated filling pressures.53 The application of this definition has been explored in several studies, as well as the pathological threshold value of CVP that is related to greater morbidity and mortality.54,55 In A2, right ventricular dysfunction is defined by the combination of severe RV dilatation (RV/LV area ratio ≥ 1) and a CVP > 8 mmHg (Fig. 2). For definitive consideration of the presence of right ventricular failure it is necessary to take into account interaction with ventilatory parameters such as excessive intrathoracic pressure or high PEEP levels.
Mandatory use of echocardiography in the early phases of resuscitation is one of the cornerstones of the ANDROMEDA-SHOCK-2 protocol. It represents the first measure of the Tier-2 interventions and should be performed when Tier-1 interventions have failed to normalize CRT. The primary objective at this point is to rule out severe left ventricular dysfunction or right ventricular failure, conditions that may command specific out-of-protocol management. However, other findings may be relevant for treatment fine-tuning beyond the 6 h intervention period.
Mean arterial pressure testIn the early phase of septic shock, circulating proinflammatory mediators induce both a decrease in arterial vascular tone, which leads to a drop in mean arterial pressure (MAP), and also venous dilation generating a relative hypovolemia, with a consequent decrease in mean systemic filling pressure, preload, and cardiac output. The decrease in systemic blood flow and perfusion pressure (MAP) may severely impair tissue perfusion and cellular oxygenation.4,56 Therefore, a critical priority in hemodynamic resuscitation is to restore minimal MAP values. Rational use of vasopressors aims to improve perfusion pressure while minimizing adverse events such as arrhythmias or ischemic events.57
In most patients, under normal conditions, in organs with autoregulatory capacity, when MAP decreases below the limits of autoregulation, between 60 and 65 mmHg, organ perfusion becomes pressure-dependent.58 In patients with chronic hypertension, these autoregulation thresholds are shifted to the right, so that the lower limit of their autoregulation range is higher.59,60 In addition, in sepsis, vascular reactivity is altered due to changes in nitric oxide production and the release of vasoactive mediators at regional level in the tissues. This alteration shifts the autoregulation thresholds upwards, making higher MAP levels necessary to preserve effective perfusion pressure. However, it is not clear what these specific autoregulatory limits are for each individual patient, and therefore the optimal MAP value is unknown.11
The Surviving Sepsis Campaign guideline recommendation to maintain a MAP of at least 65 mmHg,5 is based on the results of the three most recent RCTs on the most appropriate MAP target in patients in shock (SEPSISPAM, OVATION, and 65 Trial).57 These studies tested the impact of higher MAP targets versus standard recommendations on major outcomes, finding no difference in mortality. However, in the SEPSISPAM trial, the subgroup of chronically hypertensive patients allocated to the higher MAP target required less renal replacement therapy.59 Richards-Belle et al. performed a systematic review and meta-analysis of these three randomized controlled trials (RCTs) to assess whether exposure to vasopressors in patients in shock was associated with increased mortality (3496 patients), finding no significant difference. However, the risk of arrhythmias may correlate with higher doses of vasopressors.61
Some territories, namely splanchnic, skeletal muscle, and cutaneous tissues, show a limited autoregulation of blood flow, which makes them more susceptible to hypoperfusion in situations with decreased MAP.58 Furthermore, arteriolar remodeling secondary to hypertension leads to a functional proximal stenosis in the vascular bed. When MAP decreases in areas with autoregulatory response, even though the thresholds might be higher in hypertensive patients, the distal bed has a certain capacity to dilate in order to maintain adequate blood flow.62 By contrast, in those territories with limited autoregulatory response, perfusion is completely MAP-dependent.
