Osteoporosis weakens the structural strength of bone to such an extent that normal daily activity may exceed the capacity of the vertebra to bear this load. Vertebral fracture and deformity is a hallmark of osteoporosis. The detriment of trabecular bone properties alone cannot explain the occurrence of osteoporotic vertebral fracture. The ability of the spine to bear and resist loads depends on the structural capacity of the vertebrae, but also on loading conditions arising from activities of daily living or low-energy trauma. This review describes the mechanical properties of the vertebral bone, the structural load-bearing capacity of the various elements forming the spine, the neuromuscular control of the trunk, as well as the biomechanics of the loads to which the spine is subjected in relation to the presence of osteoporosis and the risk of vertebral fracture. A better understanding of biomechanical factors may help to explain both the high incidence of osteoporotic vertebral fractures and their mechanism of production. Consideration of these issues may be important in the development of prevention and management strategies.
La osteoporosis debilita la resistencia estructural del hueso hasta tal punto que la carga generada en la actividad diaria normal puede exceder la capacidad de la vértebra para soportar esta carga. La fractura y la deformidad vertebral son características distintivas de la osteoporosis. El detrimento de las propiedades del hueso trabecular por sí solo no puede explicar la aparición de la fractura vertebral osteoporótica. La capacidad de la columna para soportar y resistir cargas depende de la rigidez de las vértebras, pero también de las condiciones de carga que surgen de las actividades de la vida diaria o de un traumatismo de baja energía. En este artículo de revisión se describen las propiedades mecánicas del hueso vertebral, las cargas a las que está sometida la compleja estructura de la columna vertebral, el control neuromuscular del tronco, así como la biomecánica de la columna osteoporótica y el riesgo de fractura vertebral. Una mejor comprensión de los factores biomecánicos puede ayudar a explicar tanto la alta incidencia de fracturas vertebrales osteoporóticas, como su mecanismo de producción. La consideración de estas cuestiones puede ser importante en el desarrollo de estrategias de prevención y manejo.
Osteoporotic vertebral fracture is the most common type of osteoporotic fracture. It is associated with pain, disability, deformity, reduced quality of life, and increased mortality. Quality of life progressively decreases as the patient suffers an increasing number of these fractures.1,2
Structural design is dictated by the shape, size, and organisation of the vertebral body. The detrimental properties of trabecular bone alone cannot fully explain the difference in the incidence of vertebral fractures between individuals of comparable bone mineral density (BMD). BMD, bone loss, relevant biomechanics such as bone geometry, mechanical properties, load transmission, and even neuromuscular characteristics of the trunk may all act on the ability of a vertebral body to support physiological loads, yet their deterioration may contribute to the occurrence of a vertebral fracture or deformity.3
The aim of this review is to highlight the biomechanical factors identified in the literature that may influence the risk of osteoporotic vertebral fracture.
Functional vertebral anatomyThe shape of a vertebra is specifically designed to support axial loading. The orientation and structure of the vertebral body ensures that, under normal circumstances, the compressive load is transferred through the vertebral body, which increases craniocaudally as the percentage of body weight increases.4,5 Thus its design provides the requirements for optimal load transfer ensuring maximum strength at a minimum weight.6
The transmission of axial compressive load between the vertebral body and the disc takes place through the vertebral endplates. The endplates have a specialised porous microstructure more like condensed trabeculae than compact cortical bone7 and function as a nutrient pathway between the disc and the vertebral body. The thickness of the vertebral endplates ranges from .4 to .8mm depending on the level of the spine, being thinner in the centre than at the periphery and at certain levels of the spine, the lower endplates are thicker than the upper.8 The vertebral endplates are commonly affected by osteoporotic fractures and are believed to be the “weak link” of the lumbar spine.9
The cortical layer is located at the periphery of the body and surrounds the trabecular bone. It is thickest at the periphery, near the vertebral endplates, and thinnest in the central region. Although the cortical layer is only .25–.4mm thick and constitutes only 10–20% of the total amount of bone tissue in the vertebral body (the vertebral body has a trabecular bone/cortical bone volume ratio of 95/5), it absorbs up to 75% of the axial compressive load.10
Trabecular bone is found inside the vertebral body. Vertebral trabecular bone has a highly porous architecture (>80% porosity) that provides a unique spatial network to receive and distribute loads effectively.11 The design of the trabecular network enables compressive forces to be distributed efficiently. This efficient distribution is through the three-dimensional orientation of the internal microarchitecture with horizontal trabeculae (such as plates or beams) and vertical trabeculae (such as tubes or struts). Vertically oriented trabeculae attenuate axial forces, while horizontal trabeculae attenuate tensile stresses transferred from the intervertebral disc.
