Vancomycin powder (VP) has been positively used in spinal surgery to reduce the rate of infections. Hardly any data have been published on hip and knee joint replacement surgery, and its usefulness is questioned. Our objective was to investigate the effectiveness of VP in reducing prosthetic infection and its possible complications.
MethodsPrimary hip (THA) and knee (TKA) arthroplasties were reviewed, performed by five surgeons in one hospital centre, between 2017 and 2018. One gram of VP was used on the implant prior to surgical closure based on the surgeon's preferences. With a 5-year follow-up in which the infection rate and local complications were analysed.
ResultsOne thousand one hundred and fifty-one arthroplasties were performed, 748 were TKA and 403 were THA. Nine patients were diagnosed with prosthetic infection, of which five received VP and four did not (p=0.555). Likewise, another 15 patients suffered wound complications, of which 11 received VP and 4 did not (p=0.412). There were no differences, either, in the rest of the complications depending on the use or not of VP (p=0.101). Likewise, the number of patients who needed reintervention was similar (p=0.999).
No systemic complications were detected due to the use of VP.
ConclusionsIt has not been possible to demonstrate that the use of VP reduces the rates of prosthetic infection in the hip and knee, so we cannot recommend its use.
La vancomicina en polvo (VP) ha sido utilizada positivamente en cirugía de raquis para reducir la tasa de infecciones. Apenas se han publicado datos en cirugía de reemplazo articular de cadera y rodilla, estando en entredicho su utilidad. Nuestro objetivo fue investigar la eficacia de la VP en la reducción de la infección protésica, y sus posibles complicaciones.
MétodosFueron revisadas artroplastias primarias de cadera (ATC) y rodilla (ATR), intervenidas por 5 cirujanos en un centro hospitalario, entre los años 2017 y 2018. Se usó 1 g de VP sobre el implante previamente al cierre quirúrgico en función de las preferencias del cirujano. Con un seguimiento de 5 años en los que se analizó la tasa de infección y las complicaciones locales.
ResultadosSe intervinieron 1.151 artroplastias, 748 fueron ATR y 403 ATC. Nueve pacientes fueron diagnosticados de infección protésica, de los que 5 recibieron VP y 4 no (p = 0,555). Así mismo, otros 15 pacientes sufrieron complicaciones de la herida, de los que 11 recibieron VP y 4 no (p = 0,412). No hubo diferencias, tampoco, en el resto complicaciones en función del uso o no de VP (p = 0,101). Del mismo modo, el número de pacientes que necesitaron una reintervención fue semejante (p = 0,999).
Tampoco se detectaron complicaciones sistémicas por el uso de VP.
ConclusionesNo se ha podido demostrar que el uso de VP reduzca las tasas de infección protésica en cadera y rodilla, por lo que no podemos recomendar su uso.
Infection is one of the most devastating complications of joint replacement surgery. Its incidence has been established between 1% and 4% in total knee arthroplasty (TKA) and between % and 2% in total hip arthroplasty (THA).1,2 The average cost of an infected arthroplasty is >300% that of a primary arthroplasty, as well as double the risk of re-hospitalisation and death.3
The main microorganisms causing prosthetic infection are gram-positive cocci, with Staphylococcus aureus and coagulase-negative Staphylococcus (CNS), contributing to more than 50% of infections, both acute and chronic, while Streptococcus spp. and Enterococcus spp. together barely account for 10%. Under 10% are caused by gram-negative or aerobic bacilli, and up to 30% of cultures are negative.4
The struggle against microorganisms that cause prosthetic infections therefore focuses on gram-positive cocci, with vancomycin being one of the most used drugs. Vancomycin is a tricyclic glycopeptide antibiotic, which is highly effective in S. aureus infections, especially in those resistant to beta-lactams, i.e. to methicillin (MRSA).5
Vancomycin has been used in different presentations such as, for example, its inclusion in biological cements, providing benefits in the prevention of prosthetic infection.6,7 It has also been used, with broad support, in anterior cruciate ligament reconstruction surgery, in diluted form, bathing the tendon plasty in a vancomycin solution, thus reducing the rate of infections, with level III evidence.8
Its topical use has recently become popular, seeking to increase concentration in the surgical bed and minimising intravenous concentration, with the aim of reducing possible complications derived from it.9 For years, vancomycin powder (VP) has been most widely in spine surgery.10–13 Numerous retrospective studies have highlighted the advantages of its use in the prevention of surgical infection but only a single prospective study has been published, which is unable to corroborate the aforementioned benefits in reducing surgical infection.14 Furthermore, its use may not be safe, as complications arising from its local use have been described.15,16
The objective of this study was to assess the influence of the use of locally administered VP in primary joint replacement surgery of the hip and knee, also observing its possible long-term adverse effects.