It is then possible that in the subgroup of chronically hypertensive patients in a situation of shock and microcirculatory dysfunction, a higher MAP may be beneficial.59 Characteristically, persistent septic shock is associated with microcirculatory abnormalities on which a relevant feature is microvascular heterogeneity. This determines variable responses to changes in perfusion pressure. Indeed, a landmark study from Dubin et al. found that changes in perfusion of the sublingual vascular bed caused by an increase in MAP in septic patients depend mainly on the baseline state of the microcirculation.62 Only those patients who initially had an impaired microcirculation improved it, and on the contrary, higher norepinephrine doses could eventually impair microcirculatory flow in patients with preserved microcirculation at baseline. This means that eventually the balance between the increase in systemic perfusion pressure may be counterbalanced by excessive precapillary arteriolar vasoconstriction in some patients.
To determine the benefit or risk of increasing MAP levels in individual patients with persistent hypoperfusion, a MAP test was proposed in the ANDROMEDA-SHOCK trial. Assuming, that CRT is an adequate surrogate of microcirculatory flow, in previously hypertensive patients with persistent abnormal CRT, a transient increase in MAP to levels of 80−85 mmHg was induced, and CRT reassessed one hour later. This MAP level was maintained only in patients that normalized the variable which occurred in 40% of the patients. This same strategy will be used in A2.
Dobutamine testThe administration of dobutamine, a synthetic catecholamine analog, that acts on alpha-1, beta-1 and beta-2 adrenergic receptors, during septic shock resuscitation, is a controversial topic. The most recent Surviving Sepsis Campaign Guidelines offer a low level of evidence with a weak recommendation for the use of dobutamine, limiting its use to patients with ventricular dysfunction and signs of persistent hypoperfusion despite adequate fluid resuscitation.5 In a recent survey of European intensivists, dobutamine was the main inotropic drug used in shock states, while 22% of respondents used it in cases of shock with persistent signs of hypoperfusion, even in the absence of ventricular dysfunction.63
Physiological studies have explored the impact of dobutamine in both microcirculatory and macrohemodynamic variables. In some studies, dobutamine, through an increase in oxygen transport, was associated with improved lactate levels and gastric mucosal pH.64,65 Other experiences demonstrated an improved perfusion of the intestinal villi and sublingual microcirculation, without changes in cardiac output or blood pressure.66 However, other studies in clinical or experimental settings provided conflicting results.
There are no clinical studies showing a direct benefit of dobutamine in patient centered outcomes, and available evidence is highly heterogeneous. In the ProCESS, ARISE, and ProMISE studies early goal-guided therapy (EGDT) was compared to standard of care.67 Although the overall number of patients treated with dobutamine was low, its’ use was significantly higher in the EGDT arms (ProCESS 5.7% vs. 1.0%, ProMISE 18.1% vs. 3.8%, and ARISE 15.4% vs. 2.6%). These studies found no differences between the two treatment groups in mortality. Some reports have showed signals of increased mortality in patients treated with dobutamine (60.2% vs. 49.4%, OR 1.55, 95% CI 1.01–2.37; P = 0.044),68 while other have favored the combination of norepinephrine with dobutamine.69,70
Given the heterogeneous response to dobutamine, it is relevant to further characterize which subset of septic patients can potentially benefit from its use. Echocardiography is an important tool not only for assessing ventricular function prior to administration, but also for monitoring response and follow-up.71 With this objective in mind, a study was performed on 23 septic patients who were administered increasing doses of dobutamine, from 2.5 to 10 µg/kg/min. Systolic volume increased in half of the patients, with a greater increase group with ventricular dysfunction. Likewise, no improvement in microcirculation was observed in this study, except in the group with the lowest density of perfused capillaries.72 A recent study in which septic shock patients were clustered according to echocardiographic and clinical parameters could further aid in this endeavor.12 Thus, identifying patients with specific cardiovascular patterns such left or right ventricular dysfunction could aid in targeting the use of dobutamine, while avoiding it in states such as hypovolemic or hyperdynamic states (which accounted for 40% in this trial).