The strength of trabecular bone is partly attributable to its large surface area-to-volume ratio. This ratio is approximately four times greater than that observed in cortical bone. Trabecular microarchitecture is characterised by bone volume fraction (bone tissue volume/total volume) and other microarchitectural parameters (e.g. trabecular thickness, number of trabeculae, trabecular spacing, structural pattern index, connectivity density, and degree of anisotropy) that refer to the structure, interconnection, and spatial organisation of trabeculae. The deterioration of trabecular microarchitecture associated with ageing or osteoporosis leads to vertebral weakness and increased fracture risk.
Morphology of the osteoporotic vertebral fractureBy simple assessment of vertebral fracture by conventional X-ray using semi-quantitative criteria, osteoporotic vertebral compression fractures can be classified according to the morphology of their deformity into anterior wedge fracture, biconcave fracture, and crush fracture.12 The wedge fracture is the most common type of vertebral fracture.
The trabeculae of the vertebral body tend to be denser in the posterior area of the vertebral body compared to the anterior. Likewise, the trabeculae are denser in the lower half compared to the upper half, possibly because they are reinforced by the trabecular arches from the pedicles. In osteoporotic vertebrae, the load-bearing capacity of the vertebral body is transformed, as it loses bone faster from the trabeculae than from the cortex. The typical osteoporotic vertebral fracture leads to a loss of height in the anterior vertebral body, often leaving the posterior vertebral wall intact, with the vertebra then adopting that typical anterior wedge morphology.
Biomechanics of vertebral fracturesWhile hip fractures are attributable to a fall in approximately 90% of all cases, many osteoporotic vertebral fractures occur under non-traumatic loading conditions.13 The non-traumatic nature of osteoporotic vertebral fractures makes them difficult to diagnose because they are silently preceded by deterioration of the microstructure and failure of bone tissue.
Osteoporotic vertebral fractures are mechanical events that occur when the applied load exceeds the load-bearing capacity of the vertebral body. Therefore, the relevant risk factors for fracture can be defined according to the load applied to the strength of the vertebral bone.14 Based on this simple biomechanical concept, the force that a structure is expected to withstand is determined by the ratio between the strength of the structure and the loads it must withstand (Φ=load-to-strength). When this ratio (Φ) approaches 1, the structure is at risk of failing. A similar concept can be used to explain fragility fracture by defining the ratio between the loads applied to the bone and its strength as a “risk factor”. If the risk factor is >1, then the vertebral body is expected to resist. Therefore, the occurrence of a vertebral fracture depends on the mechanical loads acting on the vertebral body and, more importantly, on the vertebral strength determined by its geometry, microarchitecture, bone tissue properties, etc.
Substantial changes will occur in the vertebrae with ageing and osteoporosis. The decrease in vertebral strength is mainly due to loss of bone density and deterioration of bone microarchitecture with age. From the fourth decade of life onwards, older men may lose up to 30% and older women up to 50% of bone density. Between the ages of 25 and 75, the average force required to produce a compression fracture decreases from approximately 8000 to 2000N in the vertebrae of the thoracolumbar spine. This drops to approximately 500N in thoracic vertebrae from cadavers of older individuals.15
While age is largely responsible for the variation in bone strength, different individuals may exhibit bones that are much stronger or weaker than would be predicted by age alone. Similarly, BMD can explain some, but not all, of the variation in bone strength. At a given BMD, the measured strength values for different individuals may be higher or lower than the expected value. Therefore, BMD measurements will not fully reflect or explain bone's resistance to fracture due to the differences observed in fracture incidence between individuals with comparable BMD.
Decreased structural strength is not only the result of reduced bone density, but is also due to changes in bone architecture, bone remodelling, and rate of repair, resulting in an accumulation of damage under continuous cyclic loading. Increased bone fragility is due to the replacement of normal trabecular structures with thinner, more open spicules. The more porous appearance of cancellous bone is a result of the reduction in bone microarchitecture of the horizontal interlocking struts, which further reduces the resistance to buckling or bowing of vertically oriented trabeculae when subjected to axial compressive forces.6
To estimate the risk of osteoporotic vertebral fracture, we must accurately estimate the load on the spine during various activities of daily living. Mokhtarzadeh et al. studied the load-to-strength ratio (LSR) of the spine for a large number of activities in a group of 250 individuals.16 The LSR is a biomechanical model to estimate the risk for fracture by comparing the force, or load, applied to a bone to its strength, where a higher LSR indicates a higher risk of fracture. They determined that the activity with the highest LSR varied by spinal level, and identified three distinct spinal regions by the activity that producing maximum LSRs: lateral bending with a weight in one hand (upper thoracic spine), holding weights with elbows flexed (lower thoracic), and forward flexion with weight (lumbar).