Material and methodsA retrospective observational case–control study was conducted. The operations were performed between January 2017 and December 2018, including patients who underwent primary hip arthroplasty (THA) and knee arthroplasty (TKA).
One thousand, one hundred and fifty-one interventions were included, with a minimum follow-up of 5 years. This sample size would enable us to detect as significant a reduction in the percentage of complications in interventions with the use of VP of 2.0% compared to interventions without VP, assuming a unilateral approach with a confidence level of 95% and a statistical power of 80%.
Data were collected by two researchers from the computer programme of the health management system used in the public health service of the health area to which the patients belonged.
The patients underwent surgery in one centre, with five surgeons performing it. Three surgeons used VP over the prosthesis bed in their patients, while two did not, all according to their preferences. The five operated on both hip and knee arthroplasties, with a minimum of 100 annual interventions each.
Data were collected for each patient regarding their age, preoperative diagnosis (primary osteoarthritis; inflammatory disease; post-traumatic osteoarthritis, avascular necrosis or hip dysplasia), their comorbidities (cardiovascular disease; inflammatory disease; diabetes or kidney disease) and their previous treatment (anticoagulant/antiplatelet treatment; antidiabetic treatment or chronic immunosuppressive treatment). Postoperatively, complications of the surgical process were recorded in the 5 years after the intervention. A deep infection was considered to be a process that met the diagnostic criteria of the International Consensus Meeting on Periprosthetic Joint Infections.17 The consideration of superficial infection or wound complication was that the surgical wound did not meet diagnostic criteria for deep or implant infection, nor presented prolonged post-surgical drainage over time that required dressings beyond the first month, surgical revision and/or empirical antibiotic treatment. The rest of the post-operative complications that affected the operated joint were recorded: aseptic loosening; periprosthetic fracture; dislocation; instability or stiffness, as well as possible death of the patient.
Surgical protocolThe antibiotic prophylaxis protocol used was the same for all patients. Cefazolin (2g) was used as the antibiotic of choice, which was administered intravenously prior to the incision, and a dose of cefazolin IV/1g/every 8h was repeated during the first 24h. In case of allergy to beta-lactams, clindamycin (600mg) was used preoperatively and in three post-operative doses/every 8h). Pre-surgical skin preparation was carried out with 10% povidone-iodine, as well as post-operative dressings. Vacuum suction drainage was placed in all cases, remaining for 1 or 2 days. In all cases, intravenous tranexamic acid was used as a strategy to minimise perioperative bleeding. An ischaemia cuff was used in TKAs and was removed after closure and bandaging. The approach of choice in the knee was anterior with medial parapatellar arthrotomy and in the hip direct anterior, lateral and posterior approaches were performed. Four different arthroplasties were implanted in the knee: Triathlon PS (Stryker Orthopaedics, Mahwah [NJ]); Vanguard PS (Zimmer-Biomet, Warsaw [In]) Optetrak PS (Exactech, Gainesville [Fl]) and Apex PS (Corin, Raynham [MA]), while two implants were used in the hip: Furlong (JRI, London [UK]) and Taperloc-G7 (Zimmer-Biomet, Warsaw [In]), according to the surgeon's preference. All knees were postero-stabilised and cemented (the cement was in all cases with vancomycin). All THAs were cementless.
The VP (1g) was spread over the intra-articular surgical bed, in contact with the prosthesis, immediately before surgical closure.
