Low-grade myofibroblastic sarcoma (LGMS) represents an atypical tumor composed of myofibroblasts with a predilection for the head and neck, especially in the tongue and oral cavity, with a high tendency to local recurrences and metastases, even after a long period. LGMS arising in the maxillary sinus and in the neck are extremely uncommon. To the best of our knowledge, only 50 cases of low-grade myofibroblastic sarcoma have been reported. We report two cases of LGMS of the maxillary sinus and neck, discussing clinical, histological, inmunohistochemical and therapeutic features.
El sarcoma miofibroblástico de bajo grado (SMFBG) representa un tumor atípico, formado por miofibroblastos, que tiene predilección por cabeza y cuello, en especial la lengua y la cavidad oral, y se caracteriza por una elevada tendencia a las recidivas locales y a las metástasis, incluso después de transcurrido un período prolongado. Los SMFBG que se originan en el seno maxilar y en el cuello son excepcionales. Hasta lo que conocen los autores, solo se han publicado 50 casos de sarcoma miofibroblástico de bajo grado. Describimos 2 casos en los que se identificaron estos tumores, uno en el seno maxilar y el otro en el cuello, y abordamos sus características clínicas, histológicas, inmunohistoquímicas y terapéuticas.
Low-grade myofibroblastic sarcoma (LGMS) represents an atypical tumor composed of myofibroblasts with a predilection for the head and neck, especially in the tongue and oral cavity.1,2 Myofibroblasts are mesenchymal spindle-shaped cells present in almost every soft tissue. LGMS usually occurs in adult patients with a slight male predominance. Children are rarely affected.2 Although LGMS has been studied extensively, determining and defining its exact role in the spectrum of proliferative and neoplastic spindle cell lesions remains problematic.2 Clinically, patients complain of a painless swelling or an enlarging mass.2 It is a slow growing neoplasm with a tendency to local recurrences and metastases, even after a long period.3 The differential diagnosis includes both benign and malignant lesions such as nodular fasciitis, fibromatoses, fibrosarcoma or leiomyosarcoma.2,4 To the best of our knowledge, only 50 cases of low-grade myofibroblastic sarcoma have been reported. Now, we report 2 patients with LGMS of the maxillary sinus and neck. Clinical, histological, inmunohistochemical and therapeutic features are described.
