The purpose of this study was to evaluate the surgical protocol and discuss possible predisposing factors of apical peri-implantitis.
Material and methodsA retrospective study was performed by analyzing a series of cases involving 11 patients, all of whom where diagnosed with, and treated for, apical peri-implantitis at La Princesa Hospital in Madrid and at Navarre University Clinic in Pamplona, Spain, between 2002 and 2013. Symptomatic patients were treated with curettage of the area, which was, in some cases, combined with bone regeneration techniques.
ResultsA total of 11 cases of apical periimplantitis were included (4 asymptomatic and 7 symptomatic). The symptoms observed were similar to dental periapical pathology, and the period of time elapsed until the patients were diagnosed with API was variable, but was less than 3 years. Complete resolution of the pathology was observed in 6 of the 7 patients treated with curettage of the periapical implant area. In the remaining case the affected implant was removed.
No surgical treatment was used in asymptomatic cases, as they were self-limiting.
ConclusionApical periimplantitis is a condition which may complicate the dental implant treatment. Conservative surgical treatment has shown satisfactory results in symptomatic patients.
Evaluar el protocolo quirúrgico y discutir los posibles factores predisponentes de la periimplantitis apical.
Material y métodoEn el presente trabajo, se planteó un estudio descriptivo retrospectivo analizando una serie de 11 casos clínicos de periimplantitis apical diagnosticados y tratados en el ámbito del Hospital de La Princesa (Madrid) y la Clínica Universidad de Navarra (Pamplona) entre 2002 y 2013. Los pacientes sintomáticos fueron tratados mediante legrado de la zona con o sin relleno.
ResultadosSe analizaron un número total de 11 casos de periimplantitis apical (4 asintomáticos y 7 con síntomas). La clínica observada fue parecida a la enfermedad dentaria periapical y el tiempo transcurrido hasta el diagnóstico fue variable, inferior a los 3 años. Se observó resolución completa del problema en 6 de los 7 casos tratados con legrado de la zona periapical del implante. En el caso restante se procedió a la explantación del implante afecto.
En los casos asintomáticos no se realizó ningún tipo de tratamiento quirúrgico, presentando una tendencia autolimitada.
ConclusiónLa periimplantitis apical es una enfermedad que puede complicar el tratamiento implantológico. La cirugía conservadora ha tenido resultados satisfactorios en los casos sintomáticos.
Despite the advances of implantology in oral rehabilitation, the feasibility of an implant may be limited by possible complications, which are of great interest.