Patients began walking the next day or after 2 days with the help of English canes, progressively removing them from the 2nd or 3rd week. Wound checks were performed every 2 days until the staples were removed, starting from the 2nd week.
The study was approved by the Galician Research Ethics Committee, with registration code 2018/018.
Statistical analysisA descriptive analysis of the collected data was carried out, the quantitative variables being expressed with mean value and standard deviation, mean and range. Qualitative variables were described by absolute and relative frequencies. The comparison of means was carried out using parametric tests (Student's T) or non-parametric tests (Mann–Whitney test) as appropriate after checking normality with the Kolmogorov–Smirnov test. The association of qualitative variables was tested with the Chi-square or Fisher's exact statistic.
Survival from complications was determined using the Kaplan–Meier methodology; the comparison of the curves was performed with the log-rank test.
ResultsThe study included 1078 patients who underwent 1151 interventions, 748 (65%) were knee joint replacements and 403 (35%) were hip replacements. The mean age of the patients at the time of the operation was 71.4 years (SD: 9.4), 60.3% of the surgeries were performed on women. VP was used in 658 (57.2%) interventions.
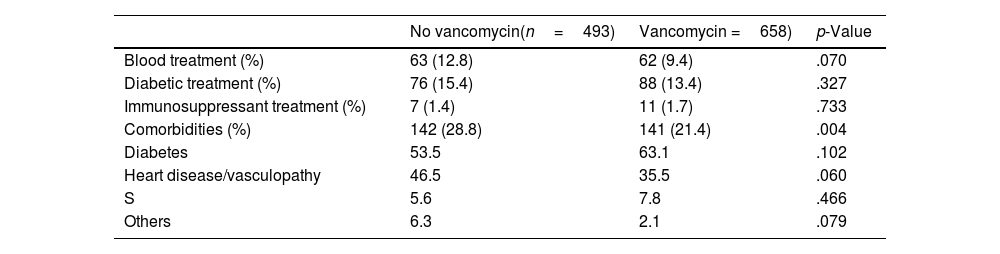
Sociodemographic and clinical characteristics were compared according to VP use (Table 1). The mean follow-up time was 5 years and 2 months (SD: 8 months). Sixty-two patients died at the end of follow-up, none of them from causes attributed to the intervention.
Descriptive data.
| No vancomycin(n=493) | Vancomycin =658) | p-Value | |
|---|---|---|---|
| Blood treatment (%) | 63 (12.8) | 62 (9.4) | .070 |
| Diabetic treatment (%) | 76 (15.4) | 88 (13.4) | .327 |
| Immunosuppressant treatment (%) | 7 (1.4) | 11 (1.7) | .733 |
| Comorbidities (%) | 142 (28.8) | 141 (21.4) | .004 |
| Diabetes | 53.5 | 63.1 | .102 |
| Heart disease/vasculopathy | 46.5 | 35.5 | .060 |
| S | 5.6 | 7.8 | .466 |
| Others | 6.3 | 2.1 | .079 |
S: stroke.
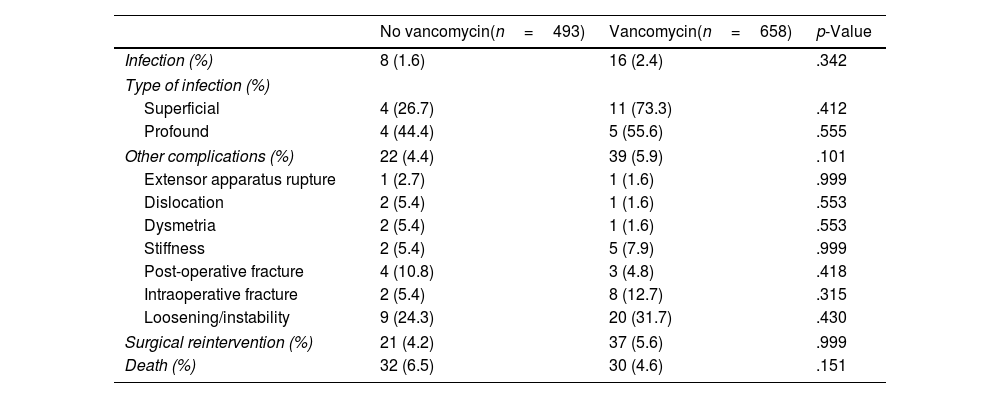
No statistically significant differences were found in the number of infections, or in the number of superficial complications, depending on the use of VP. Two point one per cent of patients during the study period suffered a complication classified as infection or wound complication. Of this percentage, 0.8% could be diagnosed with deep infection, with the previously established criteria, and 1.3% could not, with the complication being considered a superficial infection or wound complication. No differences were detected between both groups of patients in terms of the incidence of infections, neither deep nor superficial, although a tendency to suffer more wound complications was found in the group that used VP (11 versus 4 [Table 2]).