Case reportCase 1 (Fig. 1)A 75-year-old female Caucasian was referred to our Oral and Maxillofacial Department from the Otolaryngology Department, for evaluation of a painful mass in the left maxillary sinus. The mass had appeared one month previously. Her personal and familial medical histories were unremarkable and she had never smoked or abused alcohol.
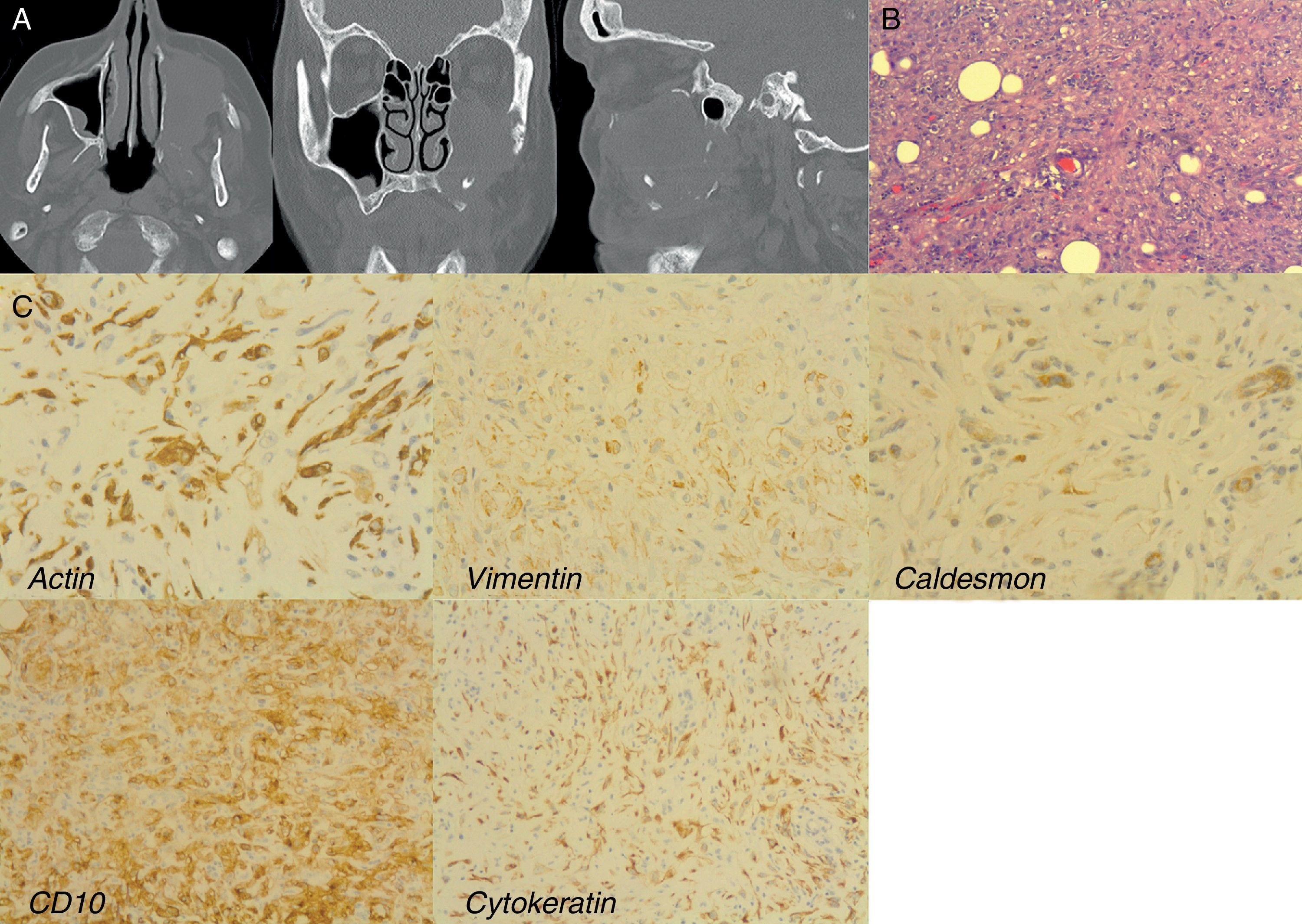
A 75-year-old female with a LGMS of the left maxillary sinus: (A) CT-Scan showing the mass into the maxillary sinus. Note the orbital floor destruction in coronal and sagittal images; (B) microscopic section showing a mesenchymal proliferation of spindle cells arranged in fascicles that infiltrates between individual muscle fibers (HE 200×); (C) immunohistochemistry. Positive stained.
Oral examination revealed a minimum but very painful swelling of the left vestibular sulcus of the maxilla. The overlying mucosa appeared normal. No clinical evidence of lymphadenopathy was observed.
CT-scan revealed a well-defined mass in the left maxillary sinus with bone destruction of the three walls and partial infiltration of the orbital floor.
An incisional biopsy of the lesion was performed. Microscopic examination revealed an expansive mesenchymal tumor characterized by a diffuse proliferation of spindle-cells with rounded nuclei, small nucleolus surrounded by an intense lymphocytic infiltrate. On the basis of these clinical, radiological and histological findings, the patient was diagnosed with fibromyxoma versus low grade sarcoma.
The patient underwent, under general anesthesia, a total maxillectomy including pterygoid region, orbital floor and zygomatic body, preserving the ocular globe, by means of a Weber-Fergusson incision. The defect was then reconstructed with two titanium meshes covered with a myofascial temporal flap.
Definitive histological examination showed a firm mass of white myxoid tissue with a central cystic area, characterized by a mesenchymal proliferation of spindle-cells, figures of mitosis, areas with myxoid and fibrosclerotic changes as well as areas of necrosis. There was extensive infiltration of the fat tissue and bone, extending into the zygomatic and palatine tissue.
