Osteoporosis is characterized by a loss of bone mass and a change in bone quality resulting in greater susceptibility to what are termed fragility or osteoporotic fractures. Recent projections estimate an annual incidence of approximately 200,000 new fractures in our country, with a financial impact of around 3000 million Euros. Moreover, it is anticipated that this number will increase by 80,000 fractures by 2025, with a parallel increase in incurred costs of approximately 30%.1 This greater incidence is due in the main to the progressive increase in life expectancy, and consequent ageing of the population, and will eventually result in a more susceptible population scenario. Age is a major risk factor; irrespective of bone mineral density (BMD). This is due not only to gradually deteriorating bone strength, but also to the fact that with age come more mobility problems, greater dependency and comorbidities which can directly contribute towards incurring fractures.2 Indeed, if we take the age factor into account in analysing the data on fracture incidence rates, there appears to be a trend towards a reduction and stabilisation of these fractures over time.3 The increase in anti-osteoporotic treatments is postulated as a plausible explanation for this4; nevertheless, in absolute terms, a rise in the incidence is projected,5 with the consequent clinical and economic repercussions.
Fractures of the proximal femur, proximal humerus, vertebrae and distal radius are considered “major” osteoporotic fractures, as they have greater consequences in terms of morbidity and mortality data and cost.6,7 It is hard to establish the real incidence rate of all these fractures, as data collection systems vary and are difficult to standardise, although proximal femur fractures can be better understood and studied because most patients require hospitalisation and surgery. It is estimated that there are around 640,000 cases annually in the European Union, while there are around 60,000 cases in Spain.8 Epidemiological data on hip fractures in Spain give an approximate incidence rate, depending on the series, of around 300 cases per 100,000 inhabitants/year, with a female:male ratio of 3.4:1.9–15
What is the clinical significance of osteoporotic fractures?The main osteoporotic fractures have an impact both on patients’ quality of life and their life expectancy.
The main clinical consequences of vertebral fractures are back pain, kyphosis and loss of height. Perceived quality of life also decreases as the amount of fractures increase.16 It is estimated that a reduced life expectancy of about 6 years17 is associated with vertebral fractures, probably due to diverse comorbidities in the elderly patient.
Fracture of the proximal humerus is the third most common fracture after the age of 65, at around 5% of all fractures. It is more common in women, generally with a high degree of independence. There has been a major increase in these fractures over the past 30 years and they are considered to be clearly associated with osteoporosis.18 They reduce functional capacity, significantly limiting daily activities 6 months after the fracture.19
Distal radius fractures do not entail increased mortality, probably because they occur in younger patients whose general health is less affected, but they may leave sequelae which can limit perceived quality of life, especially in older patients.20
Fracture of the hip is a serious clinical process which has significant morbidity and mortality.21 Depending on the series, it is estimated that these patients have an intrahospital mortality rate of around 5%, with 20% of patients dying in the first year. Thirty percent of patients are left with a permanent disability, 40% have serious difficulties in walking independently, and 80% are unable to undertake at least one independent activity per day. The mortality rate of these patients is double that of patients of the same age with no fracture; respiratory and cardiovascular problems are the main causes of death in the first 30 days.22
It should not be forgotten that the presence of an osteoporosis-related fracture constitutes a major risk factor for subsequent fractures, especially during the first year.23 Nonetheless, in a study of the burden associated with hip fractures in Spain,24 in which 22.2% of patients with fractures had suffered a prior fracture, only 1.8% of the patients had been densitometrically diagnosed with osteoporosis and only 15.6% reported having received treatment for osteoporosis at some time in the past.
Diagnostic assessmentWhat is osteoporosis?The definition that could be considered “official”, as agreed at a consensus conference, is described as a generalised, skeletal disorder characterised by compromised bone strength predisposing to an increased risk of fracture.25 The concept of bone strength is the integration of bone quantity and quality, adding qualitative criteria to previous definitions which imply that this fracture risk is influenced not only by “how much” bone there is, but also by “what” this bone is “like” and “how” it is organised.
What is an osteoporotic fracture?An osteoporosis-related fracture is a fracture following low-energy trauma such as a fall from standing height, or no identifiable trauma,26 located in any bone of the axial or peripheral skeleton, except for the skull and facial bones, once other causes of skeletal frailty have been ruled out (pathological fractures).27 As mentioned above, the most common and relevant are fractures of the vertebral column, proximal femur, distal forearm and proximal humerus. Other sites are less common, such as fractures of the distal end of the femur, rib fractures, fractures of the distal end of the humerus, proximal fractures of the tibia and fractures of the pelvic bones.9
What are the risk factors for osteoporotic fractures?We could consider that osteoporotic fractures occur in a specific context: the patient has fragile bones and suffers a fall. Therefore, all situations involving a loss of bone substance and situations where falls are more likely could be considered risk factors for this type of fracture. Numerous risk factors have been identified for both osteoporosis and osteoporotic factors, and undoubtedly, when several of these factors coincide, the risk increases considerably.28 The principal epidemiological studies undertaken in our field29–32 place advanced age, sedentary lifestyle, personal history of fracture, family history of fracture, early menopause, diseases causing osteopaenia such as rheumatoid arthritis, use of cortico-steroids, falls and densitometry results as the main risk factors.29–32
In our speciality, the presence of a prior fracture is significant as a predictive factor for subsequent fractures, as mentioned above, because traumatologists regularly manage these types of patients and are in a good position to act to prevent further fractures.
Although it is acknowledged that the result of measuring bone mass by densitometry can be considered a risk factor, it is also acknowledged that most fractures occur with a normal BMD or osteopaenic bone density.30 Therefore, it is considered a measurement with no predictive capacity in populations which are asymptomatic or not at high risk of fracture.33
What is the FRAX® tool and of what use is it?FRAX® is a fracture risk assessment tool for men and women between the ages of 40 and 90. It gives the 10-year probability of absolute risk of hip fracture and main fractures (vertebral, hip, humerus and wrist). It is based on a series of meta-analyses which identify the clinical risk factors associated with a greater risk of fracture, and on fracture incidence rate and prevalence data in each country. It takes into account risk factors of age, gender, weight, height, the presence of prior fractures, history of fracture in father/mother, smoking, steroid treatment over more than 3 months, presence of rheumatoid arthritis, a diagnosis of secondary osteoporosis and alcohol consumption. The calculation can be made adding the BMD at the femoral neck, although it is also valid without densitometric criteria.34
The FRAX® tool is useful because it makes it possible to establish an intervention threshold which could be useful in diagnostic and therapeutic decision-making. One of the main problems with the tool is that it has not been validated in our country; therefore, the optimal values from which to implement a treatment strategy have not been determined.
The main studies undertaken in our country to assess the tool's discriminative capacity32,35–37 reflect that the Spanish version of FRAX® underestimates the risk of major and hip factures by half, therefore its predictive capacity is low. It is recommended that the cut-off points of FRAX® UK – which has been validated – should be followed, because they are similar to the corrected Spanish FRAX®.35 The test's other weaknesses are that it can only be used on patients who have not received treatment previously, it does not assess falls and it does not differentiate between the different fractures and their number; nevertheless, it can be a useful tool in establishing a risk scenario for each patient.
What does a high risk of osteoporotic fracture mean?It is difficult to define “a patient at high risk of fracture”. In a consensus document drawn up in our country setting out the different perspectives of the specialities involved,38 it was concluded that advanced age, personal and family history of fracture and very low bone mass are factors which contribute significantly to an increased fracture risk, and therefore it is important that these are taken into account when establishing strategies to manage this type of patient. Using the FRAX® tool, we can consider that there is a high fracture risk if there is a risk of hip fracture ≥3% or major fracture ≥20%.39 Studying each patient individually, with their detailed clinical history, should provide us with a risk scenario which we can identify, especially when several of these factors coincide, in order, as far as is possible, to prevent a fracture.
Which analytical data are important in the diagnosis of osteoporotic fracture?We are able to establish that there is a primary, postmenopausal or senile osteoporosis, and a secondary osteoporosis which encompasses a series of disorders which cause a loss of bone mass, a change in bone structure, a reduction in bone strength and, in short, an increased risk of fracture.
In males, osteoporosis is secondary in up to 60% of cases,40,41 the most common causes being, hypogonadism, alcoholism, steroid treatment, androgen deficiency treatment in prostate cancer and myeloma.42 By contrast, only 30% of women apparently presenting with primary postmenopausal osteoporosis have an identifiable cause of secondary osteoporosis.41 This circumstance should not mean that tests are not carried out for differential diagnosis to exclude the main secondary causes of the disease.43,44 The most common causes of secondary osteoporosis in women are, hypoestrogenaemia, hyperthyroidism, primary hyperparathyroidism, type I diabetes mellitus, rheumatoid arthritis, steroid and anticonvulsant treatment.
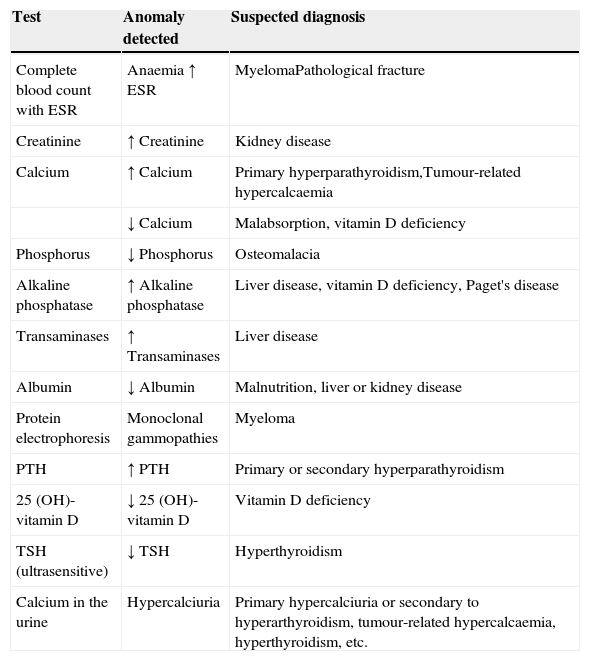
The basic analyses which should be requested to rule out the most common causes of secondary osteoporosis should include a basic analytical study (complete blood count with ESR, serum biochemistry with calcium, phosphorus, alkaline phosphatase, transaminases, urea, creatinine and calcium (24h urine)) and a specific study to screen for the major causes of secondary osteoporosis from a clinical perspective (albumin, proteins, proteinogram, serum levels of TSH, PTH, 25-OH vitamin D) in order to generally identify any haematological, mineral or electrolyte disorder, and uncover any other systemic or endocrine/metabolic disease not discovered during the patient's initial assessment. The systematic use of bone markers is not recommended for the differential diagnosis of patients with osteoporosis.45 They can be useful in identifying patients at greater risk of fracture, especially for prompt assessment of response to both antiresorptive and bone-building treatment. We recommend using the analytical tests shown in Table 1 as a methodological approach to rule out the most common causes of secondary osteoporosis.
Analytical tests in differential diagnosis of osteoporosis.
| Test | Anomaly detected | Suspected diagnosis |
|---|---|---|
| Complete blood count with ESR | Anaemia ↑ ESR | MyelomaPathological fracture |
| Creatinine | ↑ Creatinine | Kidney disease |
| Calcium | ↑ Calcium | Primary hyperparathyroidism,Tumour-related hypercalcaemia |
| ↓ Calcium | Malabsorption, vitamin D deficiency | |
| Phosphorus | ↓ Phosphorus | Osteomalacia |
| Alkaline phosphatase | ↑ Alkaline phosphatase | Liver disease, vitamin D deficiency, Paget's disease |
| Transaminases | ↑ Transaminases | Liver disease |
| Albumin | ↓ Albumin | Malnutrition, liver or kidney disease |
| Protein electrophoresis | Monoclonal gammopathies | Myeloma |
| PTH | ↑ PTH | Primary or secondary hyperparathyroidism |
| 25 (OH)-vitamin D | ↓ 25 (OH)-vitamin D | Vitamin D deficiency |
| TSH (ultrasensitive) | ↓ TSH | Hyperthyroidism |
| Calcium in the urine | Hypercalciuria | Primary hypercalciuria or secondary to hyperarthyroidism, tumour-related hypercalcaemia, hyperthyroidism, etc. |
Plain radiography is the basic test for diagnosing osteoporotic fractures. Not only does it offer a confirmation diagnosis of most fractures, but it also enables an approximation of the degree of bone loss and qualitative disintegration of the affected bone. Although plain radiography is only able to detect changes in bone mass when there has already been BMD loss of above 30%, it does generally enable an approximate assessment of the extent of regional osteoporosis. For example, the Singh index has been traditionally used in the hip for this purpose; this is no more than a semi-quantitative assessment of the degree of regional osteoporosis of the proximal end of the femur based on radiological patterns of progressive loss of compressive and tensile trabeculae, as bone mass decreases.46
Occasionally diagnosis can be difficult, of vertebral fractures for example, as there is no identifiable fracture line.47 Diagnosis is based on morphometric criteria based on measuring the size of the vertebra at the anterior, middle and posterior points. Several assessment methods have been described, the most accepted being Genant's48 semi-quantitative method. There is considered to be a mild deformity when there is a reduction of 20–25% of the anterior, middle or posterior height of the vertebral body, moderate deformity when there is a decrease of 25–40% of the anterior, middle or posterior height of the vertebral body, and severe deformity when there is 40% or more reduction of the anterior, middle or posterior height of the vertebral body.
However, occasionally vertebral fractures are not very clinically expressive, or can pass unnoticed, a diagnosis can be made incidentally during radiological examination for other reasons, on a chest X-ray, for example49; this might be important to consider in screening for the disease.
On other occasions tests such as computed axial tomography (CAT) and magnetic resonance (MR) can be useful. CAT scans can be of use in diagnosing hidden fractures, especially some femoral neck fractures which cannot be seen correctly on a plain X-ray, and also some complex fractures, where the three-dimensional reconstruction of the segment of fractured bone can help us in our choice of surgical technique. Magnetic resonance can also be useful in certain slow fractures (sacrum, hip…) where radiographic manifestations are usually delayed, and in cases where there is doubt as to whether a vertebral fracture is acute, or old, because it is possible to visualise bone oedema. It can also assist us in some spinal fractures where there is doubt as to the integrity of the posterior spinal wall and a technique is being considered to increase bone with methyl methacrylate cement, since a loss of integrity of the posterior wall can constitute a relative contraindication to the procedure.
How do we diagnose osteoporosis densitometrically?Dual energy X-ray absorptiometry [DXA] is recommended as the benchmark technique for measuring BMD. DXA has good precision, a low radiation dose and enables BMD measurement in both the axial and peripheral spine.
It provides two values: T-score (number of standard deviations [SD] of an individual's BMD compared to a normal reference population) and Z-score (number of standard deviations of an individual's BMD in relation to a population of the same sex, race and age). Although the T-score can be measured in the lumbar spine, femoral neck and hip, the WHO recommends that femoral neck values are used for classifying osteoporosis. The values obtained apply to both men and women.
The Z-score is not used to define osteoporosis, but it can identify patients with BMDs that are lower than expected for their age, in children and adults under 50 where it is recommended for interpreting results. If it is used in follow-up, the same technique should be employed and the T-score assessed in the same anatomical area.50
A diagnosis of osteoporosis can be influenced by various factors, such as the region where the measurement is taken, potential artefacts (fractures, arthritis, scoliosis, vascular calcification, osteomalacia, etc.), the number of regions explored and the reference values applied, it is therefore advisable to explore at least two regions of the skeleton. If it is not possible to perform a DXA on the lumbar spine or the hip, it is recommended that the DXA is taken on the distal third of the non-dominant radius of the forearm. This can occur if there are anatomical changes (major scoliosis, degenerative disorders, multiple vertebral fractures, morbid obesity) or technical problems (the presence of metal-type elements from rachis surgery, hip arthroplasty).
Ultrasound, peripheral DXA equipment and central or peripheral quantitative computed tomography are useful in predicting a high risk of fracture but should not be used for diagnosis, follow-up or to assess therapeutic response in patients with osteoporosis.
The traditional values to define bone mass are: normal BMD with a T-score≥−1.0 SD, osteopaenia with a T-score between −1.0 and −2.5 SD, and osteoporosis with a T-score≤−2.5 SD.
When should bone densitometry be requested?Densitometric screening of the general population is not cost-effective and there is much variation with regard to the indications for densitometry. However, the evaluation of risk factors and the presence of fracture have been demonstrated to be cost effective, and this efficacy increases with the added use of DXA.51 As with any complementary test, before requesting bone densitometry it is essential to be certain that the result will influence the therapeutic decision. It is a further factor to be taken into account in the overall assessment of fracture risk. We could consider that it is indicated in the following situations52,53:
- a)
Women and men over 65 with moderate fracture risk, established through their risk factors or with the FRAX® tool, when the value of their result could mean a change in therapeutic approach.
- b)
In patients under 65 with high fragility fracture risk factors.
- c)
In patients under 40 with very high fracture risk such as a history of several osteoporotic fractures, major osteoporotic fracture or treatment with high doses of steroids.
- d)
Before starting treatments which might affect BMD.
It is recommended that densitometric checks are performed every 2–3 years. In general it is not recommended to perform densitometric checks before 2 years, as it has been observed that some treated patients lose bone mass in the first year but can gain it in the second.
Therapeutic approachHow should treatment of an acute osteoporotic vertebral fracture be approached?Once the diagnosis of vertebral fragility fracture has been confirmed, and having ruled out other processes which could start in this way, initial treatment is conservative, and the priority objective is to achieve appropriate control of symptoms in order to reduce rest time as far as possible and prevent muscular atrophy. Control of pain, prevention of complications such as paralytic ileus or progressive deformity, which this type of fracture can entail, rehabilitation therapy to promote functional recovery as soon as possible, and secondary prevention of further fractures are the basic pillars to approach treatment. Occasionally admission to hospital can be necessary to ensure better control of symptoms.
Satisfactory pain control improves the patient's wellbeing and contributes to prompt mobilisation, and this reduces the consequences of bed rest, which include bone loss.54 Initial treatment should be paracetamol and non-steroid anti-inflammatories, using opiates if required, in an attempt to control pain which usually persists from 6 to 12 weeks.55 Rest on a hard mattress can sometimes be necessary, but only for a short period of time; the patient should start with a seated position and begin gradual walking during the first week.
Despite the widespread use of ortheses as treatment for the acute phase of vertebral fractures, there is only one randomised, prospective study on these types of devices.56 In this study an improvement in pain, quality of life and return to daily activities was observed and better muscular development. The dropout rate from wearing the corset is usually very high, and therefore light, easy-to-wear corsets are recommended. Jewett-type devices and CAMP-type semi-rigid devices for lumbar lordosis, with shoulder straps, are generally better tolerated.
There are two scenarios which can be considered in terms of surgical treatment: the first, when there is neurological damage secondary to the fracture, which is rare in osteoporotic fractures, and the second, when conservative treatment has failed, pain relief in particular. In the first scenario, it is necessary to resort to conventional surgery, either with posterior approach or anterior and posterior combined approach, taking into account the poor strength of these patients’ bone tissue which can result in the failure of the equipment and arthrodesis. In the second, we can resort to minimally invasive techniques such as vertebroplasty and kyphoplasty.
The objective of these techniques, which consist of placing methyl methacrylate cement inside the fractured vertebral body, directly or with a balloon which has corrected the vertebral height beforehand, is to stabilise the fracture and ease pain. A recent study which analyses the risks and benefits of both techniques compared with conservative treatment57 concludes that although there may be an initial improvement in pain control, in the long term the analgesic and functional benefits are modest. Furthermore, there is an increased risk of subsequent fractures in the vertebrae adjacent to those treated using these techniques, especially in patients with a greater number of fractured vertebrae, those on whom a greater number of procedures have been performed, those of advanced age, those with a lower BMD and in patients with leakage of cement. For this reason, the techniques should be used with strict clinical criteria, when appropriate analgesic treatment has not been effective in pain control, and always if bone oedema in the vertebra has been demonstrated on magnetic resonance imaging. Kyphoplasty differs from vertebroplasy in that it provides better reconstruction of the anatomy of the vertebra and reduction of the kyphotic angle; but the cost is higher.
How should treatment of an acute osteoporotic non-vertebral fracture be approached?As mentioned above, the most common non-vertebral fractures are those of the proximal femur, proximal humerus and the wrist. Leaving to one side the specific treatment for each, which is beyond the remit of these guidelines, it is important to stress that, in general, these fractures have characteristics which make them different to those in bones with normal bone density and connectivity. Therefore it is important that they are taken into account, especially if we are opting for surgical treatment, in order to prevent, as far as possible, failures in their management. There are mechanical and biological factors which distinguish them from fractures that appear in patients without osteoporosis. The mechanical factors include the site of the fracture, which essentially affects the metaphyseal area, largely structured from trabecular bone, which if lost through osteoporosis, especially in the horizontal trabeculae, will result in reduced resistance to lateral pressure, making it sometimes difficult to achieve stable internal fixation. The cortical bone is also important, because it appears thinned in the osteoporotic process, with a greater number of pores and a larger diameter; these characteristics can also impact negatively on the stability of implants. With regard to biological factors, it seems that osteoporotic bone solidifies in a different way to healthy bone, although there are few clinical studies to demonstrate this.58 The fracture patient's general state of health also needs to be considered: they are usually of advanced age, frequently have several comorbidities and are routinely polymedicated, all of which can have a negative impact on the end result of treatment.59
The principal objective of treatment of these types of fractures is to recover function as soon as possible, and this requires solid fixation of the fracture. This will not always be easy for the abovementioned reasons. To achieve this goal, we will sometimes need to take a different therapeutic approach to that required for young patients. The treatment of fractures in patients with osteoporosis poses a challenge for orthopaedic surgeons.
Which non-drug treatments should be used?There are several treatments which are considered universal for the primary and secondary prevention of fragility fractures. The first is to limit toxic habits, not just because of their effect on bone, but also because of the impact on general health. Stopping smoking, reducing alcohol consumption to less than 3units a day and cutting down on high-caffeine drinks help to maintain bone mass.60,61 A suitable diet, avoiding excess salt which encourages the kidney to excrete calcium, with an adequate intake of protein (1g/kg/day), and calcium around 1000–1200mg/day, and a sufficient amount of vitamin D (around 800IU per day), reduces the risk of fracture.39,62 It has been suggested that calcium supplements might be associated with cardiovascular events,63 although there is not sufficient evidence to confirm this.64 It is recommended that patients should have an adequate calcium intake, preferably in their diet, otherwise they should take supplements. Avoiding a sedentary lifestyle, taking regular physical activity throughout life, adapted to each age and individual, can strengthen muscles, and improve balance, agility and general health. In the first decades of life activity promotes an optimal peak of bone mass, while impact and weight bearing exercises improve and maintain bone mass in adults.65,66 Strategies to prevent falls, encouraging multicomponent exercises, reducing or stopping medication which might cause falls, correcting sensory deficits, treating diseases, particularly cardiovascular disease, addressing risks in the home and poor nutrition, especially with regard to vitamin D, are some of the pillars for the non-pharmacological management of these patients.67
How important is vitamin D in orthopaedic and trauma surgery?Vitamin D is a steroid-type hormone which acts on the receptors in a great variety of tissues and cells. It is involved in several cellular processes in bone; it promotes mineralisation of the osteoid matrix and regulates plasma calcium levels and also, by extension, parathyroid hormone levels. A severe deficiency reduces calcaemia and increases parathyroid hormone resulting in increased bone remodelling and reduced bone mass which can cause rickets in children and osteomalacia and osteoporosis in adults.68
A relationship has been established between low levels of vitamin D and a greater prevalence of osteoporotic fractures with hypovitaminosis rates in hip fracture patients, in some series, of more than 90%. Nevertheless, it appears that administering the hormone in isolation is unlikely to prevent fragility fractures, but not if given in conjunction with calcium supplements, which does reduce the risk of hip fractures, especially in institutionalised patients.69
Vitamin D also influences the maintenance of muscle tone, of particular importance in the prevention of falls. From a clinical perspective, vitamin D levels of 20ng/ml have been associated with increased body sway, poor limb proprioception and greater displacement of the centre of gravity which affects gait control.70 When these levels fall below 10–12ng/ml a marked decrease in muscle strength occurs, and frank myopathy becomes established or proximally sited loss of muscle mass, generally in the lower limbs,71 when these levels fall below 8ng/ml. This myopathy caused by a vitamin D deficiency can also contribute to an increased risk of falling, and therefore, of incurring a fracture.
With regard to bone callus formation, administering vitamin D increases the quality of collagen, accelerates the organisation of collagen fibres and the proliferation and differentiation of fracture callus osteoprogenitor cells. Similarly, it promotes better vascularisation of the callus in the first stages of fracture healing without interfering in the natural repair process, improving its consistency and strength, chiefly in spongy bone,72 which invites us to think that adequate levels of vitamin D have a positive effect on the repair process after a fracture.
Another important point is the association between low levels of vitamin D and the development of arthritis of the hip and knee,73 there are also studies which have found significantly higher implant survival rates after joint replacement surgery in patients with adequate levels of vitamin D.74
With regard to daily dose and blood levels, a recent meta-analysis75 has established that a daily dose of 800IU might be sufficient to maintain adequate bone mass, in order to obtain levels around 40ng/ml, ensuring a correct intake of calcium of 1000–1200mg at all times – preferably in the diet – although some authors defend supplementing with higher doses.76
Which drugs are available at the moment to prevent and treat osteoporotic fractures?Antiresorptive and anticatabolic treatmentsHormone replacement therapyThis is not recommended at present as treatment for osteoporosis; its use should be evaluated for treating menopause-related symptoms, using the lowest dose for the least amount of time possible, under the supervision of a gynaecologist. The patient should be informed of the beneficial effects of this treatment on the skeleton.
RaloxifeneMechanism of action77: This is a selective oestrogen receptor modulator (SERM). When acting on target cells these behave as tissue-specific oestrogen receptor agonists/antagonists. Favourable oestrogenic effects have been described with this pharmacological profile, avoiding unfavourable effects on the breast and endometrium. Raloxifene can be considered a second-generation SERM in this therapeutic group.
Anti-fracture efficacy78: Raloxifene is of proven efficacy in reducing the risk of vertebral fracture with grade A recommendation at an oral dose of 60mg/day.
Eligible patients: Non-fertile females at low risk of hip fracture with no cardiovascular risk factors for whom we are seeking to reduce the risk of vertebral fracture and concomitantly reduce the risk of breast cancer in the absence of endometrial cancer and non-filiated metrorrhagia.
Safety aspects to take into account: We can conclude from the available evidence that the two most relevant side effects are:
- 1.
Thromboembolic phenomena: 0.9% (with the necessary number of treatments to incur an event at 157 with a 95% confidence interval).
- 2.
Hot flushes: 13.5% (with the necessary number of treatments to incur an event at 20 with a 95% confidence interval).
Mechanism of action: This is a selective oestrogen receptor modulator (SERM) with high affinity to alpha and beta oestrogen receptors, but with a clearly superior binding affinity with the alpha receptors. Bazedoxifene can be considered a third-generation SERM in this therapeutic group because of its selective mechanism of action, although the clinical significance of this has not been clearly established.
Anti-fracture efficacy79: Bazedoxifene, at an oral dose of 20mg/day, is of proven efficacy in reducing the risk of vertebral fracture which has been established with morphological criteria.
Eligible patients: Similar to those eligible for raloxifene.
Safety aspects to take into account: The safety profile is similar to that of raloxifene, although some agencies have set up cardiovascular safety monitoring plans.
CalcitoninThis is a polypeptide hormone which partially inhibits the formation and activity of osteoclasts. It has been withdrawn as treatment for osteoporosis under the recommendations of the European Medicines Agency, due to an increased risk of cancer with its long term use as a nasal spray.80 Currently indications for its use as a solution for injection and infusion are to prevent acute bone loss due to sudden immobilisation, to treat Paget's disease in patients who do not respond to alternative treatments and in hypercalcaemia caused by cancer, and treatment should be limited to the shortest time possible.
BisphosphonatesMechanism of action: These are chemical pyrophosphate analogues which bind to bone hydroxyapatite. First-generation or non-nitrogenated bisphosphonates inhibit bone resorption of osteoclasts, causing their aptosis by producing toxic ATP-metabolites, currently they are barely used. Whereas second-generation bisphosphonates, which have an amino group which gives them much more powerful anti-resorptive activity, act to interfere in the metabolic mevalonate pathway, which alters the undulating edge of the osteoblast and eventually results in its apoptosis. Depending on the drug, they can be given orally and/or intravenously (Table 2).
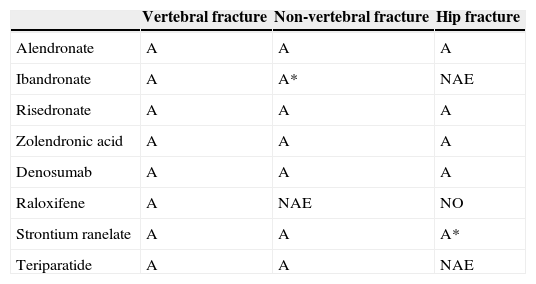
Summary of the efficacy of the different available drugs.
| Vertebral fracture | Non-vertebral fracture | Hip fracture | |
|---|---|---|---|
| Alendronate | A | A | A |
| Ibandronate | A | A* | NAE |
| Risedronate | A | A | A |
| Zolendronic acid | A | A | A |
| Denosumab | A | A | A |
| Raloxifene | A | NAE | NO |
| Strontium ranelate | A | A | A* |
| Teriparatide | A | A | NAE |
A, recommendation grade A; A*, post hoc study; NAE, no available evidence.
Anti-fracture efficacy: Several clinical trials have demonstrated that bisphosphonates increase BMD and reduce the risk of fracture.
The pivotal FIT study with alendronate81 demonstrated a 47% reduction in relative risk (RR) of subsequent vertebral fractures (RR: 0.53; CI95%: 0.41–0.68), a 51% reduction in hip fractures (RR: 0.49; CI95%: 0.23–0.99) and a 48% reduction in the risk of fracture of the distal end of the radius (RR: 0.52; CI95%: 0.31–0.87).
The VERT study with risedronate82 demonstrated a significant reduction of 41% in the incidence rate of vertebral fracture (RR: 0.59; CI95%: 0.42–0.82) and a 39% reduction in non-vertebral fracture (RR: 0.61; CI95%: 0.39–0.94) in women presenting with at least one baseline vertebral fracture.
The HIP study83 evaluated the efficacy of risedronate compared to a placebo, which demonstrated a significant reduction of 30% in RR of hip fracture (RR: 0.7; CI95%: 0.6–0.9; P=0.02).
In the BONE84 study, ibandronate significantly reduced RR of subsequent morphometric vertebral fractures compared to a placebo by 62% for the continuous daily group (RR: 0.38; CI95%: 0.25–0.59).
The HORIZON85 study with zoledronate demonstrated a 70% reduction of vertebral fractures (RR: 0.30; CI95%: 0.24–0.38) and a 41% reduction of hip fractures (RR: 0.59; CI95%: 0.42–0.83).
Eligible patients: Postmenopausal woman at high risk of fracture. Ibandronate has not been demonstrated to reduce the risk of hip fracture in prospective studies. Risendronate and zoledronate have also been approved in Spain for the treatment of osteoporosis in males and corticosteroid osteoporosis.
Safety aspects to take into account: Bisphosphonates are generally well tolerated, with few side effects. Changes in the upper gastrointestinal tract have been described, which can be minimised if the drug is taken correctly (on an empty stomach and sitting or standing) and using weekly or monthly doses. Prolonged use of bisphosphonates could be a risk factor for stress fractures in the femur and mandibular osteonecrosis after jaw surgery. These are serious processes but are rare. Prospective studies of quality are required to define the optimal length of treatment (see below: “For how long should patients receive treatment?”).
Intravenous infusion of zoledronate can cause fever, arthralgia and myalgia in the first 24–72h from the first infusion; the rate reduces in successive infusions.
DenosumabMechanism of action: Denosumab is a human monoclonal antibody which binds with high affinity to the RANK ligand, inhibiting the activation of its receptor (RANK) on the surface of the osteoclast precursors and on the osteoclasts. On blocking the RANKL/RANK mechanism, the formation, function and survival of the osteoclasts are inhibited, which results in reduced bone resorption.
Anti-fracture efficacy: In the pivotal trial86 a statistically significant reduction was observed of 68% in vertebral fractures, in non-vertebral fractures (RR: 20%: CI95%: 0.67–0.95) and hip fracture (RR: 40%; CI95%: 0.37–0.97). A subanalysis of the FREEDOM87 study has been published recently on patients at high risk of fracture where a significant reduction (of 62%) was observed in hip fracture risk in patients over 75. This reduction was still significant 12 months after starting treatment.
Eligible patients: Postmenopausal women and men88 at high fracture risk, especially if bisphosphonates cannot be used. In males with prostate cancer for treating bone loss associated with hormone suppression and high fracture risk.
Safety aspects to take into account: The principal safety aspects relate to the molecule's mechanism of action. On one hand, since RANKL is not expressed in osteoclasts alone, inhibition of the RANK receptor can manifest as an increased risk of cellulitis and infection. In their sections on the side effects of treatment with denosumab, several clinical trials have highlighted a greater incidence rate of infections, skin infections (cellulitis, including cases of erysipelas) and internal organ infections (pneumonia, diverticulitis, urinary tract infections), although with a small absolute number.89 For these same authors, these types of events have a heterogeneous aetiology, with no clear clinical pattern to suggest a relationship with the time or length of exposure to denosumab. And on the other hand, the molecule's possible long-term, hyper-suppressive effects on bone remodelling should be monitored. Recently the Spanish Agency for Medicines and Health Products issued an information note90 warning of the risk of maxillary osteonecrosis and hypocalcaemia after use of the drug, the latter especially in cases of kidney failure, and recommending that oral hygiene should be checked and invasive dental procedures avoided during treatment to prevent the former, and that pre-existing hypocalcaemia should be corrected, maintaining an adequate intake of calcium and vitamin D to prevent the latter. The incidence rate of maxillary osteonecrosis in the osteoporotic population treated with the drug is very low; with rates which vary between below 0.001% and 0.15% per year.91 In the STAND92 and DECIDE93 studies the safety profile is similar to that of bisphosphonates.
Anabolic drugsIn the purely anabolic drug group we currently only have 1.34 PTH or teriparatide, excluding 1.84 PTH, which has now been withdrawn from the market. Teriparatide is the first anabolic agent, a human parathyroid hormone (PTH) analogue, which acts to stimulate bone formation. It is the active N-terminal portion (amino acid sequence 1.34) of PTH of recombinant DNA origin.
Mechanism of action: Intermittent administration of teriparatide stimulates osteoblasts via multiple mechanisms. Its chief effect is to increase their number, differentiation and activity, while inhibiting their apoptosis. It increases bone quantity, quality and strength.
Anti-fracture efficacy: 1.34 PTH is effective in reducing vertebral and non-vertebral fractures, there are no specific studies designed to analyse its efficacy in reducing hip fractures. The pivotal or benchmark study was performed on postmenopausal women with at least one previous vertebral fracture, and found a reduction in the risk of subsequent vertebral fracture of 35% (RR: 0.35; CI95%: 0.22–0.55) and a reduction in non-vertebral fracture risk of 47% (RR: 0.47; CI95%: 0.25–0.88).94
Eligible patients: The drug is indicated for the treatment of postmenopausal women with a high fracture risk, which has been established by low BMD and/or the existence of prior osteoporotic fractures. Its datasheet states that it is indicated for the treatment of osteoporosis associated with sustained glucocorticoid therapy and for osteoporosis in males. Likewise, it can be indicated for patients who have been found to have an inadequate response to anti-resorptive treatment,95 and patients who are intolerant to other drugs used to manage the disease.
Although at a clinical level teriparatide has been demonstrated to improve distal radius fracture repair,96 further well-designed studies are necessary in order to recommend the drug for this purpose. Most of the studies in literature which show its beneficial effect on the development of fracture callus are experimental or case series,97 and this indication is not shown on its datasheet. Likewise, some studies suggest the use of teriparatide to promote repair of the atypical fractures which can occur in patients under sustained bisphosphonate treatment but at present, according to the latest recommendations of the American Society for Bone and Mineral Research,98 there is insufficient evidence to recommend it systematically. Use of the drug should be reserved for cases where repair has not been achieved with conservative treatment, after stopping treatment with bisphosphonates and maintaining an appropriate calcium and vitamin D balance.
Safety aspects to take into account: Teriparatide is a safe drug. Despite the fact that an increased incidence rate of osteosarcomas was found in preclinical experimental studies in ovariectomised Fisher rats (at doses very much higher than those used in humans and treated over 80% of the animals’ lives), there are no described cases of osteosarcoma associated with the drug in humans. The recommended posology is 20μg/day subcutaneously for 24 months, preferably given in the morning.99 It is contraindicated in patients with metabolic diseases other than osteoporosis, including hyperparathyroidism and Paget's disease, in patients who have received previous radiotherapy on the skeleton, who have unexplained raised levels of alkaline phosphatase, with pre-existing hypercalcaemia or severe renal failure. The most common side effects, which are generally mild, are dizziness, nausea, headaches, cramps and leg pain. It can cause a temporary increase in serum calcium levels.
Mixed action drugs: strontium ranelateStrontium ranelate (SR), an active, oral medicine for osteoporosis, has a dissociating effect on bone metabolism: on the one hand, reducing resorption, while on the other, increasing bone formation; this is why it is considered a dual or mixed action agent.100
Mechanism of action: In terms of mechanism of action, SR appears to stimulate the calcium-sensing receptor (CaSR) of osteoblasts, inducing activation of the signalling pathway of mitogenic protein-kinase thus promoting osteoblast cell proliferation and differentiation. In addition, it might also activate osteoprotegerin, reducing expression of the ligand bound to the receptor activator of nuclear factor kappa-B (RANK-L), the transmembrane receptor involved in the differentiation and maturation of osteoclasts, and thus reduce bone resorption.101
Anti-fracture efficacy: In its pivotal studies Spinal Osteoporosis Therapeutic Intervention (SOTI)100 and Treatment of Peripheral Osteoporosis (TROPOS),102 SR has been demonstrated to be capable of reducing the risk of vertebral and non-vertebral fractures, compared to a placebo.
Although the TROPOS study was not designed to evaluate this parameter, in women in the high-risk subgroup (aged over 74 with femoral neck BMD T-score −3 or less) the risk of suffering a hip fracture was reduced by 36%.
Eligible patients: At present it is considered a hospital diagnosis drug, which should only be used in patients with severe osteoporosis and at high risk of fracture and for whom no alternative treatment can be used, and the treatment decision should be taken by a doctor with experience in the management of this disease. This is because there are several safety issues which are outlined below.
Safety aspects to be considered in the use of the drug: The drug currently has very restricted use after three health alerts. The first, in 2007, warned of the possibility of DRESS syndrome, a very rare hypersensitivity reaction which appears 3–6 weeks after starting treatment, characterised by a fever, rash, eosinophilia and systemic involvement. The second, in 2012, was due to a risk of venous thromboembolism, based on the publication in France of a study identifying 199 serious adverse reactions with SR,103 of which half were venous thromboembolism (VTE). Its use was contraindicated in patients with current or previous thromboembolism (deep vein thrombosis and pulmonary embolism) and in temporarily or permanently immobile patients. The third, in 2014, after clinical trials with this drug, flagged up a greater risk of myocardial infarction in patients treated with SR compared to those treated with a placebo. The drug's current indications104 are for patients with severe osteoporosis and at high risk of fractures who cannot use alternative drugs because they are contraindicated or not tolerated, and who have no history of ischaemic heart disease, peripheral arterial disease or cerebrovascular disease. They should not be used in patients with uncontrolled arterial hypertension or who are permanently or temporarily immobile.
Which drugs are under development for the prevention and treatment of osteoporotic fractures?Cathepsin K inhibitors: odanacatibCathepsin K is a protease mainly expressed in the osteoclasts and responsible for the degradation of the bone matrix, 90% composed of type i collagen. A cathepsin K dysfunction causes pycnodysotosis, a rare, sclerosing autosomal recessive disease which is associated, amongst other clinical characteristics, with frequent fractures. Odanacatib's mechanism of action, in oral, weekly doses, is to selectively and reversibly inhibit cathepsin without affecting the number of osteoclasts and it is not stored in the bone long term. Patients who had been given odanacatib presented, 2 years after treatment, with a significant increase in BMD of 5.5% in the lumbar spine, 3.8% in the femoral neck and 3.2% in total hip compared to a placebo.105 This increase was maintained at 5 years, with 7.9% improvement in the lumbar spine and 5.8% in total hip106 with a good safety profile. BMD also increased in patients who had taken alendronate previously, and for at least 3 years, with results at 2 years,107 and it appears in preclinical studies that it does not affect bone repair.108
Sclerostin inhibitors: blosozumab and romosozumabSclerostin inhibits bone formation by its effect on the Wnt-beta-catenin pathway. Van Buchem's disease or generalised cortical hyperostosis and sclerosteosis are a result of a change in the SOST gene which produces it. In preclinical studies, the administration of anti-sclerostin antibodies was demonstrated to increase formation markers, BMD and bone strength.109 Blosozumab and romosozumab are different sclerostin-inhibiting human monoclonal antibodies which are being clinically evaluated (with regard to efficacy and safety) as possible anabolic agents for the treatment of osteoporosis. In this regard, blosozumab treatment (in both single and multiple doses) produced dose-dependent responses on sclerostin and on the formation and resorption markers, as well as significant increases in BMD in the spine and hip.110 Prior use of bisphosphonates did not appear to significantly alter the response, either on the bone formation markers or on the increase in BMD, it was well tolerated, and has a good safety profile. Antibodies to blosozumab were found in some patients, but with no pattern in relation to dose or route of administration. A study has also been published very recently which presents how romosozumab acts as a powerful anabolic agent, which can stimulate bone formation and reduce bone resorption, so as to rapidly increase BMD in the vertebral spine and the hip.111 Because the design of the study included alendronate and teriparatide as open-label comparators, romosozumab was demonstrated to achieve in 12 months greater increases than those achieved by the other two molecules. From a safety perspective, romosozumab did not present major adverse effects, apart from reactions at the injection site. 20% of the patients treated with this molecule presented antibodies, 3% with neutralizing activity.
Other drugsOther drugs are under development, such as chloride channel inhibitors, Src-kinase inhibitors, like saracatinib, anti-Dkk1 antibodies, calcium-sensing receptor antagonists and PTHrP, but they are still in the very early stages.
Is combined or sequential therapy possible?Combined therapyCombined therapy or the simultaneous use of two or more drugs is a treatment strategy which should be carefully evaluated because of the potential sum of adverse effects and the increased cost involved.
Many drug combinations have been studied: oestrogens with different bisphosphonates, oestrogens with teriparatide, alendronate with teriparatide and PTH 1.84, raloxifen with alendronate, raloxifen with teriparatide, and recently, denosumab with teriparatide. Most of these combinations achieve an improvement in BMD above that obtained with monotherapy, but this does not result in a reduction in fracture rate, apart from the use of oestrogens and teriparatide, which has been demonstrated to produce a substantial reduction in subsequent vertebral fractures.112 Some combinations might even be harmful, such as alendronate with PTH; the hypothesis is that alendronate could reduce the anabolic effects of this hormone.113,114
One combination which could be interesting for some patients at particularly high risk of fracture is that of denosumab and teriparatide, since in a recently published study, although there is no data on fracture reduction, both drugs were found to have an added effect on BMD of the lumbar spine, total hip and femoral neck.115
Sequential therapyThe fact that osteoporosis can require drug treatment for several years, combined with the side effects which occur with prolonged treatment with some drugs (such as bisphosphonates) and the limited therapy time of some drugs (such as teriparatide), makes it necessary to change or alternate drugs in many patients.
There are therapy sequences which appear clear, such as going from bisphosphonates to teriparatide and continuing with bisphosphonates after teriparatide. These are drugs which together, as mentioned above, do not present any advantages, but sequentially can complement one another, although it is true that prior treatment with bisphosphonates might in some way slow down the action of PTH.
There is much uncertainty about how long to maintain therapy with a particular drug, with the exception of teriparatide; its datasheet states that it should not be taken for longer than 24 months. In the case of bisphosphonates, considered standard first-line treatment for the disease, it appears reasonable to maintain therapy for 5 years after which, depending on the patient's fracture risk, it might be possible to give the treatment for longer, periodically reassessing the patient, to prescribe “drug holidays” or to stop therapy.116 There are studies on strontium ranelate which vouch for the efficacy of the drug after 8 years of treatment,117 although recent presentations regarding its cardiovascular safety might limit its use. With regard to denosumab, there are studies which refer to the efficacy of the drug with regard to a progressive increase in BMD in the lumbar spine, the hip and the radius distal third after 8 years of treatment.118
Therefore it seems reasonable to individualise treatment initially, according to each patient type and their fracture risk. Subsequently, a time has to be established for maintaining treatment, in accordance with the literature. If we need to change drug, it is preferable to use active ingredients with a different mechanism of action.
When should drug treatment be started?Drug treatment should be started in patients who:
- 1.
Have densitometry values in the femoral neck with a T-score of −2.5 or greater.
- 2.
Present with prior fragility fracture.
- 3.
Present a high risk of fracture according to the FRAX® tool. It is difficult to establish a threshold for starting drug treatment based on the results obtained using this tool. Traditionally, a high fracture risk has been considered as a risk of hip fracture of 3% or greater or a risk of major fracture of 20% or greater.39 Nonetheless, as mentioned above, its predictive fracture risk for the Spanish population is low. Each country should adjust their risk levels according to their characteristics, and in this regard we consider a recent review of the risk of a Spanish cohort119 of interest. It establishes mixed screening using FRAX® and BMD measurement, according to which patients with a risk considered high (≥7.5%, and medium (≥5% and <7.5%) would be candidates for BMD measurement, which if the diagnosis is confirmed, would indicate the need for drug treatment. This course of action would be much more cost-effective.
Once the decision has been made to start drug therapy, it is crucial to make the right choice. The following aspects should be taken into account when choosing a drug:
- 1.
Efficacy, this is understood as the capacity of a drug to produce a clinical action. In the case of osteoporosis, the clinical action that we have to assess is anti-fracture efficacy, and secondly, efficacy over its surrogate indicators (e.g., BMD or bone remodelling markers) which, although they are associated with the fracture variable, do not necessarily have a clear and complete relationship with it.
We know anti-fracture efficacy from the results of clinical trials. Although it would be desirable to have controlled trials comparing drugs with each other, with anti-fracture efficacy as the principal variable, to date most information from clinical trials is obtained by comparing the molecule to a placebo. These types of studies show anti-fracture efficacy as RR, relative measurement of the effect which indicates how many times the event (fracture) tends to take place in the group of patients exposed to the drug. We should understand this as the amount of risk which remains present despite the pharmacological measure, since an RR of the unit indicates that there is no relationship between the fracture and the medicinal action.
We know the level of vertebral, non-vertebral and hip anti-fracture efficacy according to the main pivotal clinical trials that have resulted in the marketing of the drugs which are currently available (Table 2).
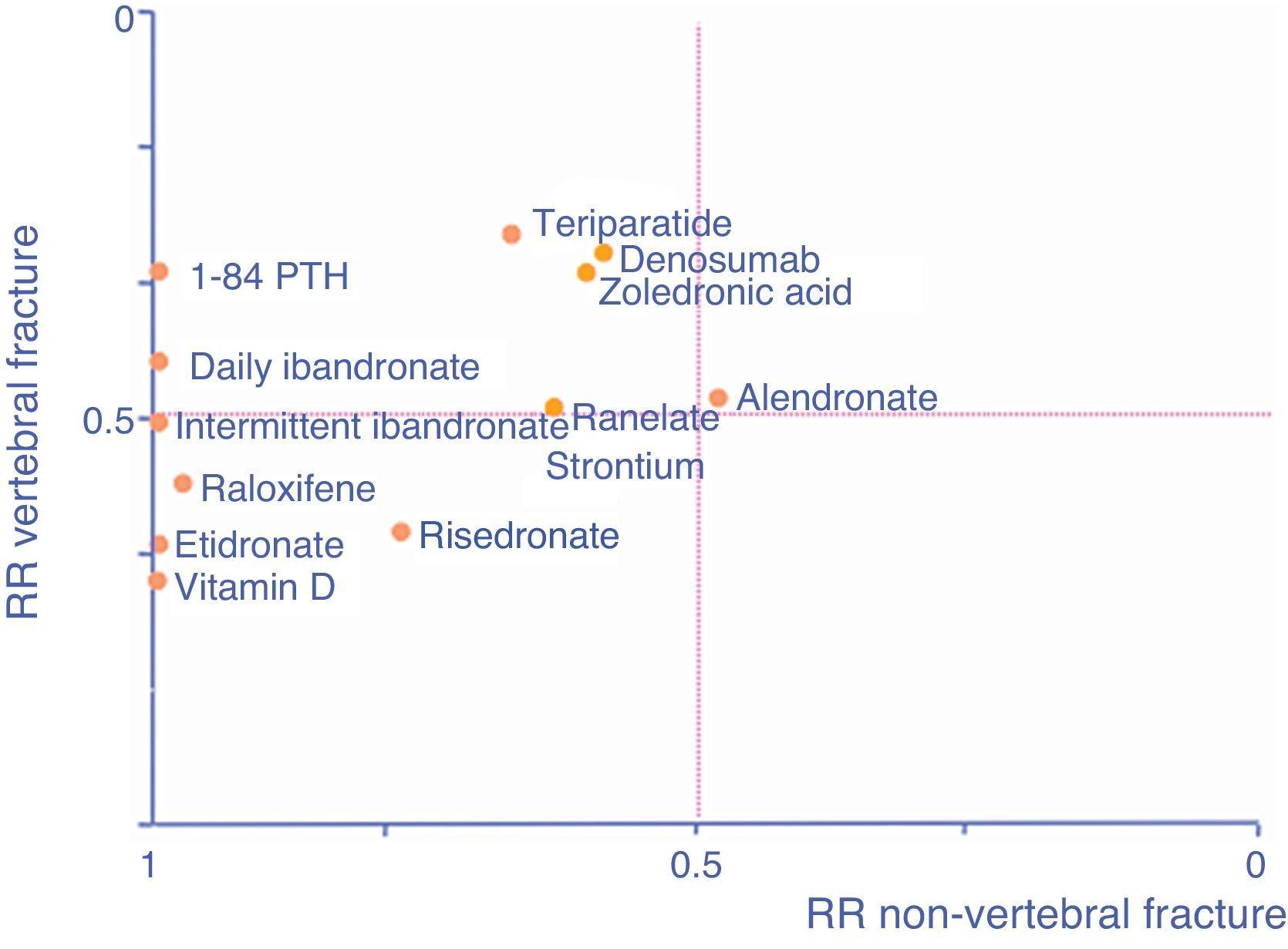
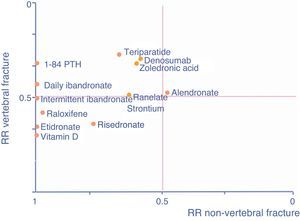
As an aid, adapted from Cranney and Guyatt,120 the graph in Fig. 1 shows the known efficacy of the molecules in the form of RR to help select a drug according to the particular fracture type we are seeking to prevent. The further a drug is to the right, the better its efficacy in reducing non-vertebral fractures, the higher it is, the greater its efficacy in reducing vertebral fractures.
- 2.
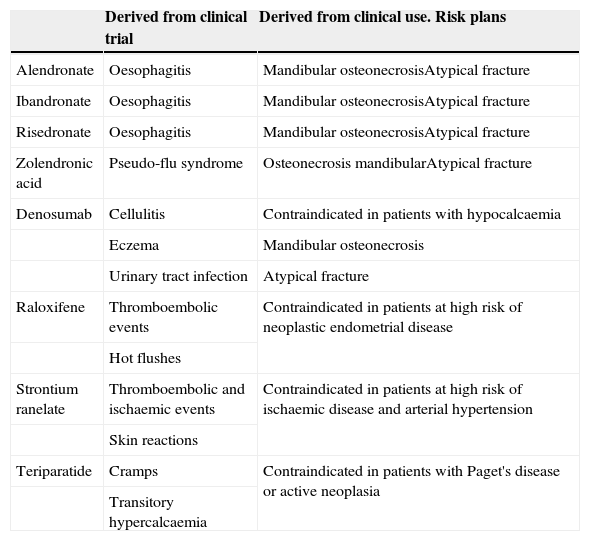
Safety, this is understood as the probability that a drug will cause unjustifiable adverse effects. It must be assumed that all drugs have the capacity to cause harm, and that the safety of a medicine is therefore a relative characteristic to be analysed in a particular clinical context. For example, chemotherapy drugs have side effects which are manageable within the framework of anti-cancer therapy, yet are unacceptable outside this context. Again, clinical trials will give us clear information on the expected toxicity of a drug, but we should not forget that the real information on the side effects of a molecule is provided to us, over time, by the drug surveillance records (Table 3).
Table 3.Principal side effects of the different drugs.
Derived from clinical trial Derived from clinical use. Risk plans Alendronate Oesophagitis Mandibular osteonecrosisAtypical fracture Ibandronate Oesophagitis Mandibular osteonecrosisAtypical fracture Risedronate Oesophagitis Mandibular osteonecrosisAtypical fracture Zolendronic acid Pseudo-flu syndrome Osteonecrosis mandibularAtypical fracture Denosumab Cellulitis Contraindicated in patients with hypocalcaemia Eczema Mandibular osteonecrosis Urinary tract infection Atypical fracture Raloxifene Thromboembolic events Contraindicated in patients at high risk of neoplastic endometrial disease Hot flushes Strontium ranelate Thromboembolic and ischaemic events Contraindicated in patients at high risk of ischaemic disease and arterial hypertension Skin reactions Teriparatide Cramps Contraindicated in patients with Paget's disease or active neoplasia Transitory hypercalcaemia - 3.
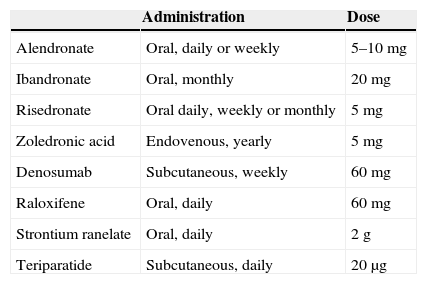
Adherence, this is understood as the extent to which a patient's behaviour corresponds to the health advice and recommendations that they have been given. There is evidence to suggest that posology which is convenient for the patient increases adherence and persistence with a treatment. The best posology is understood by the patient and adapts to their lifestyle. Therefore, good communication between the doctor and their patient will enable them to agree on the most appropriate medication to suit the patient's preferences in terms of posology and their absolute risk of fractures. Non-adherence not only results in therapeutic inefficacy, which is its major consequence, but also constitutes an unjustifiable health cost (Table 4).
Table 4.Approved posology of available treatments.
Administration Dose Alendronate Oral, daily or weekly 5–10mg Ibandronate Oral, monthly 20mg Risedronate Oral daily, weekly or monthly 5mg Zoledronic acid Endovenous, yearly 5mg Denosumab Subcutaneous, weekly 60mg Raloxifene Oral, daily 60mg Strontium ranelate Oral, daily 2g Teriparatide Subcutaneous, daily 20μg - 4.
Economic impact. Just as there are randomised clinical trials to learn the efficacy of a drug, there are probabilistic, mathematical models to determine whether the use of a drug is cost-effective. It is in this context that the health authorities should take decisions in relation to funding the different groups of patients. In general, we can conclude that treating patients at high risk of fracture is cost-effective, almost irrespective of the drug selected.121–123
We can take a logical approach and establish that treatment should be maintained as long as the patient is at risk of fracture. As mentioned above, there are no doubts as to the duration of treatment with drugs such as teriparatide, which is clearly set at 24 months. There is uncertainty with regard to the other drugs and this is sometimes difficult to resolve, especially for young patients who might require treatment over several years.
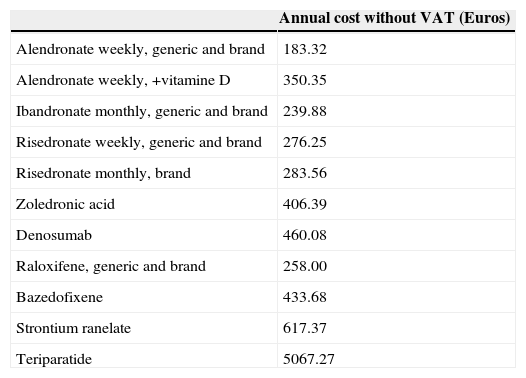
With regard to bisphosphonates, the appearance of a series of adverse effects such as atypical fractures98,124 and maxillary necrosis have raised alarm bells as to how appropriate it is to maintain treatment over a long period of time(Table 5).125
Annual cost of the different drugs.125
| Annual cost without VAT (Euros) | |
|---|---|
| Alendronate weekly, generic and brand | 183.32 |
| Alendronate weekly, +vitamine D | 350.35 |
| Ibandronate monthly, generic and brand | 239.88 |
| Risedronate weekly, generic and brand | 276.25 |
| Risedronate monthly, brand | 283.56 |
| Zoledronic acid | 406.39 |
| Denosumab | 460.08 |
| Raloxifene, generic and brand | 258.00 |
| Bazedofixene | 433.68 |
| Strontium ranelate | 617.37 |
| Teriparatide | 5067.27 |
Clinical trials denote that the efficacy of these drugs, based on their anti-fracture effect and increased BMD over 3 years of treatment, maintains a clearly favourable profile with no noteworthy adverse effects, and with drugs such as alendronate and zoledronate the beneficial residual effects are maintained after stopping the drug after it has been given over 3–5 years. It is considered that patients with a T-score below −2.5 and who have suffered a subsequent fracture could continue the treatment, and patients with a T score above −2.5 could stop treatment, within the concept of a “drug holiday”, but the subsequent risk of new fractures should always be reassessed.126 There are studies with strontium ranelate and denosumab which vouch for their efficacy and safety at 8 years,117,118 and although there have been isolated cases of atypical fractures with the latter, it appears in extension studies that this tendency has not been confirmed with bisphosphonates.
We can consider that treatment should be individualised, using sequential therapy if it is necessary to maintain therapy because there is a risk of fracture, using drugs of a different mechanism of action, as mentioned above.
The concept of treatment failureWhen a patient is receiving drug treatment, it should be taken into account that these drugs reduce the risk of fracture, but do not eliminate it.127 In order to assess whether a drug is having no effect, we should consider whether adherence is good, whether levels of calcium and vitamin D are adequate and whether the duration of the treatment has been sufficient (a bone remodelling cycle varies in duration from 3 to 6 months, at least 1 year of treatment is required to assess failure). It should also be considered that with these conditions, only a small number of patients can be said not to respond to treatment.127 There can be many causes for a lack of response, although most cases might be associated with very damaged previous condition of the bone, very low levels of vitamin D, prior fractures resulting in very fragile bone structure or a persistent risk of falling.128 We measured failure based on 3 considerations: the appearance of fractures during treatment, no favourable evolution of BMD and no response of bone markers. The appearance of a fracture does not necessarily imply a failure of treatment, as osteoporosis per se increases this risk and drugs can reduce it by up to 50%, therefore the probability of fracture persists. With regard to BMD, a loss of 4–5% compared to previous measurements is considered inadequate.129 Drugs reduce resorption markers by 50–60% and formation markers by 30%. Their limitations are that they vary according to sex, age, race, the time that the sample was taken, and whether the patient had an empty stomach, therefore there is high intra-individual variability (20–30%).129 Three scenarios can be proposed to define what might be considered therapeutic failure127: when there are two or more fractures, with one fracture without reduction of bone markers and/or significant decrease in BMD, and a situation where there is no reduction of markers and significant decrease in BMD.
How can male osteoporosis and secondary glucocorticoid-induced osteoporosis be diagnosed and treated?Male osteoporosis has distinctive characteristics compared to postmenopausal osteoporosis: it is less common but has higher rates of morbidity and mortality, it is usually secondary (hypogonadism, corticotherapy, alcoholism, androgen deprivation treatments and myeloma being its most common causes),130 and it has a lower fracture rate, especially hip fractures, as men have a shorter life expectancy.131 The risk of fracture, indication for densitometry and diagnostic cut-off points are established using the same criteria as for women.53 The drugs which have been approved in Spain for treatment are risedronate, zoledronate, teriparatide, denosumab and strontium ranelate. Denosumab is also indicated for the treatment of bone loss associated with hormone suppression in men with prostate cancer with a high risk of fracture. Therapy with testosterone is not recommended as standard treatment due to its associated side effects.132
Glucocorticoid-induced osteoporosis is the most common cause of secondary osteoporosis. There is evidence that daily doses of 5mg or more of prednisone or an equivalent dose, with a duration of 3 months or more, increase the risk of fracture; this is considered the pharmacological intervention threshold in most guidelines.133 Diagnosis by means of clinical history is straightforward. It should be borne in mind that the tools to assess fracture risk usually underestimate this risk in corticoid-induced osteoporosis, especially when high doses are used. Measurement of bone mass by DXA is essential before starting treatment, although the serious changes in bone micro-architecture caused by corticosteroids can cause fractures with normal bone mass values. This should be considered when forming therapeutic plans. Fractures can occur early, closely associated with rapid bone mass loss during the first year of treatment with steroids, and therefore prophylaxis should start early. Treatments which have been approved in Spain for this purpose are alendronate, risedronate and zoledronate. Teriparatide can be considered for patients at high risk of fracture.
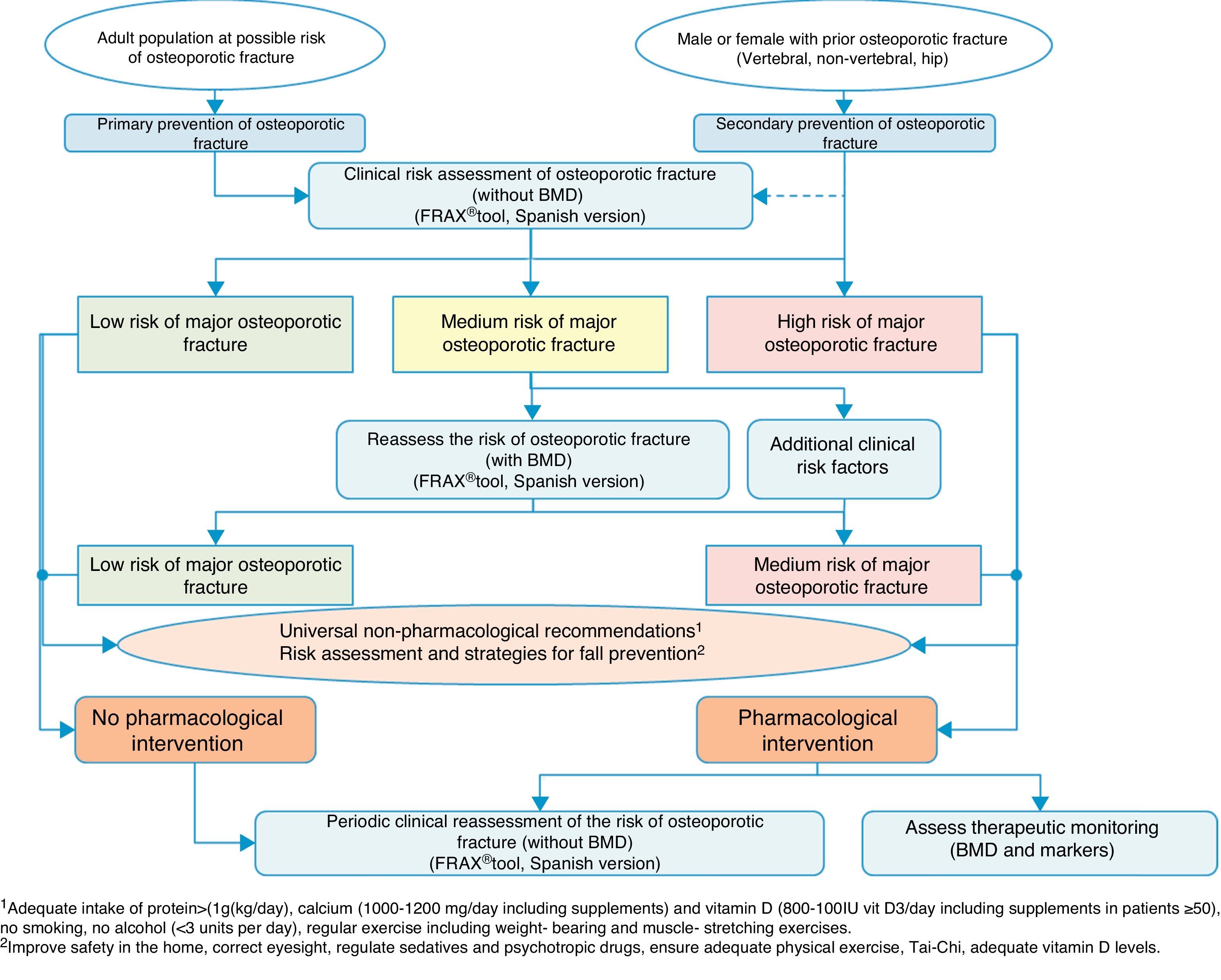
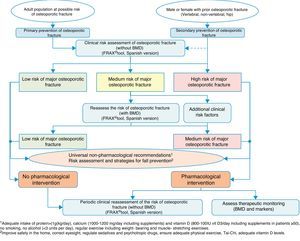
Clinical decision algorithmsAlgorithm for identification and therapeutic indicationFig. 2 shows the system for identifying patients who are candidates for pharmacological intervention, based on two basic prevention levels: primary prevention, measures to be taken for patients who have not yet suffered a fracture and secondary prevention, the approach for patients who have already suffered an osteoporotic fracture.
From the perspective of our speciality, we believe it vitally important to address secondary prevention, since, on the one hand, we deal with these types of fractures ourselves, and we are often the only people to do so, and on the other, there are worryingly few references in literature on management after fracture,134–137 despite the fact that in practice almost all national and international guidelines recommend establishing preventive treatment against subsequent events after a fragility fracture.138
This treatment recommendation is based on the fact that the increased risk of subsequent fractures in patients who have already suffered a fracture is known.139 This is easy to check using fracture rate studies, but it is also a clinical situation which is very familiar to traumatologists; it suffices to review the patient's clinical history to find that it is very likely that there will have been other fractures prior to the one being treated.
For this reason, in the algorithm we consider these patients to be at high risk of major osteoporotic fracture, and pharmacological intervention is almost compulsory, without the need for further studies to confirm bone fragility. Obviously, it will depend on the patient's life expectancy, quality of life, and their clinical determinants, however the large majority of patients will be candidates after a fracture for measures to prevent further fractures. Furthermore, we assume that universally recognised non-drug treatments should also apply, and as an essential element, a risk assessment of falls and the implementation of measures to reduce them.
We consider that primary prevention is possibly less applicable in daily practice in our area of work; nonetheless, we believe that the FRAX® tool can be very useful in drawing up a risk map for the patient, which is done initially without using densitometry to measure bone mass.
As mentioned above, this tool provides an estimate of absolute risk of fracture of the hip and the principle fractures (vertebral, hip, humerus and wrist) at 10 years. We consider the cut-off points established in a Spanish cohort119 of interest. In this cohort high risk was determined in patients with a measurement result ≥7.5%, and medium risk was determined in patients with a measurement result ≥5% and <7.5%. With this data, we can select the patients for whom measurement of BMD is most cost-effective and reassess the risk whether or not they will suffer a major osteoporotic fracture, and take the consequent action.
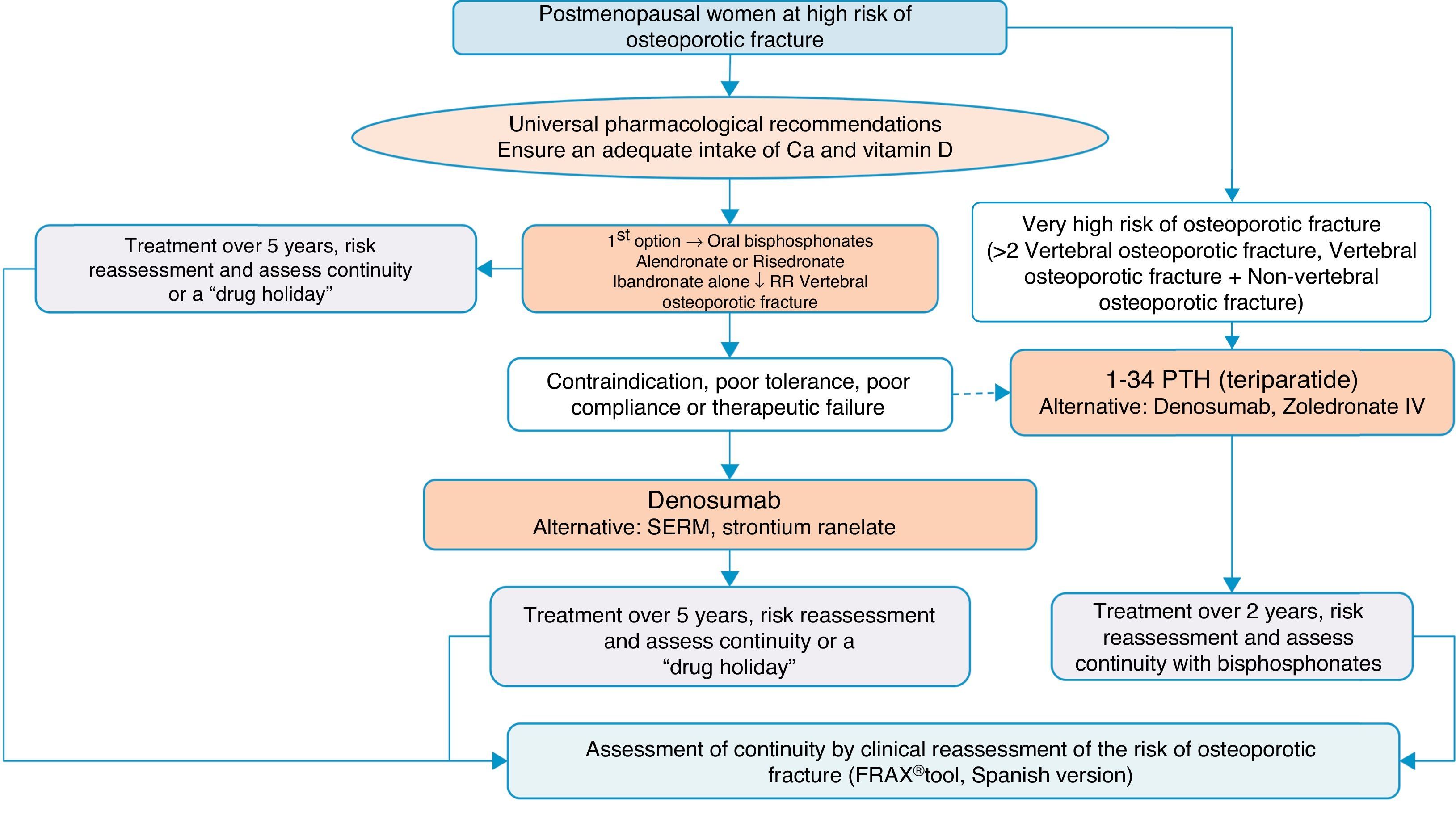
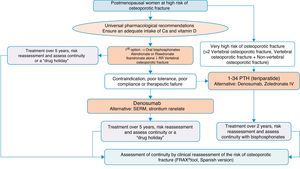
Algorithm for the treatment of postmenopausal women at high risk of fractureThis is one of the most common clinical situations, and the treatment algorithm is shown in Fig. 3. As a basic premise, before initiating therapy, we have to ensure compliance with universal non-pharmacological recommendations, and ensure an adequate intake of calcium and vitamin D, a daily 1000–1200mg of the former and a daily 800IU of the latter.
We recommend oral bisphosphonates as a first treatment option, with alendronate and risedronate as the preferred option due to their efficacy in vertebral, non-vertebral and hip fracture reduction. Selectively, if we are interested in reducing vertebral fractures, the first option could be ibandronate. In the event of contraindication, poor oral tolerance, poor compliance or a lack of therapeutic efficacy, we suggest denosumab as the preferred option. It is a monoclonal antibody which also has recognised efficacy in vertebral, non-vertebral and hip fractures. Alternatively, raloxifen could be used, a selective oestrogen receptor modulator, for which there is only recognised evidence in the reduction of vertebral fractures, and strontium ranelate, although with a very restricted indication, as mentioned above.
On the other hand, in patients at particular risk, considered in the algorithm as at “very high risk of osteoporotic fracture”, whom we can define as having suffered more than two vertebral fractures or one vertebral and another non-vertebral fracture, bone-forming treatment with teriparatide could be a good option for 2 years, for subsequent maintenance with an anti-resorptive drug. As an alternative to this treatment, we consider a bisphosphonate more powerful: zoledronate, given parenterally once a year, or denosumab, injected subcutaneously once a week.
The summary could be clear: correct indication and correct follow-up of the patient and their fracture risk; treatment regimes might be varied to maintain anti-fracture cover.
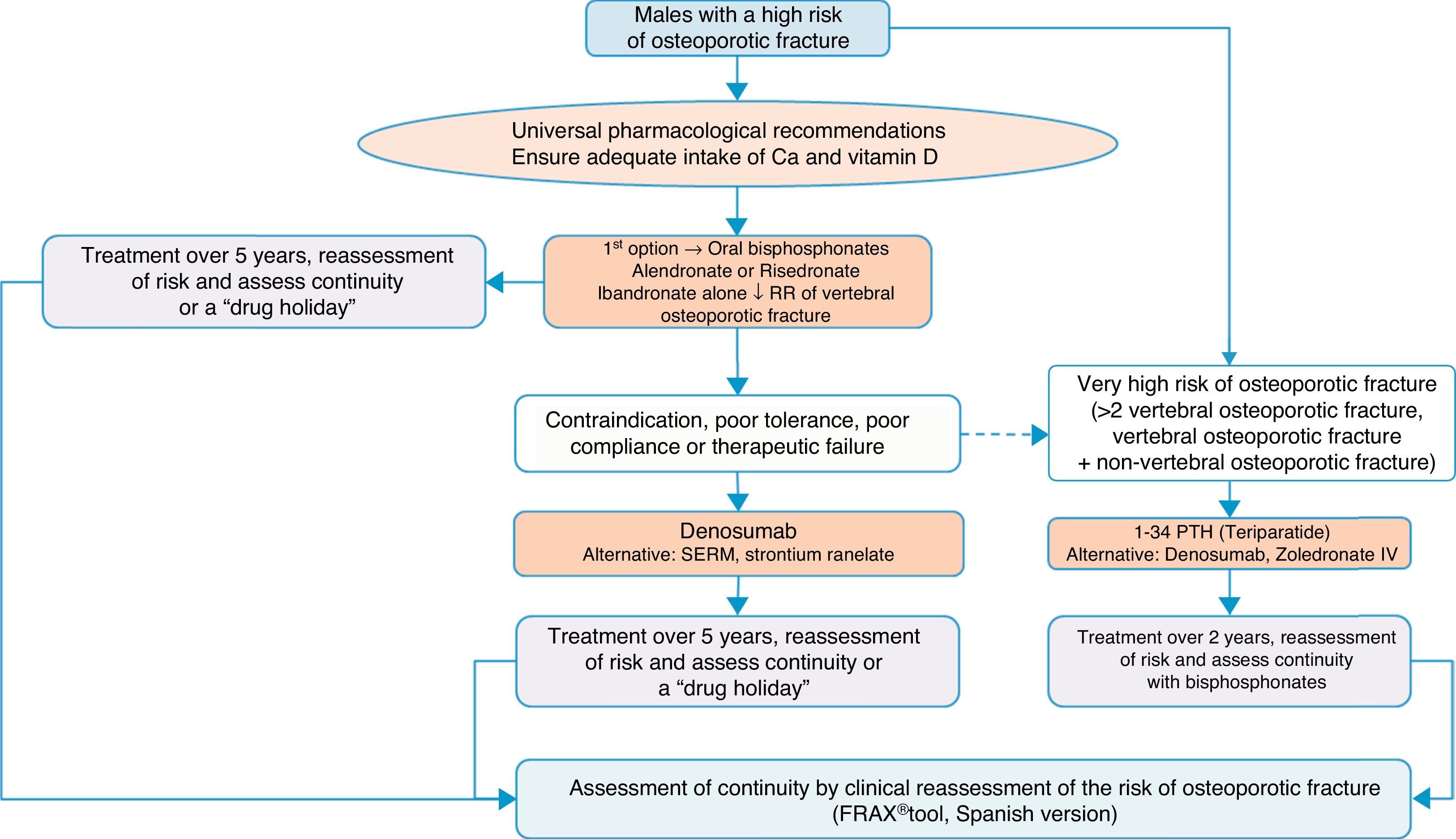
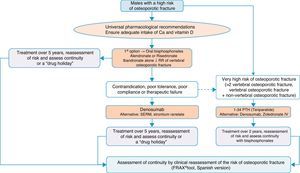
Algorithm for the treatment of males with a high fracture riskThe drugs approved for the treatment of males with a high fracture risk in Spain are risedronate, zoledronate, teriparatide, strontium ranelate, and recently, denosumab. Alendronate is approved in the United States.
Based on this availability of treatment, and as with postmenopausal osteoporosis, in our algorithm in Fig. 4 we again place bisphosphonates as the first treatment option, in this case with risedronate as the preferred option. And, in the event of contraindication, poor oral tolerance, poor compliance or adherence or therapeutic failure, we consider weekly denosumab or annual zoledronate as options. In parallel, if the patient is at particular risk, under the aforementioned definition, our preferred option is treatment with teriparatide for 2 years.
We consider osteoporosis in males of particular interest, as it usually excites less interest in the treatment and prevention of subsequent fractures than in women, and has significant comparative undertreatment rates,140 however, it undoubtedly causes fractures which, although of a lower absolute number, have a greater impact on morbidity and mortality.
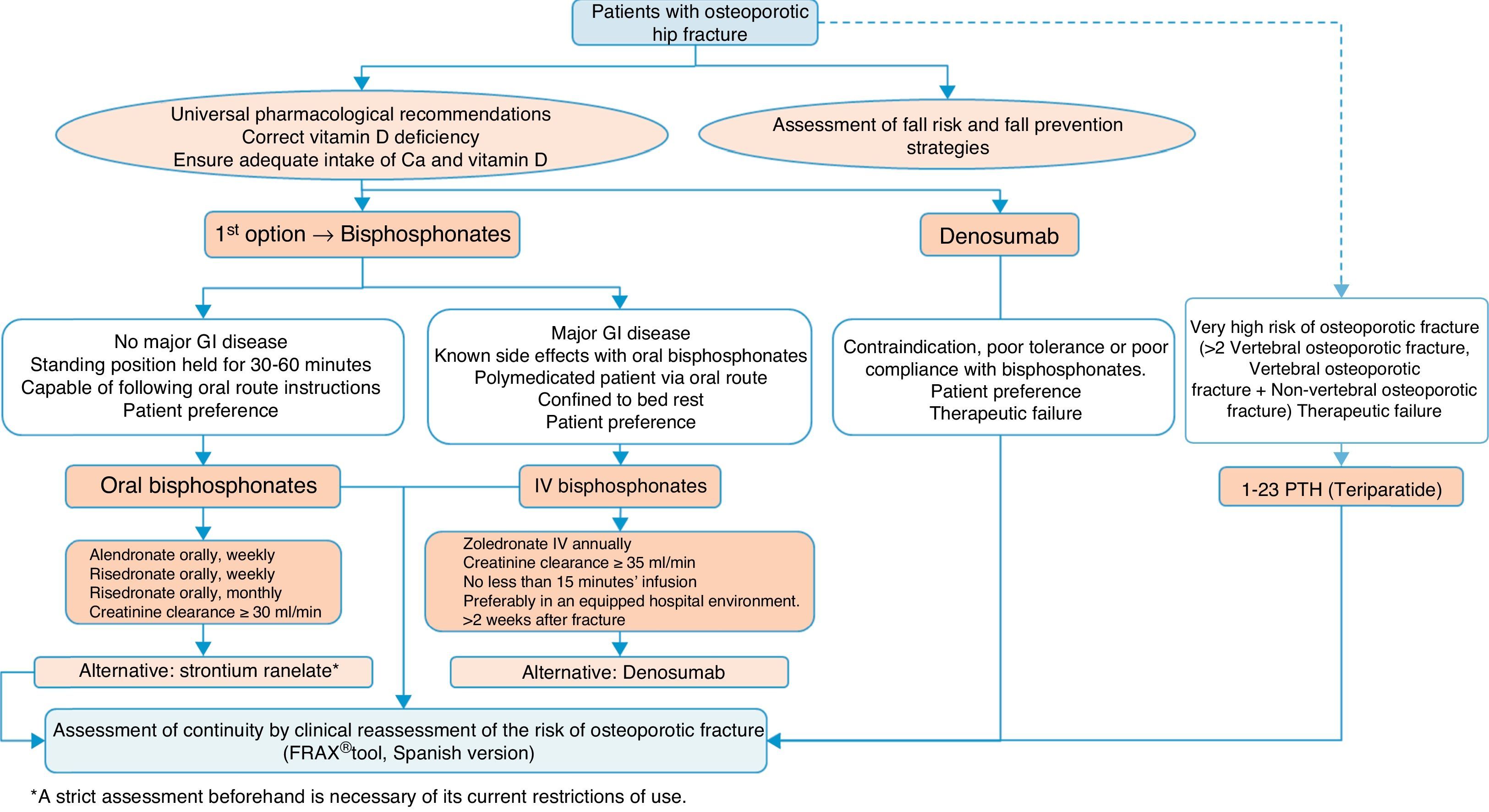
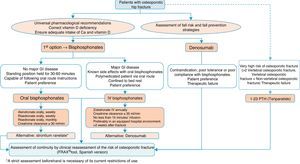
Algorithm for the treatment of hip fractureThe preferred options shown in Fig. 5 are drugs of proven efficacy in the reduction of hip fractures; these are bisphosphonates, except ibandronate and denosumab. Of the former, the indicated drugs are alendronate and risedronate, except in the case of major gastrointestinal disease, known side effects with oral bisphosphonates and polymedicated patients. Bed rest and patient preference are also determinants, in which case the most suitable option would be once-yearly intravenous zoledronate, which should be given slowly, in hospital and always as indicated on the datasheet, at least 2 weeks after the fracture has occurred so as not to interfere with the repair process. Denosumab is an alternative to bisphosphonates, especially in situations where they are poorly tolerated, there is poor pharmacological adherence or prior therapeutic failure.
We suggest teriparatide in patients at particular risk. It has no recognised anti-fracture efficacy in the hip, although it does have proven pharmacological effect at this level,141 since in patients who have suffered a hip fracture along with a history of another fragility fracture, the risk of a subsequent fracture is very significant, and therefore it could be a valid option.
Level of evidenceLevel of evidence V.
Ethical responsibilitiesProtection of humans and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their centre of work regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestI.E.F. has participated as a speaker at scientific events of the companies Lilly, Amgen and MSD. He has been a researcher in clinical trials sponsored by Amgen.
J.R.C.R. has participated as principal investigator and/or collaborator in clinical trials sponsored by the companies Amgen and Lilly, and has been a speaker at scientific events of the companies Amgen, Lilly, MSD, Pfizer and Servier.
R.L.G. has participated as principal investigator in studies sponsored by Lilly and Amgen. He is a consultant for MSD.
L.R.R. has participated as principal investigator for trials sponsored by Amgen. He has been a speaker at scientific events of the companies Lilly, Amgen and MSD. He is a consultant for Amgen.
The other authors have no conflicts of interests to declare.
Please cite this article as: Etxebarria-Foronda I, Caeiro-Rey JR, Larrainzar-Garijo R, Vaquero-Cervino E, Roca-Ruiz L, Mesa-Ramos M, et al. Guía SECOT-GEIOS en osteoporosis y fractura por fragilidad. Actualización. Rev Esp Cir Ortop Traumatol. 2015;59:373–393.