Complications.
| No vancomycin(n=493) | Vancomycin(n=658) | p-Value | |
|---|---|---|---|
| Infection (%) | 8 (1.6) | 16 (2.4) | .342 |
| Type of infection (%) | |||
| Superficial | 4 (26.7) | 11 (73.3) | .412 |
| Profound | 4 (44.4) | 5 (55.6) | .555 |
| Other complications (%) | 22 (4.4) | 39 (5.9) | .101 |
| Extensor apparatus rupture | 1 (2.7) | 1 (1.6) | .999 |
| Dislocation | 2 (5.4) | 1 (1.6) | .553 |
| Dysmetria | 2 (5.4) | 1 (1.6) | .553 |
| Stiffness | 2 (5.4) | 5 (7.9) | .999 |
| Post-operative fracture | 4 (10.8) | 3 (4.8) | .418 |
| Intraoperative fracture | 2 (5.4) | 8 (12.7) | .315 |
| Loosening/instability | 9 (24.3) | 20 (31.7) | .430 |
| Surgical reintervention (%) | 21 (4.2) | 37 (5.6) | .999 |
| Death (%) | 32 (6.5) | 30 (4.6) | .151 |
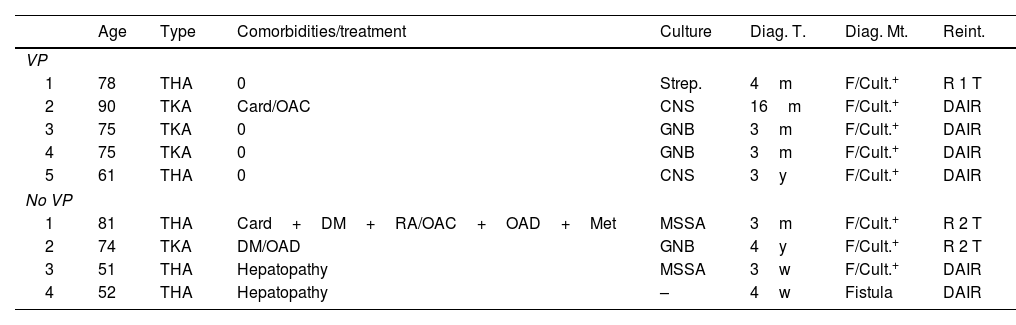
Of the 9 patients diagnosed with deep infection, 3 of them had positive cultures for gram-negative bacilli; 2 for S. aureus, both methicillin sensitive (MSSA); 2 for CNS and one for Streptococcus spp. In one case there was no microbiological documentation, although there was active drainage with an intra-articular fistula. Regarding the surgical strategy carried out, in five cases a DAIR (debridement+antibiotherapy+implant retention) was performed, one with one-stage rescue and three with two-stage rescue, of which only one was completed. The other two were not because one patient died before the 2nd intervention after a year of waiting, due to a heart problem, and the other still remains with the spacer and refuses to undergo further surgery. It should be noted that two of the infections attributed to the group without VP were in the same patient, a patient with advanced chronic liver disease. Both infections were resolved with a DAIR, but the patient died 2 years after the 2nd procedure, due to their underlying disease (Table 3).