Immunohistochemistry revealed that most spindle-cells were stained diffusely for vimentin, smooth muscle actin, CD10 and cytokeratin and focally for caldesmon. Staining for other markers, such as desmin, CD34, ALK, EMA and S-100 protein was not observed. Proliferative index (Ki-67) was moderate.
Given the age of the patient and the presence of tumor in the surgical margins, postoperative radiotherapy was performed. After 1 year of follow-up, the patient had a distant metastasis into the left humerus bone that was resected by the Traumatology Department. On the other hand, the patient has no facial or cervical pain and swelling.
Case 2 (Fig. 2)A 74-year-old man was referred to our Department for evaluation of an oropharyngeal squamous cell carcinoma relapse after three previous surgical excisions in another Department. Physical examination showed a total absence of the tongue and the presence of an intraoral pectoral flap with no evidence of oropharyngeal lesions. CT-scan showed a high density heterogeneous mass in the tongue base measuring 4.1cm×2.5cm that obliterated the left vallecula. The patient was diagnosed with tumor relapse and underwent a total laryngectomy.
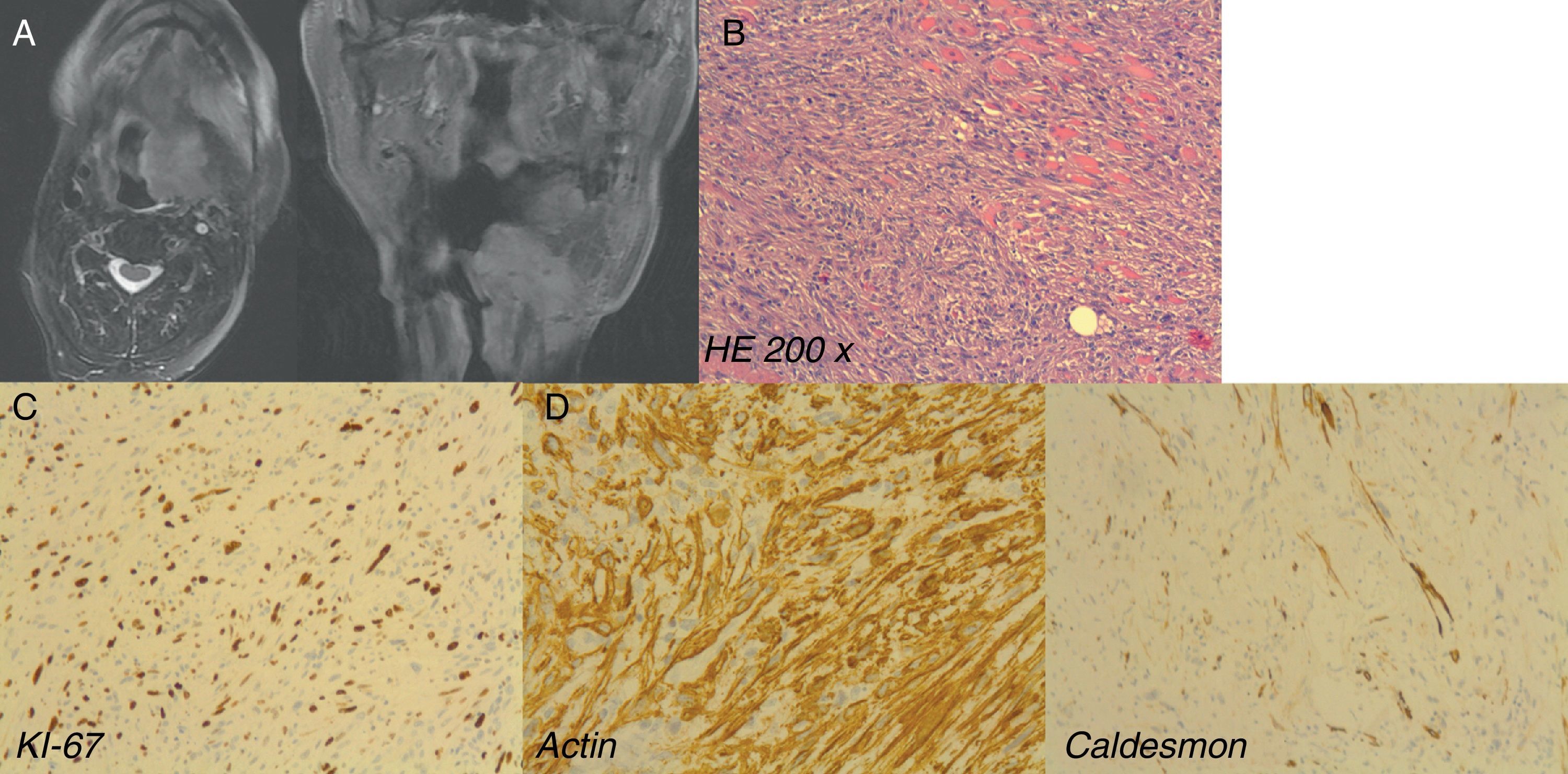
LGMS arising in a 74-year-old man after previous oropharyngeal squamous cell carcinoma relapse: (A) MRI showing the relapse of the tumor. Note the proximity of the tumor to the larynx; (B) microscopic examination (HE 200×); (C) note high grade proliferation index Ki-67; (D) immunohistochemistry. Positive stained.
Histological examination demonstrated a diffuse mesenchymal proliferation composed of spindle cells arranged in fascicles of varying length that infiltrates between individual muscle fibers. The nuclei were mostly irregular. Most tumor cells showed moderate atypia and there were many mitotic figures (more than 20 in 10 high power fields) and focal areas of necrosis. Proliferative index was high (Ki-67 80%). Immunohistochemical examination showed diffuse staining for smooth muscle actin with focal staining for caldesmon. No other stains were taken up, including desmin, cytokeratin, EMA and S-100 protein.
