Vertebroplasty has been shown to be effective for improving quality of life and pain of osteoporotic vertebral fractures (OVF) without neurological deficit and not susceptible to conservative treatment. It is advisable to perform them on recent fractures with 50% crush and without the involvement of the canal, although there are no standard recommendations. In some cases these limits are exceeded. We analyse the outcomes of percutaneous vertebroplasty (PVP) in OVF with relative/limit indication.
Materials and methodsRetrospective analysis of 88 patients (126 fractures) who underwent surgery by PVP; 95/126 were osteoporotic fractures. Thirty-four cases (35%) were included in the relative indication group, with at least one of the following: canal involvement, >50% collapse, and >12 months of evolution of the fracture. The rest of the cases were included in the standard indication group. We performed clinical-radiological follow-up, collected intraoperative data on techniques and complications, occurrence of leaks, postoperative clinical improvement (according to VAS), new adjacent fractures, and satisfaction.
ResultsMost fractures were between D11-L2 (66%) with 6–8 months follow-up. No significant differences were observed regarding clinical improvement in either group. A higher percentage of leaks were detected in the relative indication group, 44% in comparison to 29.5% in the standard indication group, without statistical significance. All leakages were asymptomatic. There were 3 new OVF after PVP in the relative indication group and 4 in the standard group, without statistically significant differences.
Discussion and ConclusionsThe use of cement in OVF with relative indication led to the same clinical benefit in our sample as those with standard indication. A higher number of leakages occurred in the relative indication group with no clinical consequences or adjacent fractures.
La cementación vertebral ha demostrado eficacia en mejorar la calidad de vida y el dolor en fracturas vertebrales osteoporóticas (OVF) sin déficit neurológico no susceptibles de tratamiento conservador. Se aconseja emplearla en fracturas recientes, con aplastamiento <50% y sin ocupación de canal, aunque no existen recomendaciones estrictas. En la práctica clínica encontramos casos en los que estos límites se rebasan. Analizamos los resultados de la vertebroplastia percutánea (VPP) en OVF con indicación relativa/límite.
Material y métodosAnálisis retrospectivo de 88 pacientes y 126 fracturas, intervenidos mediante VPP. Del total de fracturas, 95 son osteoporóticas; 34 casos (35%) forman el grupo de indicación límite, con al menos uno de los siguientes: ocupación de canal, hundimiento >50% y >12 meses de evolución de la fractura. El resto constituye el grupo de indicación estándar. Realizamos seguimiento clínico-radiológico, recogimos datos intraquirúrgicos de técnica y complicaciones, fugas, mejoría clínica posquirúrgica (según EVA), nuevas fracturas adyacentes y satisfacción.
ResultadosLa mayoría fueron fracturas entre D11 y L2 (66%), con seguimiento entre 6 y 8 meses. No hubo diferencias significativas en la mejoría clínica en ambos grupos. Se produjo mayor porcentaje de fugas en el grupo de indicación límite (44% frente al 29.5% del grupo de indicación estándar), sin significación estadística. Todas las fugas fueron asintomáticas. Se produjeron 3 nuevas OVF tras la VPP en el grupo límite por 4 en el grupo estándar, sin diferencias estadísticamente significativas.
Discusión y conclusionesLa cementación en OVF sintomáticas con indicación límite dieron lugar al mismo beneficio clínico en nuestra muestra que aquellas con indicación estándar. Observamos más fugas en el grupo límite, sin repercusión clínica ni más fracturas adyacentes.
Percutaneous vertebroplasty (PVP) consists of the injection of cement (polymethylmethacrylate or PMMA) into the fractured vertebral body. This technique was initially developed for the treatment of aggressive vertebral angiomas. It was described by Galibert and Deramond in France in 1987.1,2 Since then, the indications for this procedure have been extended to other vertebral entities, including osteoporotic compression fractures and vertebral metastases.3,4
After more than 2 decades of frequent practice, there are currently no strict indications as to the maximum time after fracture and the severity of crush for indication of this surgical technique. Different authors and experts advise vertebroplasty in recent fractures, with less than 50% crush and without occupation of the medullary canal,5,6 due to the greater risk of cement leaks. However, the good results in terms of pain control and the absence of clinical repercussions of possible complications such as cement leaks6–8 have increased confidence in practicing these techniques, even in more complex fractures. In addition, the possible complications of vertebral instrumentation surgery in osteoporotic and elderly patients also tip the scales, for doctors as well as for patients and relatives, towards less aggressive surgical techniques such as vertebroplasty. We should also not forget that these are procedures with lower financial cost, surgery time and length of hospital stay than vertebral instrumentation surgery.
In our daily clinical practice we find cases in which these theoretical limits are exceeded due to different circumstances, such as the patient’s comorbidities, they and their relatives refusing aggressive surgery, delay and saturation on surgical waiting lists in a public centre such as ours, as well as specific situations regarding the availability of the patient or organisational reasons of the health institutions.
With this study our aim was to analyse and compare the clinical and radiological results of PVP in patients with osteoporotic vertebral fracture with relative indication or, as we have termed it, relative indication, i.e., outwith the usual indication.
Material and methodsWe performed a retrospective observational analysis of a cohort of 88 patients and 126 vertebral fractures, who consecutively underwent PVP in our centre. All these patients were extracted from the database of prospective follow-up of patients operated in our Spinal Surgery Unit.