Myers et al.5 used mean values from thoracolumbar models for a cohort of 120 women over 65 years of age (mean weight 65kg and height 1.59m) to estimate forces on the vertebrae during normal activities of daily living. The estimated forces to the spine at T8, T11, and L2 during various activities ranged from approximately 400 to 2100N for the body habitus typical of a woman over 65 years of age. In general, it is difficult to quantify the forces acting on the spine at any given time, as these forces cannot be measured directly. Instead, these forces are usually measured indirectly by quantifying the intradiscal pressure or by biomechanical modelling. Here, finite element analysis based on a computed tomography (CT) scan (resolution of approximately 1mm) of the human spine can predict new vertebral fractures in both women and men17,18 even better than DXA or quantitative BMD measured by CT scan.
Effects of bending and compression on vertebral fracture riskAxial compression and flexion loads are common loading conditions that our vertebral bodies undergo during daily life. Despite the high prevalence of vertebral wedge fractures in the population, the mechanics of these fractures are not well understood. The morphology of wedge fractures, in which the anterior side is shortened in the order of 15% more than the posterior side, clearly suggests that spinal flexion plays an important role. Flexion of the mobile segment increases the loading stress within the anterior vertebral body, including the cortex and trabecular bone. Pressure transducer experiments have measured increased pressure in the anterior half of the intervertebral disc when the disc-vertebra-disc segment is loaded in flexion.19 However, it is not yet clear whether this flexion-induced increase in anterior disc pressure would increase tissue stresses within the underlying cortical and trabecular microstructure. This is due to the technical difficulty of measuring such stresses in vivo. Finite element modelling based on high-resolution micro-computed tomography (micro-CT) imaging is very suitable to address this problem.
To better understand the aetiology of wedge fractures, studies20 have been conducted to investigate the distribution of high-risk tissue within the human vertebral body under both flexion and uniform axial compressive load conditions. Yang H et al.20 designed a finite element model of 22 human vertebral bodies with compliant disc (elastic modulus of 8MPa) for the study of axial flexion and compression. The model used was linear elastic. The results showed that flexion increased the compressive load on the anterior half of the intervertebral disc. The spatial distribution of the underlying vertebral bone tissue demonstrating an increased risk of failure shifted only slightly towards the anterior aspect of the vertebral body, identified as 10% of the highly stressed bone tissue in the vertebral model. Nevertheless, with flexion, the high-risk bone tissues were located primarily within the central regions of the trabecular bone and the endplates. These results suggest that loading in flexion does not appreciably change the spatial distribution of stress within the vertebral body in the presence of a healthy, compliant disc adjacent to the vertebral body. The distribution of load on vertebral bone tissue, or at least the distribution of the bone tissues most subjected to stress, is insensitive to axial compression versus flexion load. This insensitivity could be explained by a simple model, in which the vertebral endplate and disc together behave like a flexible beam resting on an elastic foundation of trabecular bone. Therefore, the maximum stresses in the trabecular bone occur below the centre of the applied load. For uniform axial compression, the centre of the applied load is in the central region of the vertebra, whereas in flexion the load acts at a point slightly anterior to the centre of the vertebral body.
In a second step, the same authors replaced the compliant disc with a rigid layer of polymethylmethacrylate (PMMA) (elastic modulus=2500MPa) to mimic a cadaveric experimental case, with a degenerated disc. When the compliant disc was replaced with PMMA, the anterior displacement of the underlying high-risk vertebral bone tissue was much more appreciable. The PMMA and the disc together behave like a rigid beam on an elastic foundation of trabecular bone, so that in flexion the peak loading stresses shift to a more anterior area of the vertebra. In patients undergoing artificial disc replacement, fusion surgery, or discoplasty, the disc space is filled with a rigid material and the adjacent vertebral bodies may be at increased risk of wedge fracture even with a moderate degree of flexion.