Patients diagnosed with profound infection.
| Age | Type | Comorbidities/treatment | Culture | Diag. T. | Diag. Mt. | Reint. | |
|---|---|---|---|---|---|---|---|
| VP | |||||||
| 1 | 78 | THA | 0 | Strep. | 4m | F/Cult.+ | R 1 T |
| 2 | 90 | TKA | Card/OAC | CNS | 16m | F/Cult.+ | DAIR |
| 3 | 75 | TKA | 0 | GNB | 3m | F/Cult.+ | DAIR |
| 4 | 75 | TKA | 0 | GNB | 3m | F/Cult.+ | DAIR |
| 5 | 61 | THA | 0 | CNS | 3y | F/Cult.+ | DAIR |
| No VP | |||||||
| 1 | 81 | THA | Card+DM+RA/OAC+OAD+Met | MSSA | 3m | F/Cult.+ | R 2 T |
| 2 | 74 | TKA | DM/OAD | GNB | 4y | F/Cult.+ | R 2 T |
| 3 | 51 | THA | Hepatopathy | MSSA | 3w | F/Cult.+ | DAIR |
| 4 | 52 | THA | Hepatopathy | – | 4w | Fistula | DAIR |
Card: heart disease; CNS: coagulase-negative Staphylococcus; Cult.+: positive culture; DAIR: debridement+antibiotherapy+implant retention; Diag. Mt.: diagnostic method; Diag. T.: diagnosis time; DM: diabetes mellitus; F: fistula; GNB: Gram-negative bacilli; m: months; Met.: methotrexate; w: weeks; MSSA: methicillin-sensitive Staphylococcus aureus; OAC: oral anticoagulants; OAD: oral antidiabetics; RA: rheumatoid arthritis; Strep.: Streptococcus; THA: total hip arthroplasty; TKA: total knee arthroplasty; VP: vancomycin powder; y: years.
Furthermore, there were 15 superficial infections or wound complications, with patients presenting with active drainage or delayed wound healing in the first month. Four cases underwent surgery, all THA, with a DAIR performed, two with negative cultures, another with a single positive polymicrobial culture, without having had a proven fistula, and the fourth with a single positive culture for MSSA, all of them being cured with the procedure. In the fifth case, a TKA, surgical cleaning of the wound was performed, without changing components, and required over 2 more months of wound care in a diabetic patient. In this case following several superficial cultures, only one was positive for MSSA, and the patient being treated with oral antibiotic therapy. We understand that the case mix shown with little microbiological documentation could have been due to the inappropriate use of prior oral antibiotic treatment by their primary care physician, with no consultation having been made with the corresponding specialist.
DiscussionOur study did not find any benefit with the use of VP in terms of reducing prosthetic infection, neither acute nor chronic. In contrast we observed a trend towards an increase in superficial or wound complications in patients who received it.
The popularity of the use of VP in orthopaedic surgery has increased exponentially in recent years, and with it, the studies in this regard. However, results are contradictory. The most recent meta-analyses contradict each other in their final conclusions, Movassaghi et al., Peng et al. and Liao et al.18–20 recommended its use, while Xu et al., Wong et al. and more recently, Martin et al.21–23 conclude that the biases found in the majority of studies may interfere with the recommendation of its use.
The conclusions we have reached are consistent with the works previously published by Yavuz et al.24 and Hanada et al.,16 who, in patients undergoing primary knee arthroplasty, did not obtain any benefit with the use of VP, but instead warned of the increase in local complications. Previously, Otte et al.25 and Dial et al.,26 had reported a reduction in infection rates with the use of VP in revision arthroplasty and primary hip arthroplasty, respectively. However, their studies included a small number of patients and a short follow-up. Along these lines, the most recent studies, with a greater number of patients recruited, did find differences in favour of the use of VP. Buchalter et al.,27 reviewed more than 18,000 primary arthroplasties from the registry of a single hospital, observing a decrease in the incidence of infections (.8% to .5%) due to MRSA and CNS, without finding differences in gram-negative bacilli. However, the follow-up was only three post-operative months and also included other changes in the hospital's preventive protocol, such as the use of diluted povidone lavage, which could have represented a bias. Hu et al.,28 recently analysed over 1600 primary arthroplasties, with prospective follow-up, although without randomisation. They also obtained a significant decrease in the incidence of acute infection at 3 months, with a decrease from .8% to 0% with the use of VP, in this case used in a diluted and infiltrated manner after joint closure. Matziolis et al.29 analysed more than 8000 arthroplasties, noting a decrease from 1.3% to .3% in the infection rate, with one year of follow-up.