All patients undergo the same surgical technique performed by the same unit team. The patients sign and expressly authorise the intervention by informed consent. A bilateral transpedicular PVP is performed in the straight prone position, under sedation and local anaesthesia of the puncture site, guided by fluoroscopy, with an approach through the corresponding working cannulas up to the union of the anterior third with the middle third of the vertebral body. The polymethylmethacrylate is then mixed with opacifying agents and injected into the vertebra in its mid-anterior third, under scopic visualisation in gradual increments of .5ml. During the introduction of the cement, the procedure is halted when the posterior quarter of the vertebral body is reached or if there is leakage. When the surgery is over, the patient remains in an observation room for 2.3h, and is allowed to start sitting and walking from the 4th hour following the intervention. We monitor all patients radiologically.
Of the 126, 95 were osteoporotic fractures. These are patients with bone fragility fractures in whom other causes have been excluded: different bone metabolism impairments, drug use or neoplasms. Fractures that developed osteonecrosis (Kümmel disease) are also excluded from this selection, since vertebral osteoporotic fractures without necrosis are considered a differentiated entity, with an indication for vertebroplasty that currently allows nuances, which is not the aim of this study.9,10 T-score values in the patients’ bone densitometry were not gathered.
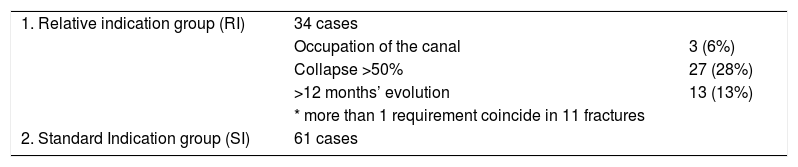
Of the total, 34 cases (35%) were included in the relative indication group (RI), with at least one of the following: occupation of the canal, collapse greater than 50% and more than 12 months fracture evolution. More than one requirement coincided in 11 fractures. The rest (61 fractures) were included in the standard indication group (SI). This distribution is shown in Table 1.
Patient distribution.
| 1. Relative indication group (RI) | 34 cases | |
| Occupation of the canal | 3 (6%) | |
| Collapse >50% | 27 (28%) | |
| >12 months’ evolution | 13 (13%) | |
| * more than 1 requirement coincide in 11 fractures | ||
| 2. Standard Indication group (SI) | 61 cases |
OVF: osteoporotic vertebral fractures.
Of the total OVF (95), 61 were included in the SI group and 34 in the RI group.
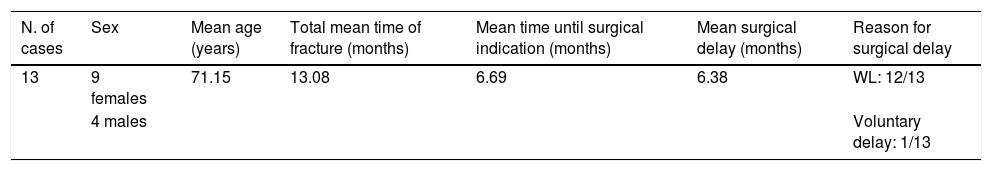
Thirteen cases with a surgical delay of more than 12 months were collected. We wanted to analyse the reasons for this striking therapeutic delay. We detail the data of these cases in Table 2. It can be observed in this subgroup that the time after the facture in which surgery was indicated was an average of 6.7 months, but the mean surgical delay in these cases was 6.36 months from their inclusion, greater than that of the rest of the patients in the study. The reasons to explain this delay are mostly delay on the surgical waiting list (12 cases), while in the other case it was attributable to the patient’s refusal to be operated from the outset: they asked to delay surgery until they finally agreed to it.
Group of more than 12 months’ fracture evolution.
| N. of cases | Sex | Mean age (years) | Total mean time of fracture (months) | Mean time until surgical indication (months) | Mean surgical delay (months) | Reason for surgical delay |
|---|---|---|---|---|---|---|
| 13 | 9 females | 71.15 | 13.08 | 6.69 | 6.38 | WL: 12/13 |
| 4 males | Voluntary delay: 1/13 |
Indication times, surgery times and reason for delay.
WL: surgical waiting list.
The patients’ affiliation data were collected: age, sex, concomitant disease, previous osteoporotic fractures. The following data were collected in relation to the fractures, after assessing the various imaging tests (x-rays, magnetic resonance imaging [MRI], computed tomography or bone scintigraphy): level of the fractured vertebra, time of fracture evolution, type of osteoporotic fracture (according to the classification by Genant et al.11); percentage of vertebral crush; presence of medullary canal occupation; degrees of local kyphosis (measured between the upper and lower plates of each fractured vertebra). The patient was considered to have canal occupation when the computed tomography or MRI scan showed any degree of retropulsion of the posterior wall or fragments towards the medullary canal. All the patients had signs of lack of consolidation when surgery was indicated on imaging tests: bone scan uptake or presence of oedema on MRI, or both. This information was collected at the time the surgical indication was established and the patient was included on the waiting list for the procedure.
With regard to the surgical procedure, the following data were collected: surgery time, volume of cement injected in cubic centimetres (c.c.), presence of intra-operative incidences, intra-or post-operative complications, cement leaks detected by intra-operative fluoroscopy and presence of other complications.
We undertook clinical and radiological follow-up, with immediate postoperative measurement and another at the end of follow-up (normally after 6 months). Follow-up time was collected. Pain was quantified using the Visual Analogue Scale (VAS). The analgesics used by the patients were not collected. Intensity of preoperative pain, immediate postoperative pain, pain at the end of follow-up (according to the VAS scale); improvement in pain (grouping the results into zero improvement (0 points), mild improvement (1–3 points), moderate (4–6 points), major improvement (7–10 points). Similarly, the onset of new fractures, the presence on x-rays of leaks, the degree of kyphosis and satisfaction with the procedure were collected.