One of them is the apical peri-implantitis (API) entity described in the 1990s1 as an infectious-inflammatory process of the tissues that surround the apex of an integrated dental implant. API has as its core element the lack of osseointegration only in the apical area of the implant.2 In a bibliographical review in 2011, Romanos et al. state that, in spite of the available diagnostic techniques, it is not currently possible to establish whether the API represents a bone scarring, a new destructive lesion of the alveolar bone or a reactivation of a prior lesion.3
Several possible aetiological factors have been suggested: bone overheating,4,5 prior alveolar or apical lesion,6 excessive implant loading,1,7 implant surface contamination,4,8 presence of radicular remains and foreign bodies,4,7 etc., although in some cases no outstanding cause is evident and several factors may coincide in some other cases. Currently, API is considered to be likely to have multifactorial aetiology.3
If the lesion does not produce symptomatology and appears as a radiological finding, it is classified as inactive and does not need treatment but follow-up, taking into account that this type of lesion represents a bone scarring determined by an excess of apical milling.7
Symptomatic API may produce pain, paraesthesia, recurrent suppurative episodes, fistulas, loss of alveolar bone, and it may condition the implant loss.3
The first cases were published by Sussman, who described the “implant to tooth” (type I) lesion when it is caused in the preparation of the implant bed and the tooth to implant (type II) lesion, when it originates from an apical lesion in the teeth adjacent to the implant.9
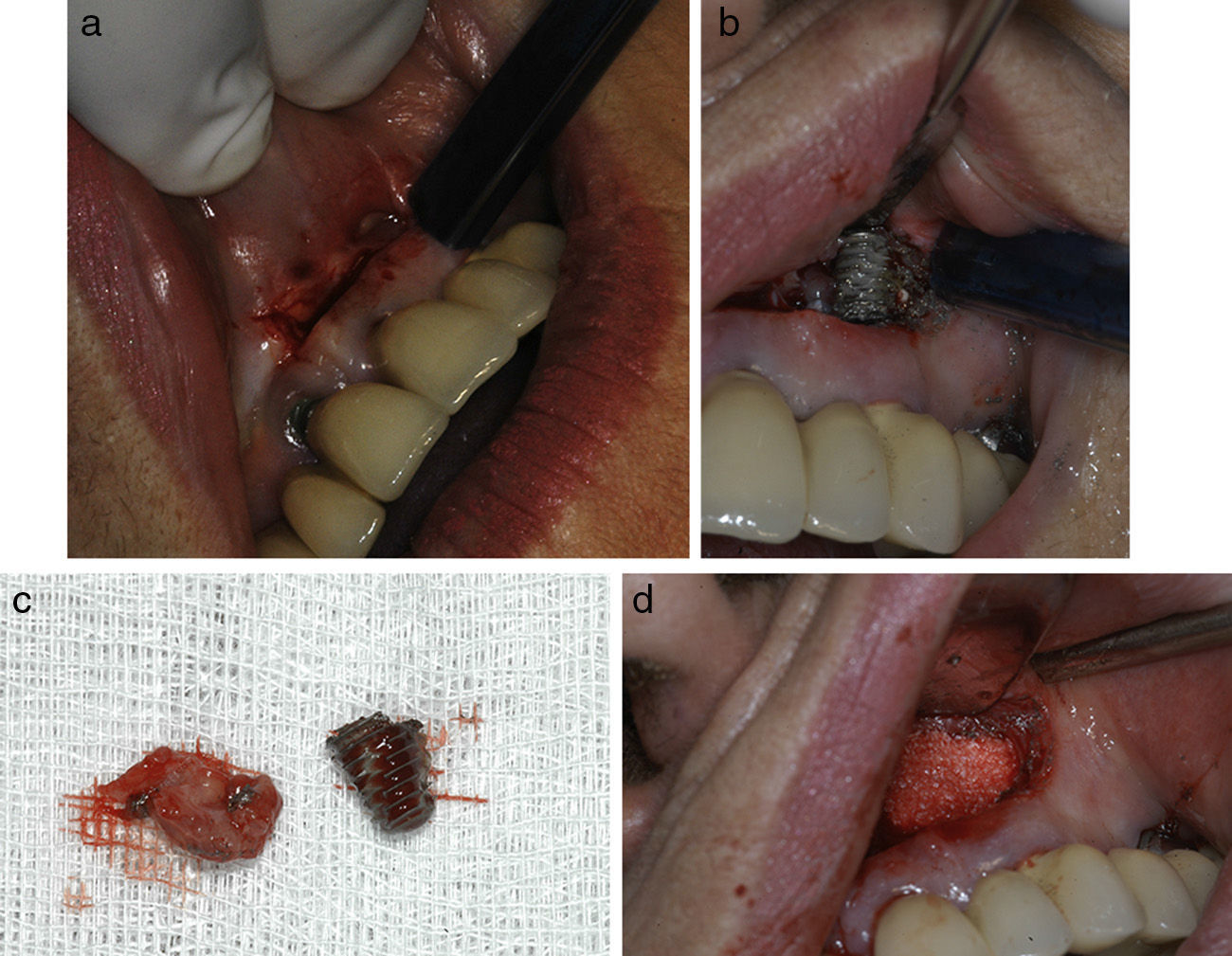
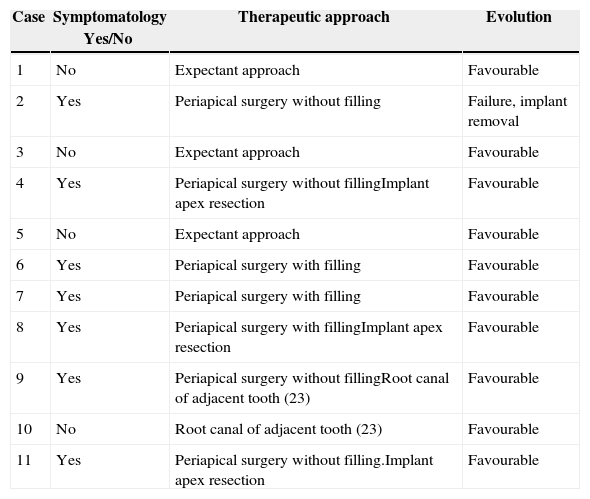
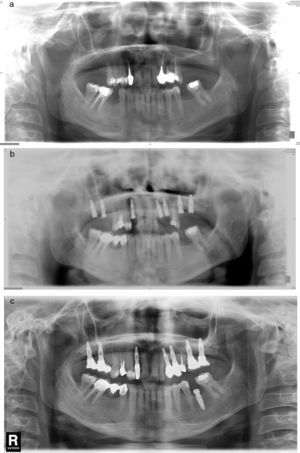
Material and methodsThis is a descriptive, retrospective study that presents a series of 11 clinical cases of API diagnosed and treated at the Hospital de La Princesa (Madrid) and the Clínica Universidad de Navarra (Pamplona.) Every case with clinically and radiologically integrated implants and those with radiolucent images at the apical level of the implants between 2002 and 2013 has been included: 4 patients were asymptomatic (there was no intervention except for a root canal of an adjacent tooth in one case) and 7 patients were symptomatic. Symptomatic patients were treated with the standard approach of “apicoectomy” through an incision at oral vestibule level, performing granulation tissue curettage, and 3 of them had an implant apex resection performed to facilitate access to the cavity and eliminate the implant's most contaminated area (Fig. 1a–c). Implant surface detoxification has not been performed. Bone filling (associated with the use of collagen resorbable membranes) was performed in 3 cases: with particulate alveolar bone autograft in 2 cases and with artificial bone of bovine origin in the other case (Fig. 1d). One case also received endodontic treatment in an adjacent tooth due to negative pulp vitality (Table 1).