6 months after surgery, the patient presented another cervical relapse treated by means of another surgical intervention and died in the early postoperative period because of a carotid artery breakage.
DiscussionLow-grade myofibroblastic sarcoma (LGMS) has a predilection for the head and neck, especially in the tongue and oral cavity.1,2 Rare cases involving the salivary glands, the paranasal sinuses and even the mandible have also been reported.4–6
Despite isolated case reports, myofibrosarcoma was defined as a distinct entity only recently.7,8 It usually occurs in adult patients with a slight prevalence in males. Children are rarely affected.2
The tumor is composed of myofibroblasts. First described by Gabbiani et al.9 in 1971, they are mesenchymal spindle-shaped cells that share ultrastructural features with both fibroblasts and smooth muscle cells.2,10 They are present in connective tissue and contribute to reparative and reactive conditions, such as granulation tissue, hypertrophic scars, myofibroblastoma2,3 and even appear in the desmoplastic stroma of carcinoma.11
Clinically, LGMS usually behaves as a slow growing low-grade malignant sarcoma and exhibits a pattern of aggressive local spread with common local recurrences and eventual metastatic dissemination, only after a prolonged period of time.2,3,10
Nevertheless, in most cases, patients complain of a painless swelling or an enlarging firm mass with pale and fibrous cut surfaces; pain or related symptoms are rarely reported,2 although in our two cases, both patients complained of severe pain, even at rest.
We must also consider the behavior of the tumor, usually described as slow growing but aggressive at the same time. In our cases, there were two different behaviors. The first patient had a very slow growth with a benign behavior but, on the contrary, the second patient had a very fast growth with two relapses in 6 months and finally died, even with similar histological features. This leads us to believe that histology could not be correlated with the clinical course of the patients.