Similarly, as in the ANDROMEDA-SHOCK trial, and considering the conflicting evidence on its microcirculatory and macrohemodynamic effects, the ANDROMEDA-SHOCK-2 study proposes the use of dobutamine as an inodilator test, during the last part of the resuscitation algorithm. In patients with normal ventricular function and persistently altered CRT, dobutamine is started at low doses (2.5–5 mcg/kg/min), and CRT is reassessed after 1 h. If there is no improvement in tissue perfusion, dobutamine infusion is stopped, otherwise, it is maintained.
ConclusionThe ANDROMEDA-SHOCK-2 trial will test a multidimensional strategy to personalize early septic shock resuscitation by using simple bedside available tools with a strong physiological background. The pillars of the proposed algorithm are the use of CRT as a target, and hemodynamic phenotyping which includes the combined analysis of pulse pressure and diastolic blood pressure to decide on further interventions in patients with persistent hypoperfusion. Patients with low versus normal PP will be managed differently by either moving to fluid responsiveness assessment and potential fluid boluses in the former, versus to increase vasopressor dose to get a minimal DBP in the latter. In addition, systematic and repeated reassessment of FR status, as well as limiting fluid boluses to a maximum of 1 liter before a formal basic echocardiography to rule out severe cardiac dysfunction is performed, may help in avoiding potentially harmful fluid administration. Finally, the use of a MAP or a dobutamine tests in patients, unresponsive to previous interventions, may also improve the risk-benefit ratio of these vasoactive interventions. The sum of all these physiologically tailored interventions may bring the best opportunity to personalize early septic shock resuscitation, maximizing the odds of a rapid reperfusion and at the same time decreasing the risk of fluid overload or over-resuscitation. The results of ANDROMEDA-SHOCK-2 will define if personalizing septic shock resuscitation with the use of simple clinical tools may improve relevant outcomes in this severe condition.
ScvO2: central venous oxygen saturation; CRT: capillary refill time; ANDROMEDA-SHOCK-2: A2; PP: Pulse pressure; DBP: diastolic blood pressure; FR: fluid responsiveness; LVEF: left ventricular ejection fraction; FAC: fractional area change; VTI: velocity time integral; RV: right ventricular; CVP: central venous pressure; MAP: mean arterial pressure; RCT: randomized controlled trial; EGDT: early goal directed therapy.
Conflict of interestThe authors declare that they have no conflict of interest.
Hospital Universitari y Politecnic La Fe, Valencia: Paula Carmona, Iratxe Zarragoikoetxea, Marta López Cantero, Daniel Pérez Ajami, Pedro José Martínez, Miguel Ródenas, José García Cantos, Clara Pascual, Silvia Polo, Alvaro Del Mazo, Victoria Johanensen, Cristina López Forte, Maria Jesús Montero, Esther Perez Sancho, Ana Vidal, Azucena Pajares.
Hospital Universitario de Cruces. Baracaldo (Vizcaya): Iñaki Bilbao Villasante, Covadonga Peralta, Gonzalo Tamayo.
Hospital Universitario 12 de Octubre. Madrid: Adriana Calderón Barajas, Claudia Olea Vielba, Víctor Zarza Fernández de Alegría, Meta Levstek, Álvaro Ramiro Ruiz, Javier Silva García, Carolina Lugo Duarte, Eloísa López López, Matilde González Serrano, Ana Hermira Anchuelo, Raquel García Álvarez, David Benguría Puebla, Aída Fernández García, Isabel de la Calle Gil, Patricia Pascual Cambero, Miguel Saiz Sánchez-Buitrago, Mª Isabel Real Navacerrada.
Hospital General Universitario Gregorio Marañón. Madrid: Silvia Ramos Cerro, Patricia Piñeiro Otero, Isabel Solchaga Sánchez, Mercedes Power Esteban, Alberto Calvo García, Sergio García Ramos.
Hospital Universitario de La Princesa. Madrid: Fernando Ramasco, Rosa Méndez, Sheila Santidrian, Jesús Nieves-Alonso, Carlos Puga, Luis Gómez-Arredondo, Juan de Ancos, Sonia Expósito, Esther García-Villabona, Carmen Vallejo, Mar Orts, Cárlos Figueroa, Julia Hernando, David Arribas, Jara Torrente, Alejandro Suarez de la Rica, Carlos Román, Amadea Mjertan, David Cordón, Andrés v.Wernitz, Iñigo Guerra, Ana Martínez-Molina.
Clínica Universidad de Navarra. Pamplona: Marc Vives, Ariel Duilio González, Iñigo Rubio, Carmen Cara, Pablo Montero, Carmen Sala, Carmen Garau, Marta Luque, José Antonio Fernández, Leire Goñi, Elena Cacho, Raquel Callejas, Ricardo Calderón, Francisco Hidalgo, Gemma Echarri, Luis López, Pablo Monedero, Cristina Honorato, Antonio Martínez, Rafael Moncada.
Hospital Universitario Vall d'Hebron. Barcelona: Antonio Barbara Ferreras, Alfonso Gómez Felices, Miriam de Nadal
Hospital General Universitario de Elche: Ana Pérez Carbonell, José Javier García Romero, Maria Mercader y Jaime Miralles.
Hospital Clínico Universitario de Valencia: Sara Martínez Castro, Gerardo Aguilar Aguilar, Esther Romero Vargas, José Antonio Carbonell, Martina Savino, Eduardo Passariello, Pablo Lorenzo, Alba Montoya, Rafael Badenes.
Complejo Hospitalario Universitario de Orense: Ariadna Rodriguez Rodriguez, Raquel Ruido Dacal, Eva Villar Arcay, Leticia Gómez Viana, Milagros Cid Manzano y María Concepción Alonso Gonzalez.
Hospital Universitario Ntra Sra. de Candelaria (Santa Cruz de Tenerife): David Domínguez García,Alberto Vera González, Daniel Piñero Prieto, Adrián Díaz Fernández, Lucía Pazos Otero, Raúl Hernández Bisshopp, Israel,Amador García, Carola Guillén Iranzo, Amelia González Beltrán, Luis Alejandro Soto Jáquez, Irina Paula Rodríguez González, Liuva Pereira Esmoriz, Marina Sánchez Navas-Parejo, Gabriela Noemí González Chiale.
Hospital Universitario de Móstoles: Raquel Fernández-García, Diana Marcela Narváez Cubillos, Rocío Ayala Soto, Saúl Vélaz Domínguez, Pablo Oliver Fornies.
Hospital Universitario Central de Asturias (Oviedo): Alberto de Juan Álvarez, Natalia Pérez de Arriba, Lorena Varela Rodríguez,
María Cristina Iglesias Fernández.
Hospital General Universitario de Ciudad Real. Francisco Javier Redondo-Calvo, Remedios Moreno, Tatiana Cuesta, Ruben Villazala, Victor Baladron, Jorge Redondo, Omar Montenegro, Patria Faba, Juan Valencia, María Gracia Villanueva, Manuel Valbuena, Ana López-Olio, María Molina, Aránzazu Pérez, Fernando Garvayo, Alejandro Sánchez.
Complejo Hospitalario Universitario de Albacete: Delia Parreño Bueno, Luisa María Charco Roca, José María Jiménez Vizuete.
Hospital Clinic. Uci Quirúrgica. (Barcelona): Jordi Mercadal Mercadal, Luigi Zattera, Ana Fervienza Sánchez, Enric Barbeta Viñas, Amalia Alcón Rodríguez, Guido Muñoz Rojas, Albert Carramiñana Domínguez, Juan Perdomo Linares, Ramsés Marrero García, María Elena del Rio Morales, Ricard Mellado Artigas, Andrea Palomeque Flores.