(a) “Apicoectomy” type approach through incision at oral vestibule level. (b) Exposure of peri-implant osteitis area and section of implant apical part to facilitate access. (c) Re-dried implant apical area and curettaged granulation tissue. (d) Bone filling (in this case with artificial bone).
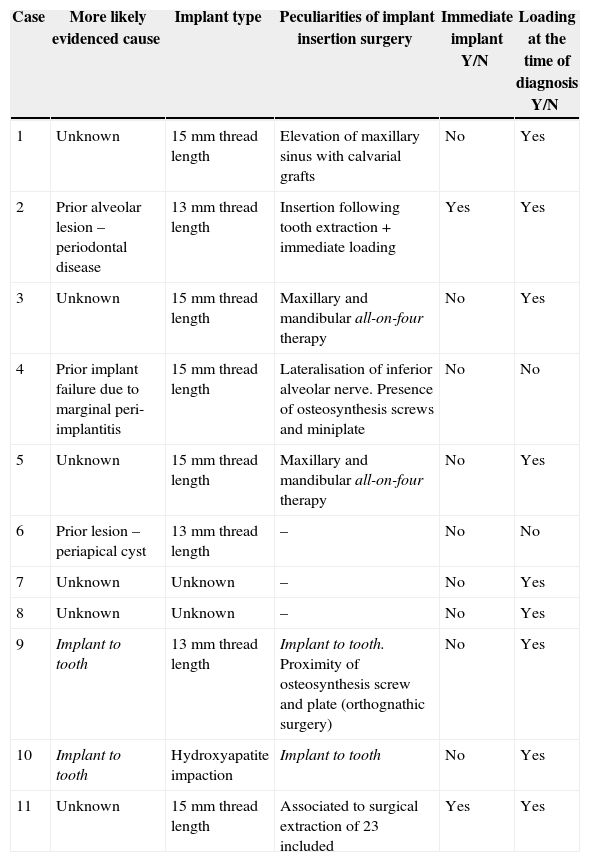
Therapeutic approach and evolution.
| Case | Symptomatology Yes/No | Therapeutic approach | Evolution |
|---|---|---|---|
| 1 | No | Expectant approach | Favourable |
| 2 | Yes | Periapical surgery without filling | Failure, implant removal |
| 3 | No | Expectant approach | Favourable |
| 4 | Yes | Periapical surgery without fillingImplant apex resection | Favourable |
| 5 | No | Expectant approach | Favourable |
| 6 | Yes | Periapical surgery with filling | Favourable |
| 7 | Yes | Periapical surgery with filling | Favourable |
| 8 | Yes | Periapical surgery with fillingImplant apex resection | Favourable |
| 9 | Yes | Periapical surgery without fillingRoot canal of adjacent tooth (23) | Favourable |
| 10 | No | Root canal of adjacent tooth (23) | Favourable |
| 11 | Yes | Periapical surgery without filling.Implant apex resection | Favourable |
The anti-inflammatory and antibiotic medical treatment was applied in acute outbreaks, or associated with the surgery for the treatment of the API.
ResultsIn the series, we have found several API-predisposing factors:
- -
presence of a prior lesion: apical cyst (in one case) and chronic marginal periodontitis with alveolar osteitis (in one case)
- -
prior implant failure due to peri-implantitis (in one case)
- -
“implant to tooth” type mechanism (in 2 cases)
- -
implant longer than 13mm (in 8 cases presented) (Table 2).