The data were analysed with IBM SPSS 20.0.0. The results were compared between the IS group and the RI group, taking as the null hypothesis that there were no differences between the two groups. Frequency parameters and proportion in each variable were described, and chi-squared tests to compare proportions and Pearson’s correlation to compare quantitative and qualitative variables. Statistically significant values were defined when p<.05. The graphs were prepared using IBM SPSS 20.0.0 and Microsoft Excel 2011.
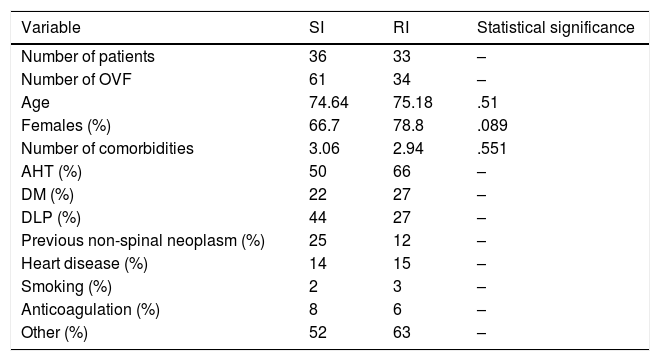
ResultsWith regard to affiliation data and concomitant diseases, both groups are comparable, as we can see in Table 3. There were no statistically significant differences in terms of age, sex or concomitant diseases.
Patients’ affiliation data.
| Variable | SI | RI | Statistical significance |
|---|---|---|---|
| Number of patients | 36 | 33 | – |
| Number of OVF | 61 | 34 | – |
| Age | 74.64 | 75.18 | .51 |
| Females (%) | 66.7 | 78.8 | .089 |
| Number of comorbidities | 3.06 | 2.94 | .551 |
| AHT (%) | 50 | 66 | – |
| DM (%) | 22 | 27 | – |
| DLP (%) | 44 | 27 | – |
| Previous non-spinal neoplasm (%) | 25 | 12 | – |
| Heart disease (%) | 14 | 15 | – |
| Smoking (%) | 2 | 3 | – |
| Anticoagulation (%) | 8 | 6 | – |
| Other (%) | 52 | 63 | – |
DLP: dyslipidaemia; DM: diabetes mellitus; OVF: osteoporotic vertebra fracture; AHT: arterial hypertension.
Two intra-operative incidences were gathered, both in the SI group. In one patient it was impossible to cement due to sclerosis in one of the pedicles; in another patient the procedure had to be interrupted due to insufficient anaesthesia. There were no intra-operative complications affecting the patient’s health.
In 5 patients (all in the SI group) the patient’ admission was prolonged for more than one day. In all cases it was due to diseases not related to the procedure, there were concomitant reasons other than the OVF during one hospital admission for a different reason.
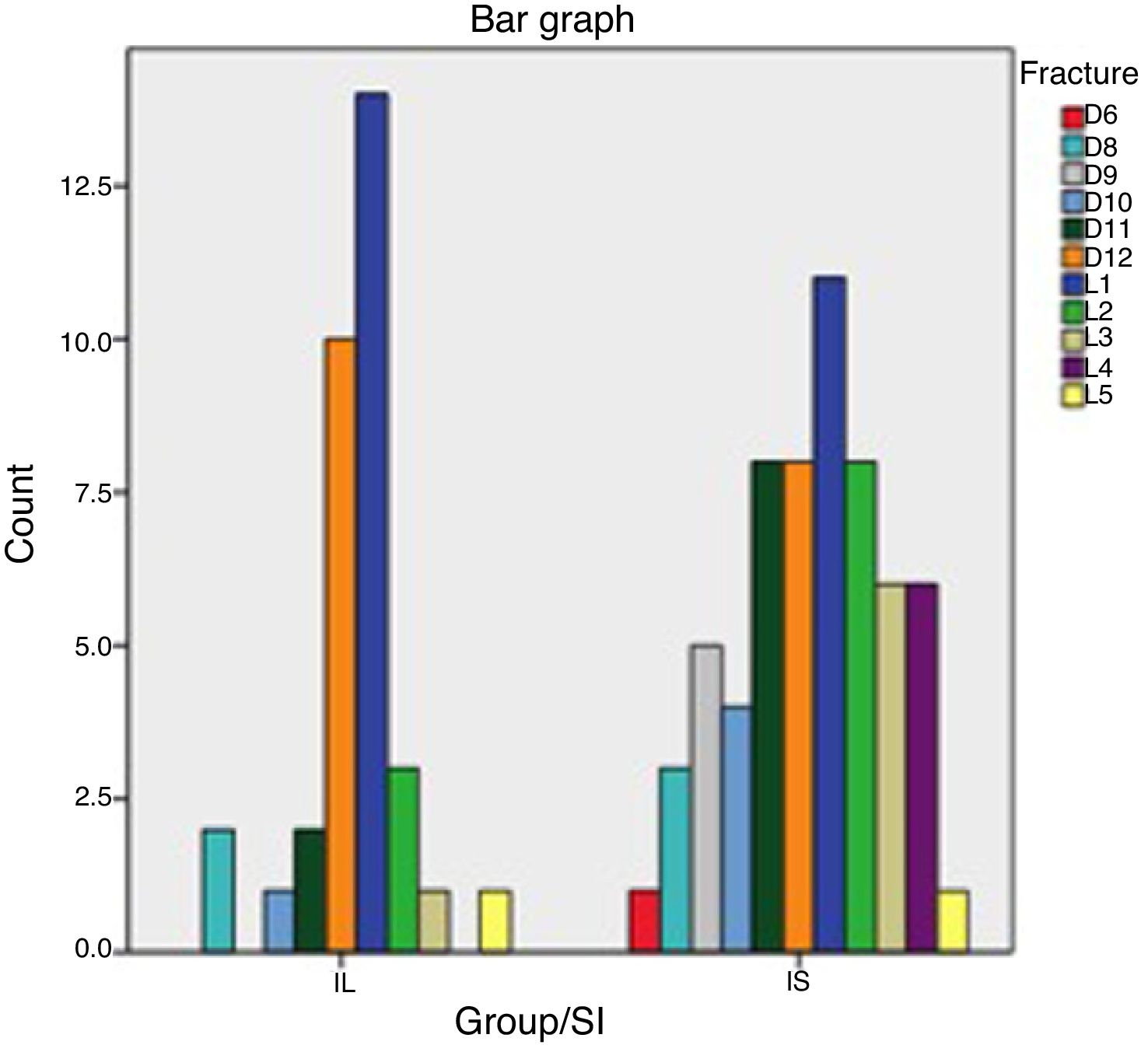
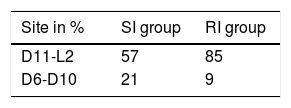
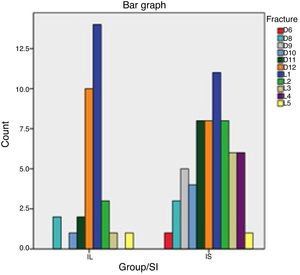
The following data were collected in relation to fractures: with respect to the level of the fractured vertebra, in the RI group 85% of the fractures occurred between levels D11 and L2. In the SI group this percentage was 57%, with more PVP in the dorsal area D6–D10 than in the RI group (21% compared to 9%), with statistical significance of p=.059. This distribution can be seen in Table 4 and Fig. 1.
The most frequent type of osteoporotic fracture (according to the classification by Genant et al.11) was wedging morphology in both groups (59% and 65%), followed by biconcave (28% and 20%) and crush type (13% y 25%) morphology. There were no statistical differences in this distribution (p=.154).
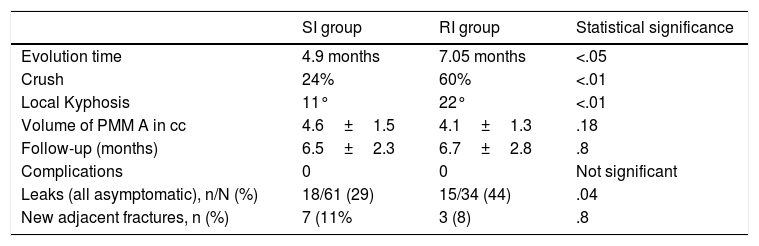