The decrease in surgical infection with VP has been published in multiple spinal surgery10,11 studies. However, only one prospective study has been carried out evaluating its use, and post-operative infection was not reduced in it.14
The use of vancomycin can, however, cause complications, which have been demonstrated intravenously, but not yet through its topical use. They include ototoxicity, kidney failure and Vancomycin flushing syndrome. We did not observe any cases of systemic complications in our study. In fact, Sweet et al.13 measured blood levels of vancomycin after its local use in spinal surgery, without observing values high enough to cause systemic problems. However, its use in the surgical bed could cause local complications, as has been previously suggested,15,16 due to the increase in local acidity because of excessively low pH levels (pH: 3–4.5)30,31 which would increase the risk of cell necrosis, with its consequent alteration of the reparative capacity of the tissues. Furthermore, a harmful effect on the function of osteoblasts has been observed, which would make the integration of prosthetic implants difficult.30–33 There is some evidence that vancomycin can be toxic to endothelial cells, as well as other types of cells that influence tissue repair.34 The possible creation of loose bodies could also influence the behaviour of prosthetic implants, as a polyethylene-degrading agent or as a pro-inflammatory mediator, which could ultimately facilitate infection. It does not seem that polyethylene is affected “in vitro”, although there are no “in vivo” studies due to the use of VP, nor are there any chromium–cobalt alloys.35 These complications have been observed in spine surgery,15 but also in primary knee arthroplasty.16 In our study, a statistically significant increase in the rate of aseptic loosening or any other local complication derived from the use of VP in the surgical bed was not observed, although cases of wound complications did increase in the group that used VP.
The use of local antibiotics raises several possible problems, not yet well studied, such as, for example, the delay in the moment of action of the same on possible pathogens. It has been studied that the moment of maximum action against pathogens is just before the surgical incision, and the moment of greatest infectious risk is also the one that exceeds 2h after the incision. It seems obvious then that administering the antibiotic just before closure would not be the best option.36
Vancomycin, like other antibiotics, can contribute to the development of resistance if its use is inappropriate.
Furthermore, it is necessary to consider the phenomenon of “arthroplasty antibiotic flora”, according to which the high concentration of antibiotics not only does not reduce infection, but can promote the growth, and therefore infection, of certain pathogens usually resistant to vancomycin, such as gram-negatives or yeasts. Prospective studies are needed to assess these effects.27
It is interesting to note that there were no differences in the type of isolated microorganisms in the existing infections in our study, regardless of the use or non-use of VP. The possible prevalence of MRSA in our environment is therefore not a factor to take into account for the indiscriminate use of glycopeptides. Additionally, the S. aureus isolated in the diagnosed infections were all MSSA.
We therefore do not consider the use of VP useful in primary hip and knee joint replacement surgery. Although its cost is not high, we have not been able to verify any benefit with its use, and it is also a possible source of local complications according to the bibliography consulted. In our study we were not able to demonstrate this in any significant way, although there was a tendency towards an increase in complications.
Our study had several limitations such as the fact that the same surgeon always used VP or did not. This may have represented a bias, since a higher infection rate in surgeons who used VP could have been obscured. However, these surgeons also did not have more overall complications than those who did not use VP. The follow-up of the patients by different doctors, other than specialist orthopaedic surgeons, did not favour the drawing of adequate conclusions, since many patients could have been underdiagnosed as a result. This was demonstrated with the significant number of patients being treated with oral antibiotic therapy by their general practitioner.
Further evidence is required for more concise recommendations, although, in our opinion, efforts aimed at reducing the rate of prosthetic infection should be made elsewhere, for which better results will probably be obtained.
Level of evidenceLevel of evidence III.
FundingThis study received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.