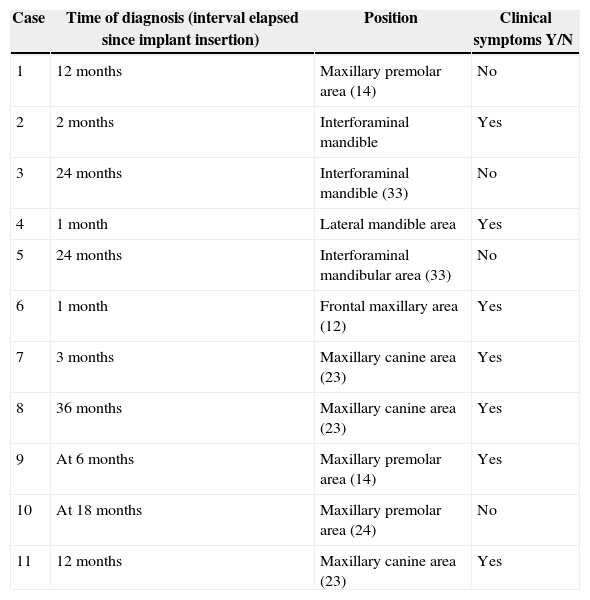
Table 2.Special elements of implants and possible associated aetiological factors.
Case More likely evidenced cause Implant type Peculiarities of implant insertion surgery Immediate implant Y/N Loading at the time of diagnosis Y/N 1 Unknown 15mm thread length Elevation of maxillary sinus with calvarial grafts No Yes 2 Prior alveolar lesion – periodontal disease 13mm thread length Insertion following tooth extraction + immediate loading Yes Yes 3 Unknown 15mm thread length Maxillary and mandibular all-on-four therapy No Yes 4 Prior implant failure due to marginal peri-implantitis 15mm thread length Lateralisation of inferior alveolar nerve. Presence of osteosynthesis screws and miniplate No No 5 Unknown 15mm thread length Maxillary and mandibular all-on-four therapy No Yes 6 Prior lesion – periapical cyst 13mm thread length – No No 7 Unknown Unknown – No Yes 8 Unknown Unknown – No Yes 9 Implant to tooth 13mm thread length Implant to tooth. Proximity of osteosynthesis screw and plate (orthognathic surgery) No Yes 10 Implant to tooth Hydroxyapatite impaction Implant to tooth No Yes 11 Unknown 15mm thread length Associated to surgical extraction of 23 included Yes Yes
Symptomatic patients referred a similar symptomatology: local soreness and pain, recurrent inflammations and fistulas. All implants presented conserved stability. We are unaware of the pre-surgical situation in 3 of the cases that came from other institutions. Two implants were performed simultaneously with the dental extraction and loading was performed immediately. The rest were deferred.
The period of time between the implant insertion surgery and the diagnosis varied between one month and 3 years, with a mean of 12.5 months.
In most symptomatic cases (5 out of 7 cases,) clinical symptoms started within the first 6 months after implant insertion (Table 3).
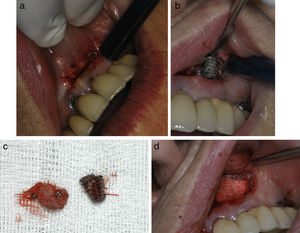
Clinical data of the series.
| Case | Time of diagnosis (interval elapsed since implant insertion) | Position | Clinical symptoms Y/N |
|---|---|---|---|
| 1 | 12 months | Maxillary premolar area (14) | No |
| 2 | 2 months | Interforaminal mandible | Yes |
| 3 | 24 months | Interforaminal mandible (33) | No |
| 4 | 1 month | Lateral mandible area | Yes |
| 5 | 24 months | Interforaminal mandibular area (33) | No |
| 6 | 1 month | Frontal maxillary area (12) | Yes |
| 7 | 3 months | Maxillary canine area (23) | Yes |
| 8 | 36 months | Maxillary canine area (23) | Yes |
| 9 | At 6 months | Maxillary premolar area (14) | Yes |
| 10 | At 18 months | Maxillary premolar area (24) | No |
| 11 | 12 months | Maxillary canine area (23) | Yes |
The most frequent location was the maxillary premolar area (4 cases) followed by the mandibular interforaminal area (3 cases) (Table 3).
The surgical intervention achieved clinical and radiological remission in 6 of the 7 cases operated on, presenting stable results at 1–4 years of follow-up. In the other case, in spite of the surgery, the periapical bone loss persisted, causing implant loss (Table 1).
A clinical-radiological follow-up was performed in asymptomatic cases, observing a self-limiting tendency.
DiscussionOne cause of API argued in the literature would be bone necrosis determined by bone overheating during the implant insertion surgery. Several published studies have indicated that the development of the apical bone lesion would be more likely to occur in a harder bone (more likely in the jaw,) which may imply the use of excessive force in performing the milling. Even so, practice does not confirm these assumptions: bibliography indicates the higher frequency at maxillary level (with a peak of incidence at maxillary premolar level),3 a finding that coincides with our observations (7 cases out of 11 at maxillary level, 3 of which occurred at maxillary premolar level and 3 in the maxillary canine area) (Table 3).
The general correlation between the deeper milling (more than 12mm) and the greater bone overheating has been proposed in several studies.10–12 Thus, there is a correlation between the greater length of the implant and the higher likelihood of API appearing. This observation, although undemonstrated, coincides with the results of our series, in which most of the implants (8 out of 11) have a minimum length of 13mm (Table 2).
However, other publications on bone necrosis indicate that there is more friction and overheating in the cortical area and not in the apical area when performing bone milling.13
There is debate regarding the presence of bacteria in implant periapical lesions.14 Some authors support this aetiology;15 other authors consider that the infectious aetiology has a secondary relevance.16
In Romanos’ bibliographical review, from the 32 cases presented (most of which are symptomatic cases with intervention), microbiological samples were taken only in 3 cases and bacterial presence was confirmed in only one case.3
Several authors recommend antibiotic treatment in acute phases, associating it with periapical surgery,17 and they even report eradication of the pathological process with medical treatment exclusively.18,19 In our series, the administration of antibiotics achieved only partial remission of the acute inflammatory symptomatology, without eliminating the inflammatory process entirely.
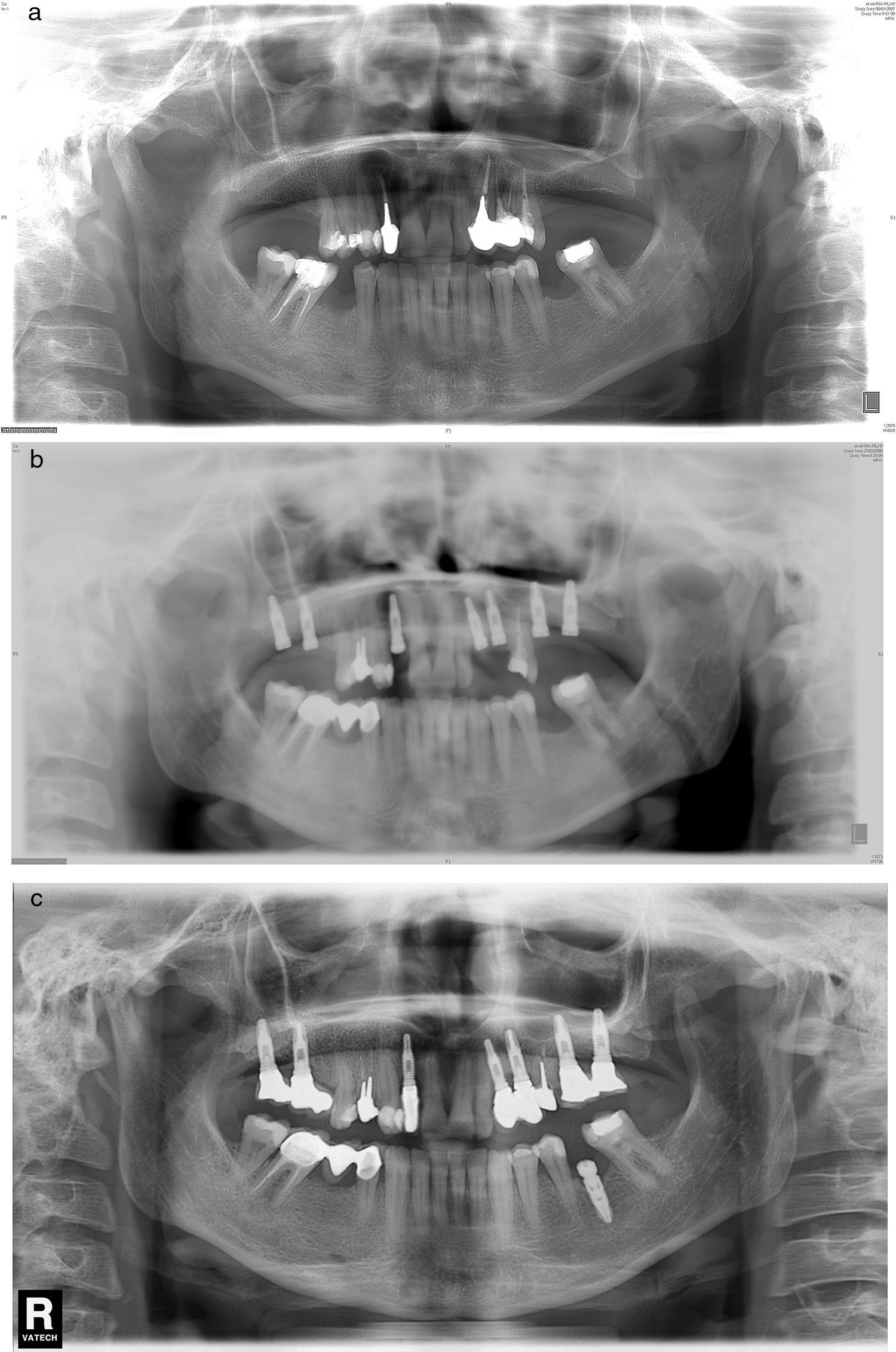
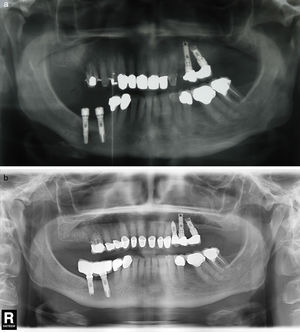
Prospective studies on the rate of success of implants inserted in alveoli with prior periapical infection do not indicate a higher rate of complications and recommend this type of procedure in the correct alveolar debridement conditions. This view is contrary to the initial assumptions regarding aetiology, which presented the prior alveolar lesion as an important aetiologic factor.20,21 In this series, we present one case of reappearance of prior apical lesion (case number 6), the only case in the series that has had an anatomopathological exam performed (result compatible with periapical cyst) (Fig. 2a–c).
(a) Case number 6: preoperative X-ray that shows cystic periapical lesion at upper right lateral incisor level. Tooth extraction of tooth 12 and curettage of the apical area were performed with good healing. (b) The periapical area reappears after the implant insertion that presents good stability; curettage was performed. (c) Final result after periapical surgery associated with bone filling.
Another factor reported is the premature and excessive loading that nevertheless fails to explain the apical location of the lesion, since excessive loading induces microfractures in the implant bone interface.3 Also, many of the cases described in the literature appear before initiating implant loading. For example, in the Peñarrocha and Zhou series, out of 7 and 6 cases respectively, all the APIs had started before loading.16,17 In our series, however, 9 out of the 11 cases were diagnosed after loading. We consider that this view is very likely caused by the late diagnosis performed in the cases in our series (average of 12.5 months) instead of the early diagnosis performed in the series mentioned in the bibliography (2–3 weeks.) Thus, at the time of diagnosis, most of the implants in our series had loading (which was performed at 3 months).
Regarding the asymptomatic lesions, our experience indicates a self-limiting tendency. Out of the 4 asymptomatic cases in the series, none presented symptoms during the radiological follow-up or tendency to progression. With the other authors,7 we consider that in the case of asymptomatic API, only follow-up is needed.
The few cases published in which an anatomopathological exam was performed indicated unspecified inflammatory changes of types: inflammatory infiltrate, bone necrosis, granulation tissue2,22,23 very similar to those found in apical periodontitis.
In regard to the therapeutic approach, some surgical technique variations have been described: curettage and irrigations with chlorhexidine,24 local application of tetracycline,6,17 application of an acid to favour a new osseointegration5 and bone regeneration with biomaterial and membranes,24,25 which are not universally accepted.
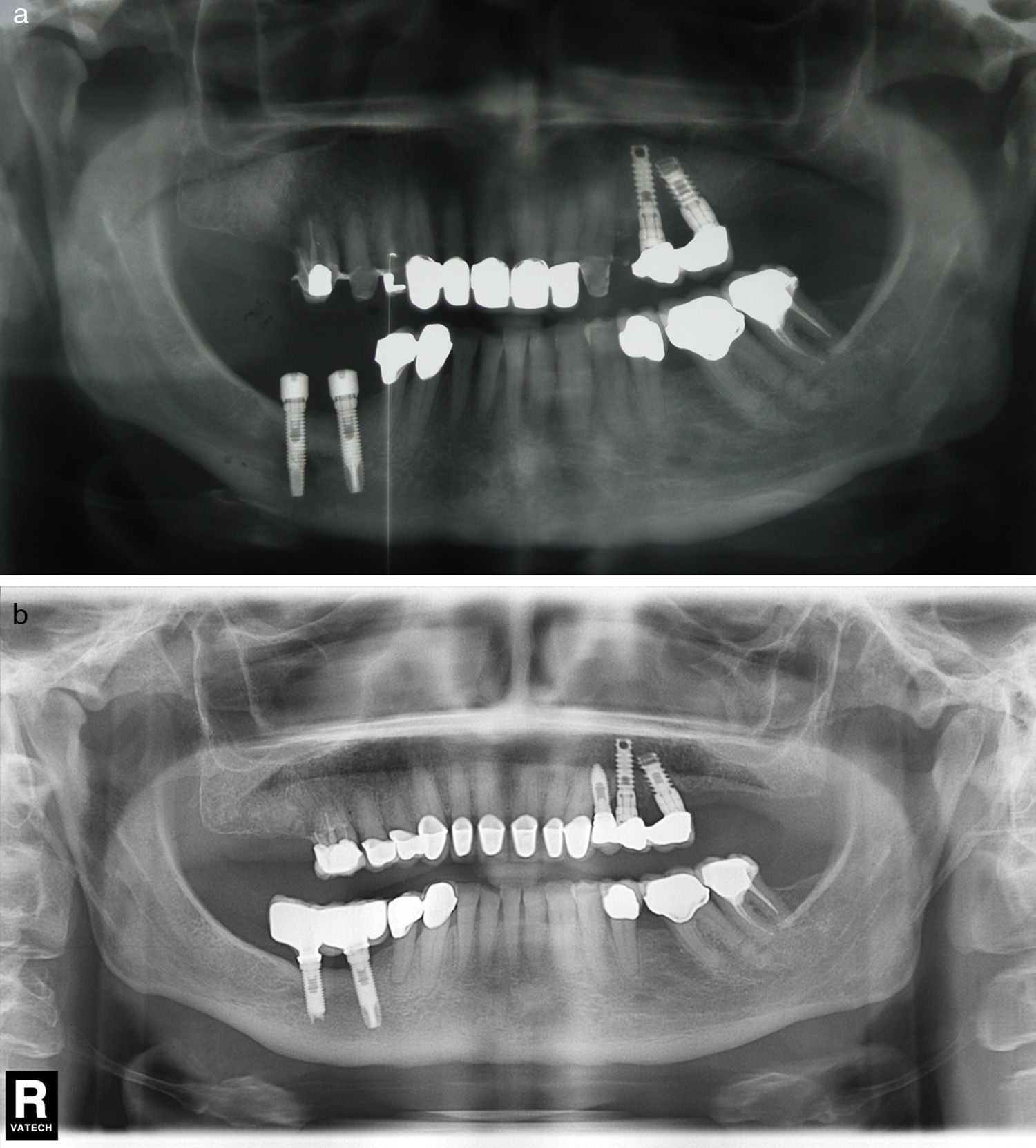
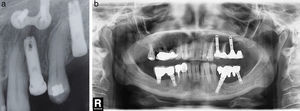
Implant apex resection is generally recommended in cases in which it prevents complete granulation tissue elimination, or when they are located in the maxillary sinus or nasal cavity26 (Fig. 3a and b).
(a) Case number 4: presence of radiolucent area round the implant apex of more posterior mandibular implant. In this case, implant insertion was performed simultaneously with the lateralisation of the right inferior alveolar nerve. (b) Radiological control after periapical surgery without bone filling. Apex resection was necessary to access the entire osteitis area correctly.
Some authors question the possibility of reosseointegration27 or osseointegration improvement with detoxification.14 In the 7 API cases intervened in our series, we have not used detoxification or demineralisation techniques, with rates of success similar to those described in the literature (83%). Bone cavity filling would prevent connective tissue migration towards the bone defect and would eliminate postoperative dead space. In our series, periapical curettage has shown good results: in 3 out of 7 cases, we used filling with scraped alveolar bone (2 cases) and artificial bovine bone in the remaining case.
The case of mandibular API reported by Li-Ching Chang in 2009 that remitted only with antibiotic treatment (stable result at 2 years)18 does not make us change our approach, but it does offer the possibility of taking into account an antibiotic treatment whenever the surgery cannot be performed immediately.
Another key element in the prevention and handling of API is the relation with adjacent teeth indicated by Sussman in 1998. Later, several authors published cases of API in edentulous patients and without any relation to the rest of the teeth, demonstrating that the apical peri-implant disease is not exclusively related to the adjacent teeth.2,15
From a practical point of view, implant to tooth and tooth to implant lesions have as common denominator the loss of vitality of the adjacent tooth, which requires the performance of root canal as a primary measure. The performance of associated periapical surgery depends on the presence or absence of symptoms.
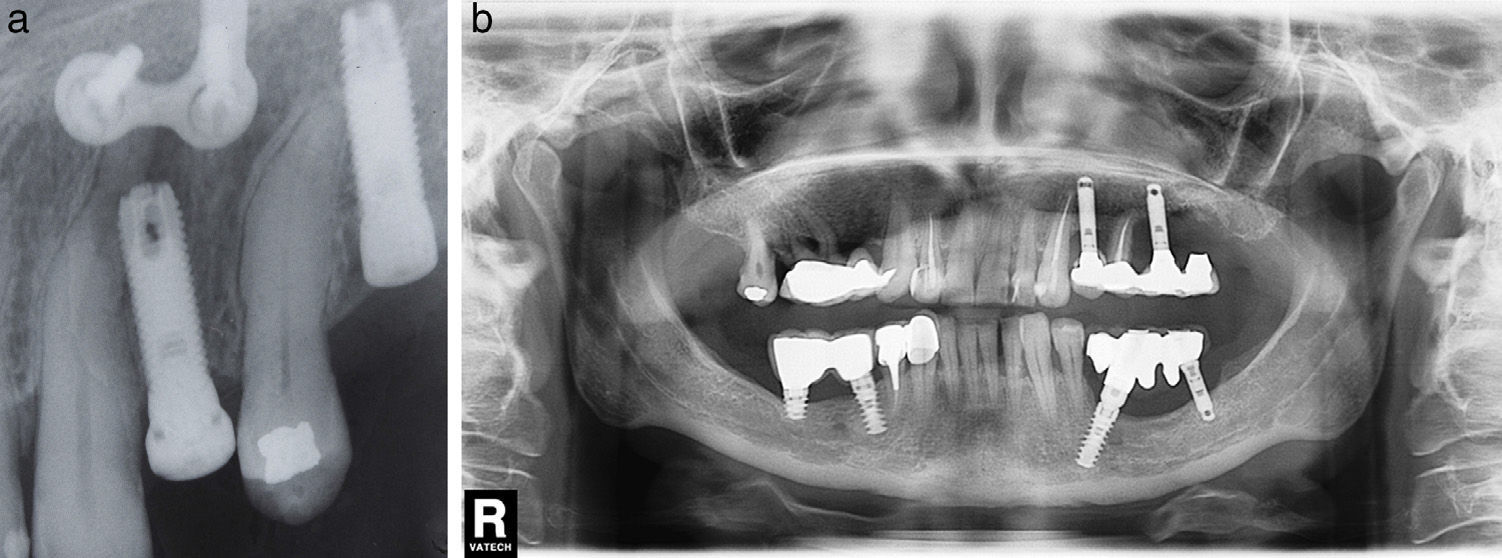
The 2 cases of type I (“implant to tooth” or injury of an adjacent tooth when placing the implant) lesion included in our series were treated following the principle stated above. After performing a root canal of the injured tooth, we intervened in a symptomatic case with posterior resolution of the periapical process (Fig. 4a). In one asymptomatic case, we decided to perform a root canal and monitor the evolution, observing the gradual disappearance of periapical radiolucency (Fig. 4b).
(a) Case number 9: implant to tooth type lesion that has been favoured by radicular curvature of the adjacent tooth. The presence of miniplate is the consequence of prior orthognathic surgery (maxillary osteotomy). (b) Case number 10: after implant insertion, the upper left canine has lost pulp vitality. A root canal was performed and the initial radiotransparency disappeared during follow-up. No periapical surgery has been performed since the patient did not present symptoms.
The API is a rare inflammatory process that may determine the loss of implants or adjacent teeth, complicating the treatment plan.
API may be considered an alveolar osteitis similar, from the anatomopathological and evolutionary point of view, to apical periodontitis. Whenever possible, the option would be to try to perform conservative periapical surgery, which offers good results.
The causes may be multiple: prior periapical or peri-implant disease, over-instrumentation of the implant bed (for example due to implants being too long).
For the purpose of expanding knowledge in this surgical field, the performance of microbiological cultures and systematic anatomopathological exams of the surgical pieces is recommended.
Ethical responsibilitiesProtection of people and animalsAuthors state that the proceedings followed conformed to the ethical standards of the Responsible Committee on Human Experimentation and according to the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors state that they have followed the protocols of their workplace about the data publication of patients and that all the patients included in the study have received enough information and have given their written informed consent to participate in that study.
Right to privacy and informed consentAuthors have obtained the informed consent from the patients or subjects referred to in the article. This document is in possession of the corresponding author.
Conflict of interestThe authors declare that there are no conflicts of interest when writing the document.
Please cite this article as: Stavaru Marinescu B, Naval Gíasb L, Herrera Calvo G. Periimplantitis apical – presentación de serie de 11 casos clínicos. Rev Esp Cir Oral Maxilofac. 2015;37:188–195.