The fracture evolution time until surgery was 4.97±3.5 months on average in the SI group and 7.05±8.9 in the RI group. This difference was statistically significant. In the RI group 13 fractures were included with more than 12 months’ evolution (38%).
As for the mean percentage of vertebral crush, there were also statistically significant differences. In the SI group this was 24.4%±13.7% whereas in the RI group it was 60.62%±17.3%. In the RI group we observed: 27 vertebrae out of the 34 (79.4%) with more than 50% crush.
The presence of medullary canal occupation was observed in 6 cases out of the 34 of the RI group (17.6%).
Local kyphosis (measured in degrees between the upper and lower plates of each fractured vertebra) was 22.1°±8° in the RI group, by 11°±5° in the SI group. This difference was statistically significant.
The volume of cement (PMMA, injected in cubic cm (cc), is less in the RI group (4.1±1.3 cc compared to 4.6±1.5 cc of the SI group), but with no statistically significant differences (p=.182).
The mean follow-up time was 6.7±2.8 months in the RI group and 6.5±2.3 months in the SI group, with no significant differences.
With regard to detected cement leaks, 44.1% (15/34) of leaks were detected in the RI group, compared to 29% in the SI group (18/61). The most frequent type of leak was towards the supra-adjacent disc, with 7 cases in the RI group and 6 in the SI group, with no statistically significant differences.
New adjacent fractures: 7 occurred in the SI group (11%) and 3 in the RI group (8%), with no statistically significant differences (p=.8). All these values are summarised in Table 5.
Results in both groups.
| SI group | RI group | Statistical significance | |
|---|---|---|---|
| Evolution time | 4.9 months | 7.05 months | <.05 |
| Crush | 24% | 60% | <.01 |
| Local Kyphosis | 11° | 22° | <.01 |
| Volume of PMM A in cc | 4.6±1.5 | 4.1±1.3 | .18 |
| Follow-up (months) | 6.5±2.3 | 6.7±2.8 | .8 |
| Complications | 0 | 0 | Not significant |
| Leaks (all asymptomatic), n/N (%) | 18/61 (29) | 15/34 (44) | .04 |
| New adjacent fractures, n (%) | 7 (11% | 3 (8) | .8 |
In our study we did observe a statistically significant relationship between the appearance of leaks and new adjacent osteoporotic vertebral fractures. This occurred by analysing all the cemented vertebrae (p=.028) and by doing so independently in the SI group (p=.029) and more intensively in the RI group (p=.007). In the RI group, all the new fractures had previous leakage (3 cases out of the 34 fractures in the RI group). In the SI group (61 fractures) of the 7 new fractures, 43% (3 cases) had previous leakage. Of the total sample (95 fractures), 60% of the new adjacent fractures had previous leaks, while, of all the leaks that occurred, 18.2% suffered a subsequent adjacent fracture. Conversely, of the cemented fractures that did not leak (56 out of a total 95) only 6.7% had new adjacent fracture. There were no other medical complications in the patients in our sample.
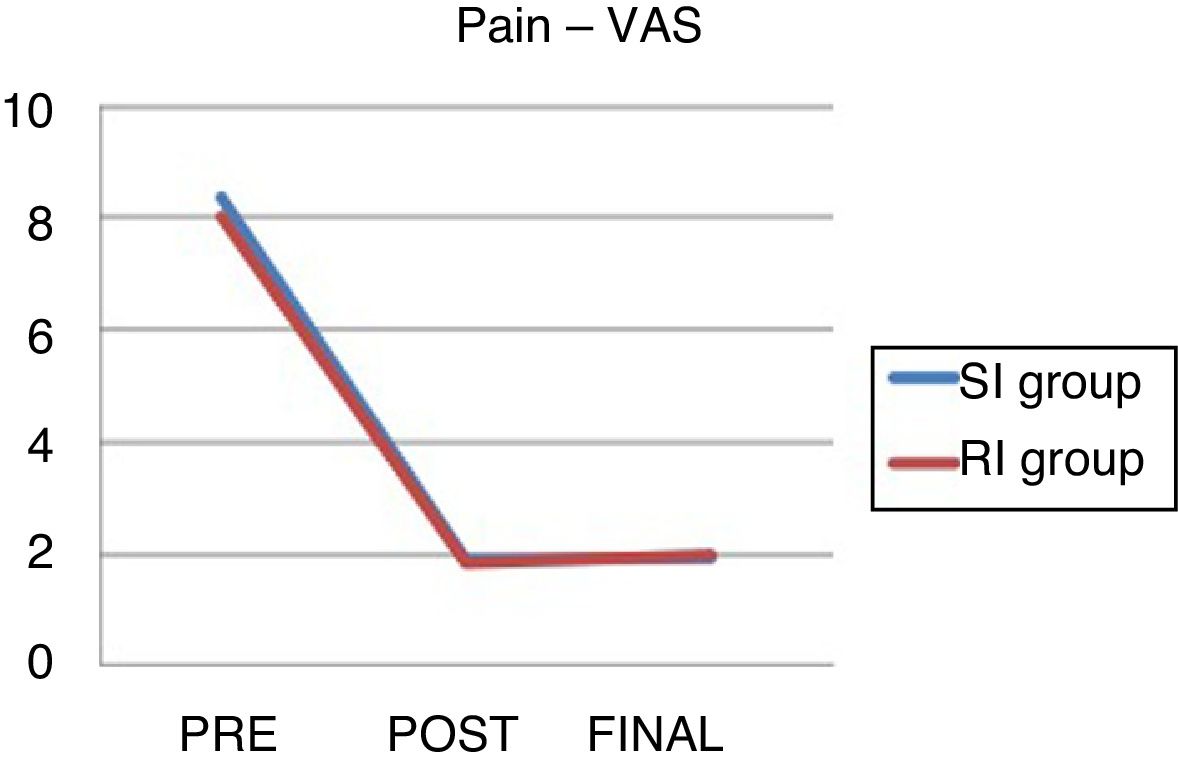
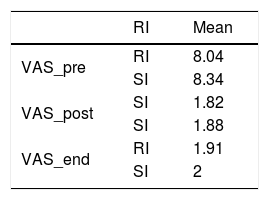
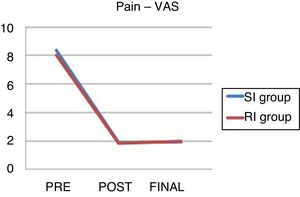
The data on pre and post-operative pain and at the end of follow-up can be seen in Table 6 and Fig. 2.
Visual Analogue Scale.
| RI | Mean | |
|---|---|---|
| VAS_pre | RI | 8.04 |
| SI | 8.34 | |
| VAS_post | SI | 1.82 |
| SI | 1.88 | |
| VAS_end | RI | 1.91 |
| SI | 2 |
There were no significant differences between the groups.
VAS: visual analogue scale; end: pain at end of follow-up; SI: standard indication group; RI: relative indication group; post: immediate postoperative pain; pre: preoperative pain.
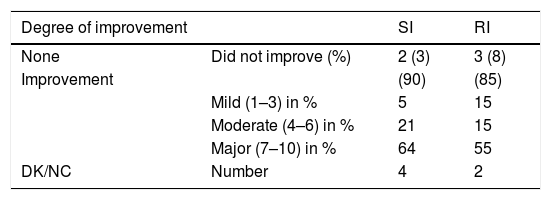
If we group our results according to magnitude of improvement, the results would be as expressed in Table 7.
Relationship between vertebral fractures and degree of improvement at the end of follow-up in each group.
| Degree of improvement | SI | RI | |
|---|---|---|---|
| None | Did not improve (%) | 2 (3) | 3 (8) |
| Improvement | (90) | (85) | |
| Mild (1–3) in % | 5 | 15 | |
| Moderate (4–6) in % | 21 | 15 | |
| Major (7–10) in % | 64 | 55 | |
| DK/NC | Number | 4 | 2 |
The patients who did not fill in the VAS adequately were included in the DK/NC section.
There are no statistically significant differences in clinical improvement in either group: it was mild (improvement 1–3 VAS points), moderate (3–6) and high (7–10) in 15%, 15% and 55% of cases, respectively in the RI group, and 5%, 21% and 64% in the SI group.
Within the RI group, we observed no statistically significant differences in clinical or radiological results between the fractures of more than 12 months’ evolution and the rest of the fractures with shorter evolution times.
A high percentage of patients were satisfied with the procedure in both groups: 82% in the RI group and 94% in the SI group. All of them would undergo the vertebroplasty again if they were able to go back in time.
The evolution of the following clinical cases are shown as examples in Fig. 3 and Fig. 4.
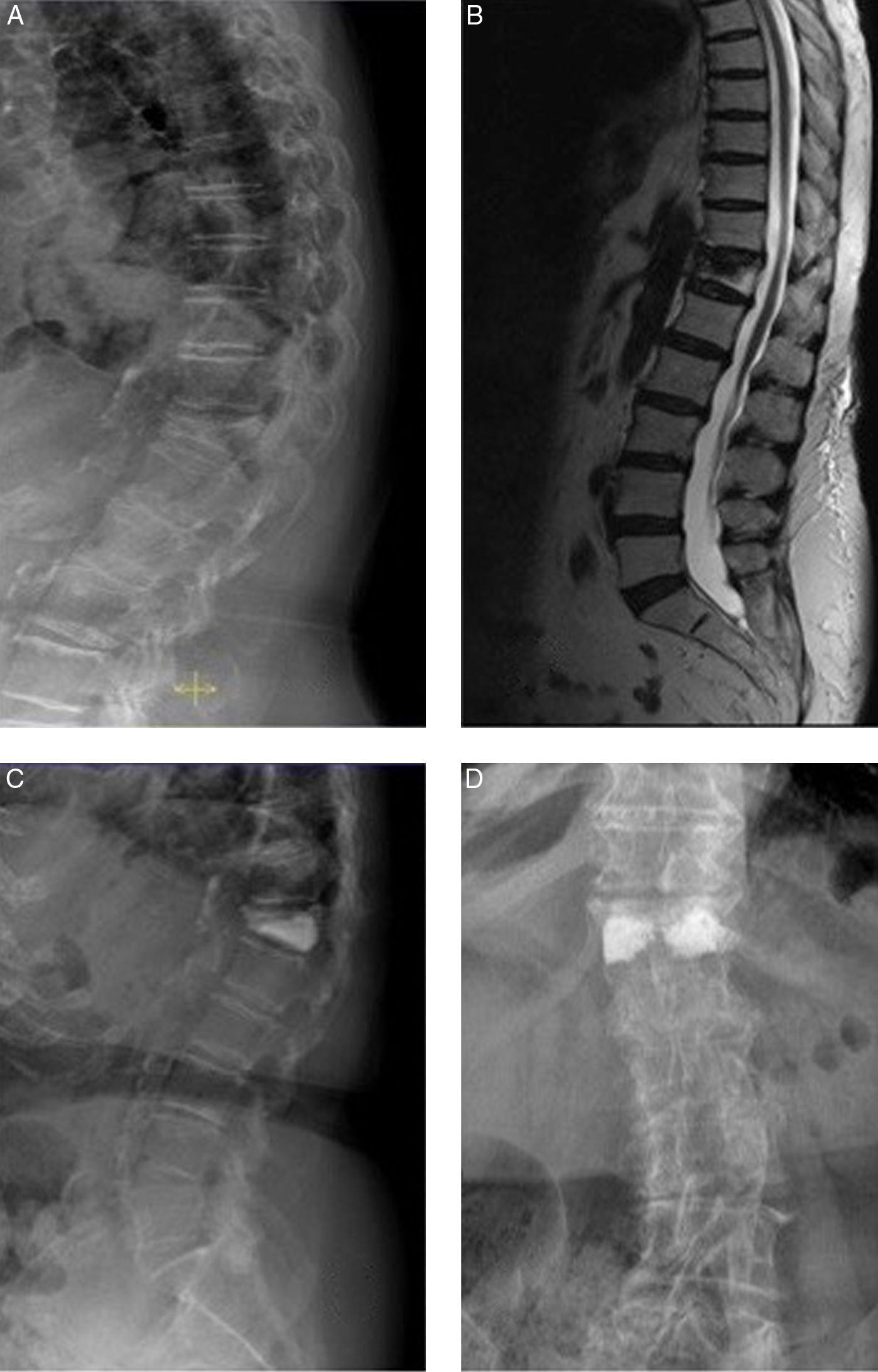
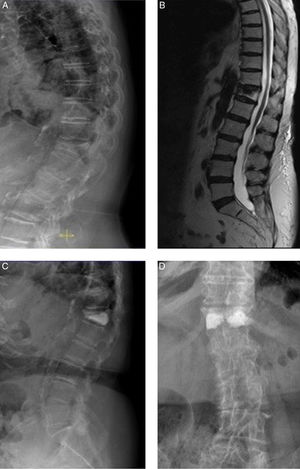
81-year-old female. A) D12 osteoporotic wedge-type fracture with 75% crush and 18° kyphosis (RI group). B) MRI showing oedema in the fractured vertebra (sign of recent fracture). C and D) anteroposterior and lateral radiological projections after bilateral transpedicular vertebroplasty. Good vertebral fill with cement. Cement leak of small dimensions is shown lateral to the right pedicle. The patient experienced improved pain of 5 points on the VAS.
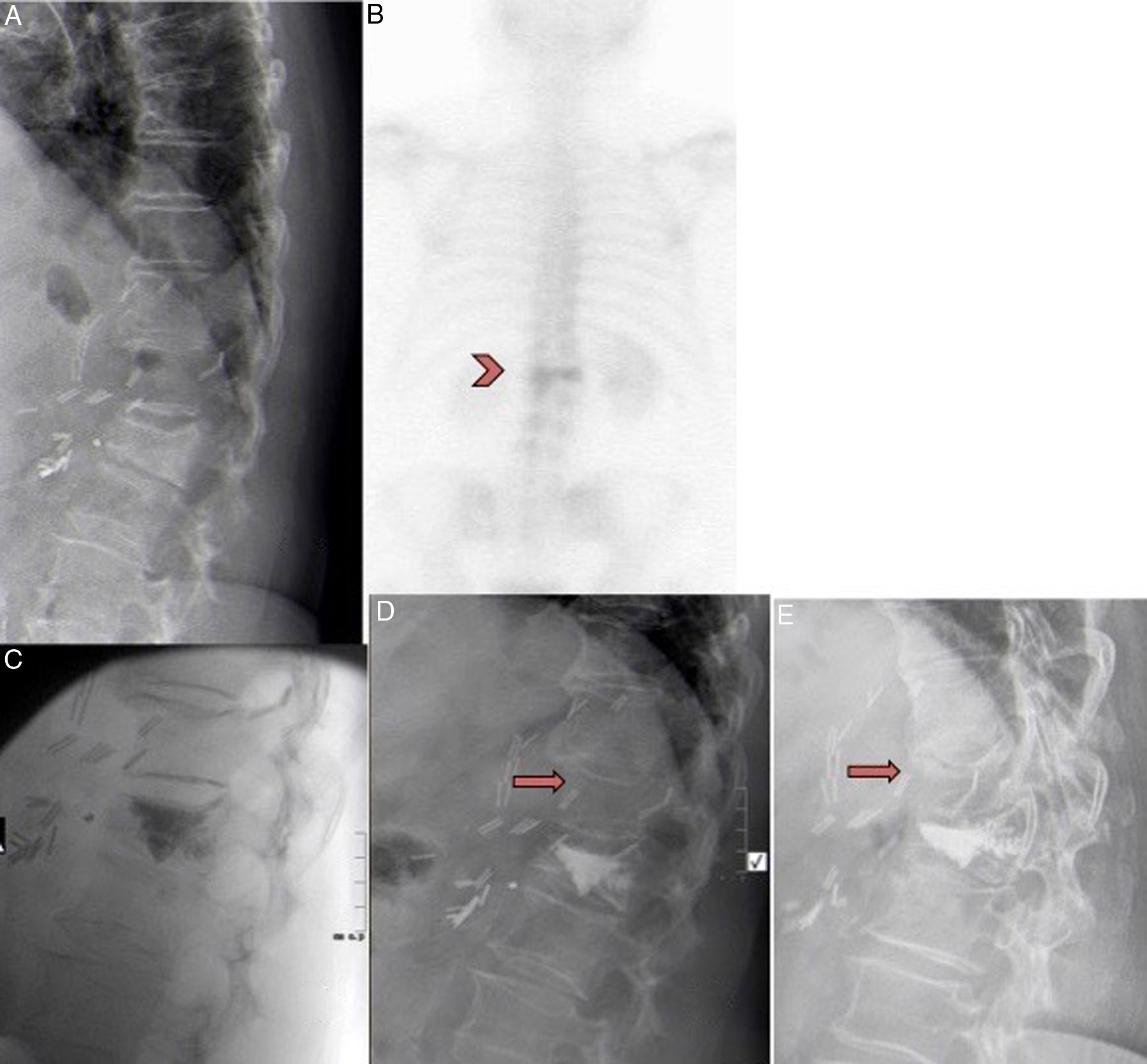
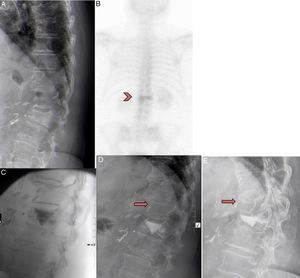
A) 71-year-old female with D12 fracture. B) Had previously undergone abdominal surgery. Bone scan showed hyper-uptake in the fracture after 12 months. C) Patient included in the RI group. Vertebroplasty performed in D12, with cement leaking into the caudal disc. D) Improvement of 4 points on the VAS was observed. One month after the vertebroplasty the patient presented intense back pain after spinal flexion. The x-ray showed a new compression fracture in D11 that was treated conservatively with corset and analgesia. E) After 3 months, the fracture had consolidated with 30% crush. The patient is currently asymptomatic.
There is consensus in the literature on the conservative treatment of osteoporotic vertebral fractures. However, there are no strict criteria on the indications of the vertebral reinforcement techniques. According to a recent review by Luthmann12 this is due to insufficient clinical evidence and the heterogeneity of patients with osteoporotic vertebral fractures. Meta-analyses and randomised control studies have been published in recent years that even advise against the routine use of vertebroplasty in osteoporotic vertebral fractures, claiming a lack of improvement compared to placebo.8,13,14 However, literature continues to be published showing the benefits of vertebral cementation techniques compared to conservative treatment15,16; it seems, therefore, that the debate is ongoing.
In our centre, patients with symptomatic osteoporotic vertebral fractures without neurological deficit are referred to the spinal unit for treatment and initial follow-up. In the cases where there has been no previous treatment with antiresorptive agents are referred to the bone metabolism unit for treatment. The usual treatment protocol for these patients is conservative, with symptomatic treatment using usual analgesics and posture control with rigid thoracolumbosacral or Jewett type hyperextension corset. Use of the corset is omitted for the cases that cannot tolerate it. We carry out clinical follow-up in the clinic until the fractures have consolidated. In the cases where pain is not controlled or the patient is not able to sit or walk, the surgical option is considered.
Our usual indications for vertebroplasty are AO type A1 compression fractures without neurological deficit, more than 3.4 months’ evolution with failure of conservative treatment; with hyper-uptake on bone scintigraphy or MRI with oedema on STIR sequences that indicate absence of consolidation, and patients with no contraindications for this surgical technique.
On reviewing the literature, the clearest indications described for performing a PVP on an OVF are fractures with poor pain control with conservative treatment, and those with signs of absence of consolidation on MRI or bone scintigraphy. However, there are no strict criteria in terms of fracture time, and we found studies with cementation of early fractures, of less than 2 weeks’ evolution, and studies of fractures of more than 6 months’ evolution.6,8,12,16
Neurological injury, infection, coagulopathies and allergy to cement are described as absolute contraindications. Relative contraindications are described as retropulsion of fragments, fractures with large collapse (type A3 and A4) and those with local kyphosis of more than 20°.5,6,17
In these cases it would be logical to move on to traditional or even percutaneous surgery with vertebral instrumentation, but greater complications and expense are well described in these patients compared to those in whom vertebroplasty or kyphoplasty is performed. Between 16% and 28% of complications are described in lumbar instrumentation surgeries in the geriatric population.18–20 Purvis et al.,18 in their study of patients over the age of 75 with thoracolumbar fractures, on a total of almost 60,000 patients, describe a 8.1% complication rate in patients treated with vertebroplasty or kyphoplasty, 8.7% in patients treated conservatively and 16.3% in patients operated using instrumentation.
These patients are frail, elderly, with comorbidities and poor bone quality. We find these types of patients in our environment, and they as well as their relatives often refuse to undergo standard spinal surgery. Vertebroplasty is a routine technique, which is performed safely, and we believe it is a fairly reasonable technique in some patients.
Hence the idea of undertaking this study. From our experience our perception is that this technique is still very useful if we extend the indications. The concept of “relative indication “in these fractures has not been used in any publications, and was coined specifically for this study in a subjective way to assess the results of this group of patients.
We consider that cases of great crush, and even fragment retropulsion, this is a technical challenge for the surgeon, but with an appropriate fluoroscopy technique and careful cementation it can be undertaken safety, given that these are chronic fractures in which we hope there is partial consolidation that prevents leakage, unlike in acute fractures. We believe that this approach avoids having to resort to the instrumentation of a fragile and osteoporotic patient with less morbidity and shorter hospital stay, with clinical outcomes that have been demonstrated as optimal.
We know that the most controversial concept is undoubtedly a fracture evolution of more than 12 months. In fact, we see in our sample that the indication was in fractures of 6.7 months’ evolution, which is common with that observed in different studies.6,8,12,16 However, circumstances led us to practice the surgery with a much longer delay. We must stress that this situation is not usual and is determined by exceptional circumstances, some of which have already been mentioned at the beginning of this paper. Once surgery was indicated, diagnostic imaging tests were not repeated in the patients who took part in the study. This could result in a bias in our results in patients with longer surgical delays. However in the a posteriori statistical analysis we found no statistically significant differences in the clinical or radiological outcomes of fractures of more than 12 months’ evolution and the rest of the cases that comprised the RI group. Despite our results, our recommendation is to always have the most recent possible diagnostic test at the time of surgery (preferably MRI), and to repeat it if a long time has elapsed or if the patient has a change in symptoms that would make us suspect consolidation of the fracture, pain of a different origin or a new osteoporotic vertebral fracture. Therefore, the fundamental criterion to indicate the technique is not a specific maximum evolution time, but the presence of clinical signs and imaging tests that indicate that the fracture is painful and has not consolidated.
In our samples we obtained results similar to other studies found in the literature in term of pain reduction, volume of PMMA used, percentage of leaks, type of leaks, complications, new adjacent vertebral fractures and follow-up time.8,17,21,22
We can highlight other limitations of the study as its being retrospective; the follow-up at 6 months could have obviated incidents such as recollapse of cemented vertebrae, which has been described in studies with longer follow-up.23–25 No baseline bone densitometry values were collected, although many of the patients did not have a previous diagnosis of osteoporosis. Neither did we study the effect of these fractures on sagittal balance, or on the quality of life of these patients. We focussed our analysis on pain reduction according to the VAS. We did not collect changes in the specific use of analgesic medication.
Conclusions- •
Vertebroplasty is a safe, effective and reproducible technique in the treatment of osteoporotic vertebral compression fractures without neurological deficit for which conservative treatment has failed.
- •
Vertebroplasty in symptomatic osteoporotic vertebral fractures with crush greater than 50%, occupation of the canal or more than 12 months’ evolution resulted in the same clinical benefit in our sample than those with standard indication.
- •
More leaks occurred in the relative indication fracture group with no clinical impact and no more adjacent vertebral fractures.
- •
Extending the indications for percutaneous vertebroplasty could be considered in osteoporotic vertebral fractures with more than 50% crush or occupation of the canal without neurological deficit, maintaining precautions and insisting on appropriate surgical technique to avoid complications.
Level of evidence III.
FundingNo funding was received to undertake this study.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Navarro-Navarro R, Fernández-Varela T, Montesdeoca-Ara A, Lorenzo-Rivero JA. Resultados de la vertebroplastia en fracturas vertebrales osteoporóticas con indicación límite. Rev Esp Cir Ortop Traumatol. 2020;64:4–12.