Hospital del Mar (Barcelona): Adela Benítez-Cano, Ramón Adalia, Jesús Carazo, Hugo Rivera-Ramos, Marta Antelo-Adran, Isabel Ramos, Lorena Rivera, Antonio Ferraroni, Leire Larrañaga
Hospital Universitario de Cáceres: Fernando García- Montoto Pérez, Inés Maria Parejo Cabezas, José Ignacio Hermoso, Manuel Francisco Mirón Rodríguez, Guillerma Pardo Romero, José Ramón Serrano Santano, Maria Pilar Caldera Miguel, Leticia Charro Hidalgo, Pedro de Alonso Andrés, Francisco Pablo Bueno Villalba, Diana Araujo Rodríguez, Valentín Moreno Carbonell.
Hospital Can Misses. Ibiza: Manuela Santos Parralo, Walter Andres Ramirez Lajones, Elena Uson Garcia, Gaspar Tuero Leon.
Hospital General Universitario Doctor Balmis (Alicante): María Galiana Ivars, Mariia Spirina, Úrsula Toral Toral, Santiago Pardines Rico, Carlos Ferrero Coloma, Francisca Eugenia Fornés, Alba Moscardó Descalzo, Manuel Romero Torres, Jaime Rodríguez Tallero.
Hospital Universitario Ramón y Cajal. Madrid: Pilar Cobeta Orduña, David Pestaña, Nilda Martínez Castro, Alberto Balvis Balvis, Diego Gil Mayo, Tommaso Bardi.
Hospital Ribera POVISA. Vigo: Mª Sonsoles Leal Ruiloba, Alberto Pintos Chamadoira, Paola Escáneo Otero, Maria Artiaga Canda, Benigno Rodríguez Estévez.
Hospital General Universitario de Valencia: Carolina Ferrer Gomez, Alvaro Cervera Puchades, Javier Hernández Laforet, Jaume Puig, Tanya Gabaldón, Jose Tatay Vivo.
Hospital Universitario de Gran Canaria Dr. Negrín: Oto Miguel Padrón Ruiz, Sergio López Ruiz, Antonio Arencibia Almeida, Nazario Ojeda Betancor, Aurelio Rodríguez Pérez.
Complejo Asistencial Universitario de León: Eva María Higuera Miguélez, María Merino García, Rafael Gonzàlez De Castro, José Miguel Marcos Vidal, Sergio Marcos Contreras y Cristina García Pérez
Hospital Universitario Rio Hortega. Valladolid: Cesar Aldecoa, Jesús Rico Feijoo, Eugenio Ruiz, Almudena Rodriguez, Isabel García, Laura Vaquero, Alicia Bordell, Rocío Perez, Irene Garcia, Alba Diaz
Hospital General Universitario de Castellón: Ignacio Catalán Monzón, Enver Rodríguez Martínez, Lluís Tormo Rodríguez, Mª Lidón Mateu Campos, Laura Galarza Barrachina.
Hospital Universitario Puerta de Hierro. Madrid: Cristina Ferreras, María Casado, Alejandra Del Campo, Reyes Iranzo.
Complejo Hospitalario Universitario de Pontevedra: Marina Varela Durán, Eva María González Babarro, Santiago Domínguez Fernández, Loreto Vidal Castro, Pilar Díaz Parada.
Hospital Clínic de Barcelona (Área de Vigilancia Intensiva): Adrián Téllez Santoyo, Pedro Castro Rebollo, José María Nicolás Arfelis, Sara Fernández Méndez, José-Ramón Alonso-Viladot, Carolina Sánchez-Marcos, Adrià Carpio-Blasco, Victor Gil-Espinosa, Daniel Nicolás Marco Prats.
Hospital Universitario Virgen de la Arrixaca. Murcia: Julio Padilla Rodríguez. Piedad Martínez Gil. Giovanni Alessandro Ercole, Carlos García Palenciano.