Vertebral body strengthVariability in vertebral compressive strength is primarily determined by vertebral size (and thus by bone mass) and bone density. However, laboratory studies show that BMD can explain only 50–70% of the variability in vertebral compressive strength.21 Other properties such as microarchitecture, collagen characteristics, microdamage accumulation, mineralisation, number, and viability of osteophytes may also play an important role, although their relative contribution to total vertebral strength remains poorly defined. While several studies have demonstrated an important role of microarchitecture in determining the mechanical behaviour of isolated trabecular bone samples, the contribution of trabecular and cortical bone microarchitecture to overall vertebral strength is not well understood and is difficult to isolate due to the pervasive influence of size, vertebral shape, and bone mass.
Due to vertebral trabecular morphology, vertebrae are generally less dense in the anterior and upper part and denser in the posterior and lower part.22 The lower density and thus lower compressive strength of the anterior vertebral body may explain the higher incidence of anterior wedge fractures in individuals with osteoporosis. During axial loading, there is usually a plastic deformation of the vertebral body (which has a lower mechanical resistance to axial loading compared to the posterior spine), generating the angular bending deformation of the spine, localised at the fracture site. In addition, the bending force component is increased by the deformation due to the fracture itself.
The behaviour of the spine under axial compressive forces will also be different in the presence of a pure axial load or an off-centre axial load due to the anatomical and structural characteristics of the vertebral bone and intervertebral discs. In the presence of an off-centre axial load, the compressive force acting on the spine causes a bending moment which is supported by the extensor muscle. It is also the load that contributes most to the compressive force on the spine, which will be absorbed by the posterior spine, which has the greatest mechanical strength.23
It has also been shown that the properties of cortical bone are a major contributor to the biomechanical behaviour of vertebrae.24 Of great interest are recent studies showing that microstructural heterogeneity can contribute to vertebral fragility independently of bone mass.25 Many clinical studies also provide evidence supporting the important role of trabecular microarchitecture in vertebral fragility, demonstrating deterioration of trabecular and cortical microarchitecture (assessed by morphometric analysis of iliac crest biopsies) in patients with osteoporotic vertebral fractures. More recently, non-invasive assessment of trabecular and cortical bone microarchitecture by high-resolution peripheral CT has shown that microstructural deficits, particularly in cortical bone, are associated with increased severity of osteoporotic vertebral fractures in postmenopausal women.26,27
Effects of the intervertebral disc on vertebral bone stressAgeing is also accompanied by degenerative changes in the intervertebral disc. The occurrence of anterior wedge fracture is thought to be associated with flexion loading, under which the bony tissues in the anterior portion of the vertebral body are at increased risk of failure. However, as we have noted previously, previous cadaver experiments have shown that, in the presence of healthy, compliant discs, bone failure typically occurs in the central regions of the trabecular bone and the vertebral body endplates, regardless of whether the compression is axial or through flexion.22,28
The magnitude of vertebral stress, risk, and even fracture morphology of the osteoporotic vertebra may also be related to the mechanical properties of the intervertebral disc. As noted above, by constructing a high-resolution finite element model of the vertebral mobile segment with a compliant intervertebral disc, it is demonstrated under axial compressive and flexion loading conditions that high-risk bone tissue is mainly distributed in the central regions of the endplate and the trabecular vertebral body. Only when the load in flexion is applied to the vertebra through a rigid PMMA layer (simulating a degenerated disc), the most bone tissues subjected to the most stress are located in the anterior aspect of the vertebra.20 These results imply that the mechanical properties of the intervertebral disc may influence the distribution of vertebral stress.
Alterations in the mechanical properties and morphometry of the intervertebral disc are associated with ageing and degeneration.29,30 Disc degeneration and loss of disc height led to an increased risk of osteoporotic vertebral fractures.31 However, it is not known whether typical variations in the mechanical properties of the disc can influence the location of high-risk tissues within the vertebra or the magnitude of vertebral stress. Yang et al. performed mechanical tests on 16 cadaveric discs (age 66±16 years) to obtain a homogeneous effective linear elastic modulus of the entire disc.32 They then introduced parametric variations in micro-CT-based finite element models of the elastic modulus (between 5.8 and 42.7MPa). The modulus of the human intervertebral disc does not exceed 100MPa; disc height ranged from 2.9 to 9.3mm. The thickness of human discs ranges from approximately 5mm in the thoracic region to 17mm in the lumbar region. They then virtually subjected the vertebral models to moderate flexion. As the elastic modulus of the disc increased and disc height decreased, the vertebral bone stresses increased, but their spatial distribution remained virtually unchanged. Most of the high-risk tissues appeared in the centre of the vertebral endplate and trabecular bone. Therefore, it can be concluded that, for a moderate degree of kinematically imposed load in flexion, increased disc stiffness (increased elastic modulus of the degenerated disc) or disc narrowing (decreased height) may increase the overall stress level within the vertebral body, but not lead to anterior vertebral body failure. Sensitivity of vertebral body elastic modulus variation to the magnitude of stress within the vertebral body to elastic modulus and disc height may have clinical implications for fracture risk assessment.
With ageing, the intervertebral discs progressively deteriorate, causing them to become more fibrous and less able to distribute compressive stress evenly and thus some parts of the vertebral body are subjected to higher concentrations of stress than others. Severe disc degeneration results in an increased load on the neural arch and posterior elements in upright position, and a decreased load on the vertebral body, which may lead to a progressive loss of bone mass in the anterior vertebral body.33 As a consequence of this altered load distribution, during standing, the anterior vertebral body would be subjected to stress shielding (the bone remodels at a lower load level than it should support, causing a process of osteopenia or loss of bone mass), but severely overloaded when the spine is in flexion.34 This sequence of changes in the spine could provide a mechanism by which the anterior region of the vertebral body becomes vulnerable to osteoporotic fracture, and explain the relationship between disc degeneration and osteoporotic vertebral fracture.35
Other age-related changes that may contribute to the development of osteoporotic vertebral fracture. Changes in neuromuscular functionThe muscles that attach to the spine have the dual function of producing motion and providing protection to the spine by stabilising its structures. Without muscle support, the spine would have a compression threshold of only 2kg before buckling.
Muscle mass typically peaks in the 20s and 30s and then declines progressively throughout life.36 Muscle strength, by contrast, is largely maintained at peak levels until the fifth or sixth decade, after which accelerated losses occur until strength declines by 25–35% by the age of 70. Not only does peak muscle force production decline with age, but the rate of muscle force and power development also declines. These changes in muscle force production could alter the normal loading capacity of the spine, as coordinated antagonistic muscle contraction is key to maintaining spinal stability during flexion and extension tasks.37
The effect of sagittal plane changes and changes due to spinal pain on the training characteristics of the thoracic paraspinal musculature in individuals with vertebral fractures also needs to be investigated. It is likely that changes in statics will influence the training characteristics of these muscles as changes in strength, force vectors, and length-tension ratios occur.
Finally, a reduction in intrinsic spinal stability may contribute to the poorer balance and postural stability observed with age and in subjects with osteoporosis. As a result, this reduced muscle function may further contribute to the occurrence of falls leading to fractures.9
Potential mechanisms for the occurrence of wedge fracturesMathematical models developed to predict compressive loads on the dorsolumbar spine indicate that the loads supported increase with flexion and with positions holding a weight at a distance from the centre of body mass. For example, the load on L2 is .5 times body weight during relaxed standing and increases to 1.5 times body weight with trunk flexion to 30° with arms extended. The force magnitudes associated with lifting weights of between 15 and 30kg are between 1000 and 2000N, in an adequate range for a person of average weight and height, however, these values would put them at high risk of fracture in the case of vertebral osteoporosis.38
However, as noted above, flexion loading may not directly result in a wedge fracture. Age-related changes or degeneration in the properties and morphometry of disc material would not alter the central distribution of high-risk tissue within the vertebral body and therefore may not cause wedge fractures. Therefore, the aetiology of wedge fractures may lie elsewhere. Adams et al.14 proposed that, with disc degeneration and narrowing, the upright position may cause some anterior unloading, as contact occurs primarily at the facet joints and load is transferred more through the neural arch. This anterior unloading may cause stress shielding and adaptive bone loss of the anterior portion of the vertebral body, thus compromising the strength of the anterior bone. Thus, when a forward flexion load acts on the vertebral body, the anterior portion would fail first and thus a wedge fracture would occur.
Alternatively, a moderate load in flexion may not be directly related to wedge fractures, but a more severe load in flexion may be. Some studies using a higher degree of flexion have found a relationship with the occurrence of anterior wedge fracture regardless of the state of disc degeneration.39 However, moderate flexion could produce initial vertebral fractures in the vertebral plates and in the underlying central trabecular bone.40 Cyclic loading in flexion in the posterior region and perhaps even friction may cause progressive collapse in the anterior vertebral body. In that case, the observed wedge-shaped fracture morphology could be the end result of a whole process that results in a fracture. It is possible that the disc responds with increased stiffness when its height decreases with degeneration or when the disc is subjected to high loads, which would generate increased stress within the central vertebral body, which could eventually progress to an anterior wedge fracture. This observation could explain why degeneration-related disc space narrowing is often associated with an increased risk of vertebral fractures, regardless of the type of fracture.41 In addition, vertebral fractures may be related to fatigue damage of bone tissues under cyclic loading. The fracture could be the end of a gradual process of cumulative fatigue failure of the vertebral body. Or fractures may occur slowly under constant loading through gradual frictional deformation. Further research is required to gain a deeper understanding of the mechanisms of wedge fracture of the spine. Thus, despite the profound individual and public health impact of osteoporotic vertebral fractures, the underlying biomechanical mechanisms remain largely unknown.
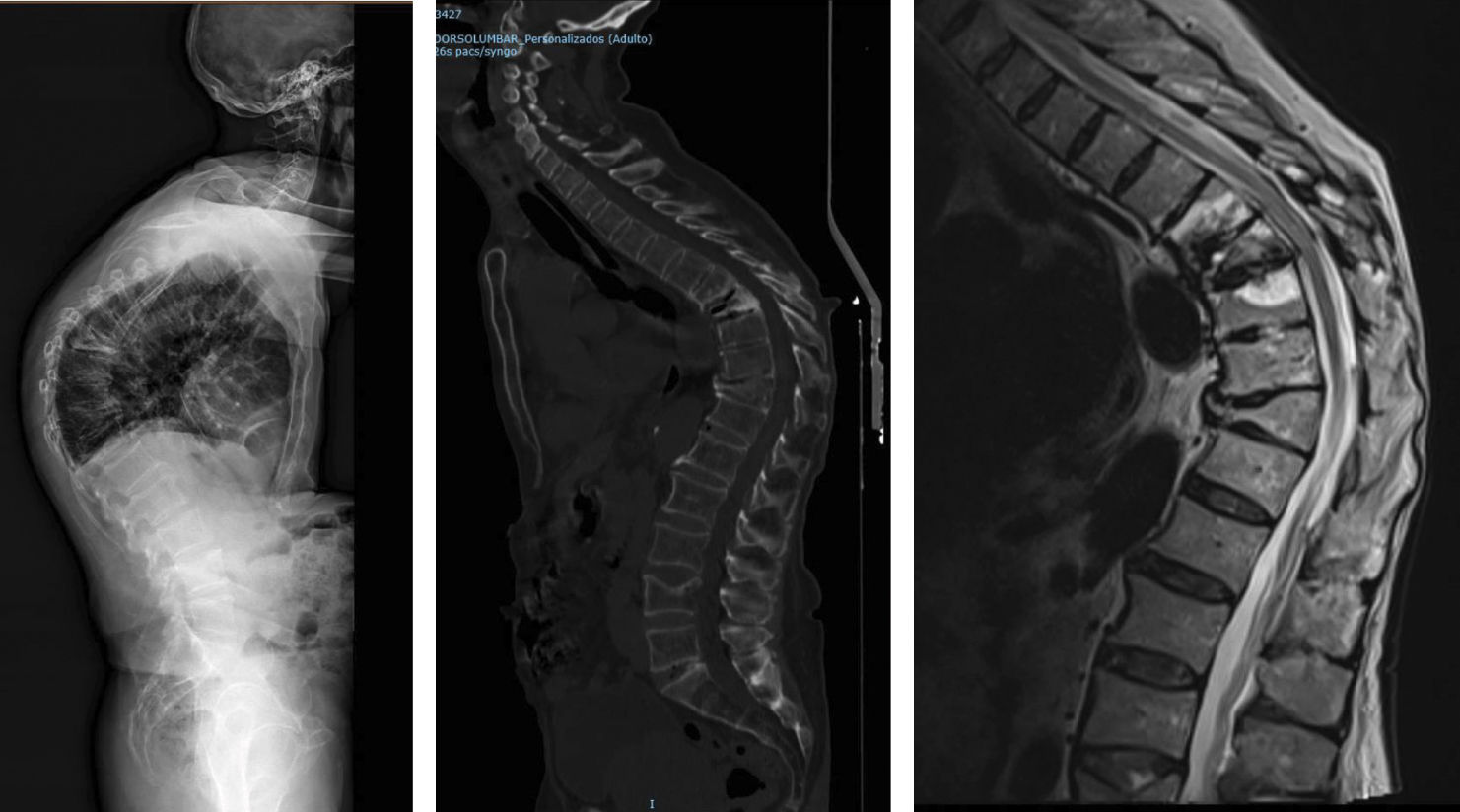
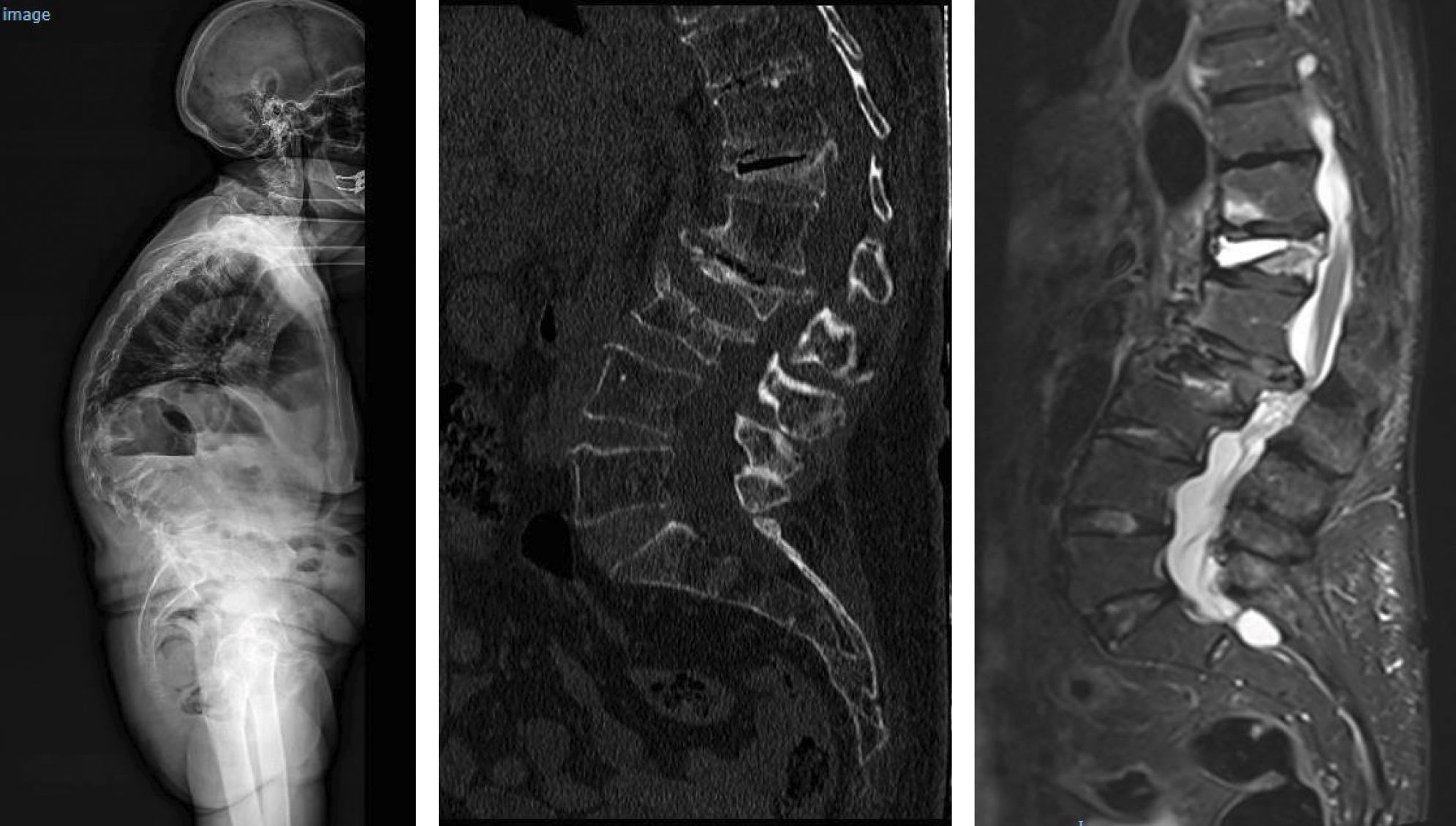
Location of vertebral fractures along the spineDespite low bone mass being a systemic disorder, osteoporotic vertebral fractures do not occur uniformly along the spine. They occur more frequently in the mid-dorsal (D7-D8) and dorsolumbar hinge (D11-L1) regions rather than in other parts of the spine. The reasons underlying this bimodal distribution of osteoporotic vertebral fractures are not fully understood, although it has been hypothesised that biomechanical factors due to variations in spinal curvature may contribute to the increased incidence of osteoporotic vertebral fractures in these regions. Maximal thoracic kyphosis occurs around D7-D8, and this may result in greater anterior bending moments and an increased risk of anterior wedge fractures in this region. Briggs et al.42 used a biomechanical model of the spine to demonstrate that older subjects with severe thoracic kyphosis have greater anterior flexion moments as well as greater compressive forces on the vertebral bodies (Fig. 1).
At the dorsolumbar junction, curvature of the spine changes from kyphotic to lordotic, and the rigid rib cage gives way to a more mobile lumbar spine. The higher incidence of osteoporotic vertebral fractures at D11-L1 may be because the rib cage no longer helps to protect and support extra loads at these immediately lower levels of the spine.43 In addition, the transition from a relatively rigid structure in the spine and rib cage to a more mobile lumbar region may contribute to an increased risk of fracture, although the underlying mechanism is not fully understood (Fig. 2).
Variations in BMD and bone strength along the spine constitute another factor that may contribute to the uneven distribution of osteoporotic vertebral fractures. Vertebral compression loads generally increase from the dorsal to the lumbar spine. However, Burklein et al.44 compared the compressive strength of the D6, D10, and L3 vertebrae in 119 cadavers, and found only modest correlations between the different levels, suggesting some heterogeneity in bone strength along the spine that may contribute to variations in fracture incidence in different regions of the spine.
Biomechanics of vertebral cascade fracturesOsteoporotic vertebral fractures are also of vital importance because they are a strong predictor of future fracture risk in any other location, regardless of BMD.45,46 The risk of suffering a new osteoporotic vertebral fracture is several times higher in those who have already suffered one, compared to those without previous fractures, and increases exponentially with the number and severity of prevalent fractures. Of great concern is the high rate of osteoporotic vertebral fractures following an initial fracture, often referred to as vertebral fracture cascade.47 Up to 20% of women with an osteoporotic vertebral fracture will suffer a new fracture within one year.48
However, it is not clear why an osteoporotic vertebral fracture predisposes a person to further fractures, although there are several factors that may contribute to this phenomenon. It is possible that osteoporotic vertebral fractures are an indicator of overall poor bone strength along with impaired trabecular and cortical microstructure that would predispose to multiple fractures. It is also likely that the presence of a vertebral fracture alters the mechanical load on adjacent vertebral bodies.49 Takano et al. studied the estimation of axial compressive and shear loading using a biomechanical model.50 They determined that secondary vertebral compression fractures are caused not only by bone fragility but possibly also by the increase in vertebral stress concentration around the site of the initial fracture. In addition, the increased thoracic kyphosis that can result from anterior wedge fractures increases vertebral loads.45 The centre of mass will also shift to an area anterior to its axis of rotation with an increase in kyphosis, which is a known occurrence in the presence of osteoporotic vertebral fracture and weakness of the extensor muscles of the back. Furthermore, kyphosis is known to increase with successive vertebral fractures.
In the dorsal spine, the increased flexor moment due to trunk flexion increases the risk of new vertebral fractures. Preservation of lumbar lordosis is key to maintaining correct balance in the sagittal plane and a fracture in the lumbar spine will accelerate the degenerative process.51 Overall, this altered mechanical loading scenario may play an important role in contributing to the development of vertebral fracture cascade.
ConclusionsVertebral fracture is a hallmark of osteoporosis. Osteoporotic vertebral fractures are mechanical events that occur when the applied load exceeds the load-bearing capacity of the vertebral body to support the load. The relevant risk factors for fracture can be determined by the fragility of the vertebral bone to the strength of the load applied.
Decreased structural strength is not only the result of reduced bone density, but is also due to changes in bone architecture, bone remodelling, and rate of repair.
Osteoporotic vertebral fractures do not occur uniformly along the spine, but occur more frequently in the mid-dorsal regions, where thoracic kyphosis is maximal, and in the dorsolumbar hinge, due to the transition from a relatively rigid dorsal structure to a more mobile lumbar region.
The risk of suffering a new osteoporotic vertebral fracture is several times higher in those who already have one, compared to those without previous fractures. These secondary vertebral fractures are caused not only by bone fragility but also by increased vertebral stress concentration around the initial fracture site.
Level of evidenceLevel of evidence v.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.