Histologically, most cases are characterized by a diffusely infiltrative growth pattern composed of spindle or stellate cells arranged in fascicles. More rarely, a prominent collagenous matrix with focal hyalinization and numerous thin-walled capillaries or inflammatory cells have also been reported.2,12 Neoplastic cells usually have contractile elements and synthesize collagen, fibronectin, laminin3 and show nuclear atypia with enlarged, hyperchromatic, irregular nuclei and slightly increased proliferative activity. Mitosis can be presented and necrosis is rare.12 The lack of significant atypia and mitosis in our first patient, led us to consider a benign diagnosis such as fibromyxoma, as the first choice.
Because this tumor shares histological features, such myofibroblastic differentiation, with other malignant neoplasms (i.e. fibrosarcoma), conventional microscopy is generally insufficient for a definitive diagnosis, so that immunohistochemical analysis, or electron microscopy may also be required.2
Immunohistochemically, cells have a variable immunophenotype.11 The markers include smooth muscle actin (SMA), desmin, muscle actin (HHF35) and calponin. Most cells are positive for SMA, while fewer than half are positive for desmin. Although calponin is usually positive, it is considered a non-specific marker. Other markers, such as fibronectin, vimentin, CD34 and CD99, may stain in some cases but are not specific.12 In contrast, LGMS does not stain for S-100 protein, laminin, epithelial markers (cytokeratin, epithelial membrane antigen), CD21, CD23, CD117, h-caldesmon and anaplastic lymphoma kinase (ALK).2,3,10 Nevertheless, one of our cases, stained diffusely for cytokeratin. Does it mean that LGMS could express epithelial markers in some cases, there are cells that could differentiate into carcinoma, or maybe there were two synchronous tumors (LGMS and carcinoma)?.
Some authors insist on ultrastructural studies for categorizing these tumors and report that positive immunohistochemical staining for at least one myogenic marker can be used to confirm a diagnosis of LGMS.2 Others feel that one or more ultrastructural features should not be the only criteria used for diagnosis, and both morphology on hematoxylin–eosin stained sections and immunohistochemistry should be used. On the other hand, other authors report that recognizing myofibroblastic differentiation is difficult without electron microscopic examination, but agree that neoplastic cells have poorly developed ultrastructural features that may not be identifiable in all cases.13,14
It has been established that sarcomas with myofibroblastic differentiation can be categorized in several entities. These include low-grade myofibroblastic sarcoma, inflammatory fibrosarcoma and high-grade or pleomorphic myofibrosarcoma.10 Given the lack of consensus regarding diagnostic criteria, myofibroblastic sarcomas are probably more common than currently noted in the literature, and may include a variety of clinicopathological forms.2 Controversies surrounding the existence of neoplastic myofibroblasts and the lack of consensus on diagnostic criteria may be responsible for this issue.3 As both is relatively rare and has heterogeneous morphologies, LGMS can be mistaken for other benign or malignant neoplasms12 such as nodular fasciitis and fibromatoses both of which may display similar immunophenotypes,10 myofibroblastic tumors, myofibroma, myofibromatosis, myopericytoma and sarcomas.2
Spindle cell carcinoma has to be considered in the oral cavity since it is composed of fasciculated spindle-cells that express SMA but, unlike LGMS, cytokeratin expression and a dysplasic epithelium may be seen.3
Other tumors to be considered in the differential diagnosis include monophasic synovial sarcoma, malignant peripheral nerve sheath tumor and spindle-cell rhabdomyosarcoma.3
Although the treatment has not been clearly defined, an aggressive surgical resection with wide tumor-free margins, and occasionally, radiotherapy or chemotherapy, is the preferred therapeutic option.2,12
Recurrences in the head and neck are late and described up to 44%, depending on the treatment used.3 So that, 75% of patients treated with local excision alone relapsed, while those who underwent a wide surgical excision with or without radiotherapy was 7%.12 Nevertheless, in our second case, the patient presented with a very rapid growth of the tumor with two recurrences in a very short period of time.
Therefore, recognition of myofibroblastic sarcoma and appropriate management with close follow-up is essential to prevent late recurrence or metastases.5
Conflict of interestWe do not report any conflict of interest.
Ethical disclosuresRight to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation