3D-printed patient-specific instruments (PSIs), also known as 3D guides, have been shown to improve accuracy in resection of pelvic tumors in cadaver studies and achieve better surgical margins in vivo. This study evaluates the clinical impact of 3D-printed guides on medium-term local and distant disease control, as well as disease-free and overall survival in patients.
Material and methodsA cohort study included 25 patients with primary pelvic or sacral sarcomas: 10 in the 3D group and 15 in the control group, with a median follow-up of 47 months. Demographic and clinical data, including tumor histology, stage, resection technique, associated reconstruction, adjuvant therapies, and complications, were evaluated. Surgical margins (free, marginal, and contaminated) and relapse-free and overall survival curves were analyzed.
ResultsThe 3D group achieved a higher rate of free margins (80% vs. 66.7%, p=.345). Local recurrence (50% vs. 60%, p=.244) and distant disease relapse (20% vs. 47%, p=.132) rates were lower in the 3D group. At the end of the follow-up, the 3D group had a higher overall survival rate (60% vs. 40%, p=.327). The complication rate was similar in both groups, with a deep infection rate of 40%.
ConclusionsThe use of 3D guides in resecting primary pelvic tumors not only achieves a higher rate of free margins compared to conventional techniques but also shows a trend towards higher local, distant, and overall disease-free survival. Further studies with larger sample sizes and higher levels of evidence are necessary to validate these clinical trends.
La utilización de guías impresas en 3D ha demostrado mejorar la precisión en osteotomías pélvicas en cadáver y obtener mejores márgenes quirúrgicos in vivo. Este estudio analiza su impacto sobre la supervivencia global y libre de enfermedad a medio-largo plazo en los pacientes con tumores del anillo pélvico.
Materiales y métodosSe diseñó un estudio de cohortes con 25 pacientes con sarcomas pélvicos o sacros primarios, 10 en el grupo 3D y 15 en el grupo control, con un seguimiento medio de 47meses. Se compararon los márgenes obtenidos (libres, marginales o intralesionales) y se analizaron las curvas de supervivencia libre de enfermedad y global.
ResultadosLa tasa de márgenes libres fue mayor en el grupo 3D (80 vs. 66,7%; p=0,345). Las tasas de recidiva local (50 vs. 60%; p=0,244) y a distancia (20 vs. 47%; p=0,132) fueron menores en el grupo 3D. La supervivencia global fue mayor en el grupo 3D (60 vs. 40%; p=0,327). La tasa de complicaciones fue similar en ambos grupos (40% de infección profunda).
ConclusionesEl uso de guías 3D en la resección de tumores pélvicos primarios no solo consigue una tasa de márgenes libres superior y un mejor control local de la enfermedad, sino que también muestra una tendencia hacia una mayor supervivencia libre de enfermedad a distancia (87 vs. 53%; p=0,293) y global (60 vs. 40%; p=0,327) a medio plazo.
Tumour resection of sarcomas located in the pelvic ring constitutes a major challenge for the orthopaedic surgeon due to their magnitude and proximity to neurovascular structures and vital organs. According to Cartiaux et al., the probability of achieving adequate tumour margins in pelvic resection (considering a free margin of 10mm with ±5mm error) is only 52% (CI: 37–67%).1 Suboptimal resection margins (marginal or contaminated) are associated with a high rate of local recurrence (28–35%), which has a highly unfavourable prognostic impact.2
In recent years, several tools have been used to improve the accuracy of these margins. Some groups have begun to use CT-assisted navigation3–5 or optical navigation6 with good results. The rapid expansion of 3D printing in the orthopaedic area has brought with it the design and manufacture of patient-specific cutting templates or patient-specific instruments (PSIs), also called 3D guides, to improve the precision of osteotomies in different locations.7–9 PSIs have been used by several groups in complex oncological resection surgery with good results.10–12 They have been shown to be as precise as navigation with the advantage of shorter surgical time13 and potentially lower cost.
Cadaveric studies have confirmed greater precision in pelvic tumour osteotomies with the use of this type of guide.14–16 Although an impact on the local recurrence rate and disease-free survival is expected with the achievement of better resection margins, analytical studies on this subject have not been previously published, except for a single work by the Belgian group of Evrard et al. in 2019.12 This group analysed the effect of using PSIs on local control of the disease (local recurrence rate), but not its impact on the disease-free interval (DFI) or overall survival (OS).
With the purpose of advancing in this line of research, we set ourselves the objective of analysing the rate of adequate resection margins using 3D guides in resection of pelvic sarcomas and their impact, not only on the local and distant recurrence rate, but also on the disease-free and overall survival of these patients. The working hypothesis of this study states that the application of PSIs in the resection of primary tumours of the pelvic ring achieves better local margins, and that this has a positive impact on local control of the disease and the disease-free and overall survival of these patients.
Material and methodPatient inclusion, design and analysisA cohort study was designed in which all patients affected by bone or soft tissue sarcomas of the pelvic ring (pelvis and sacrum) operated on in our centre between 2011 and 2018 were included. Those cases with a diagnosis of pelvic sarcoma that were not treated surgically and those lost to follow-up were excluded.
From a sample size of 25 patients, two groups were identified: a group of 10 patients in whom PSIs were used and a second group of 15 in whom conventional resection was performed. We had prior approval from the ethics committee of our centre.
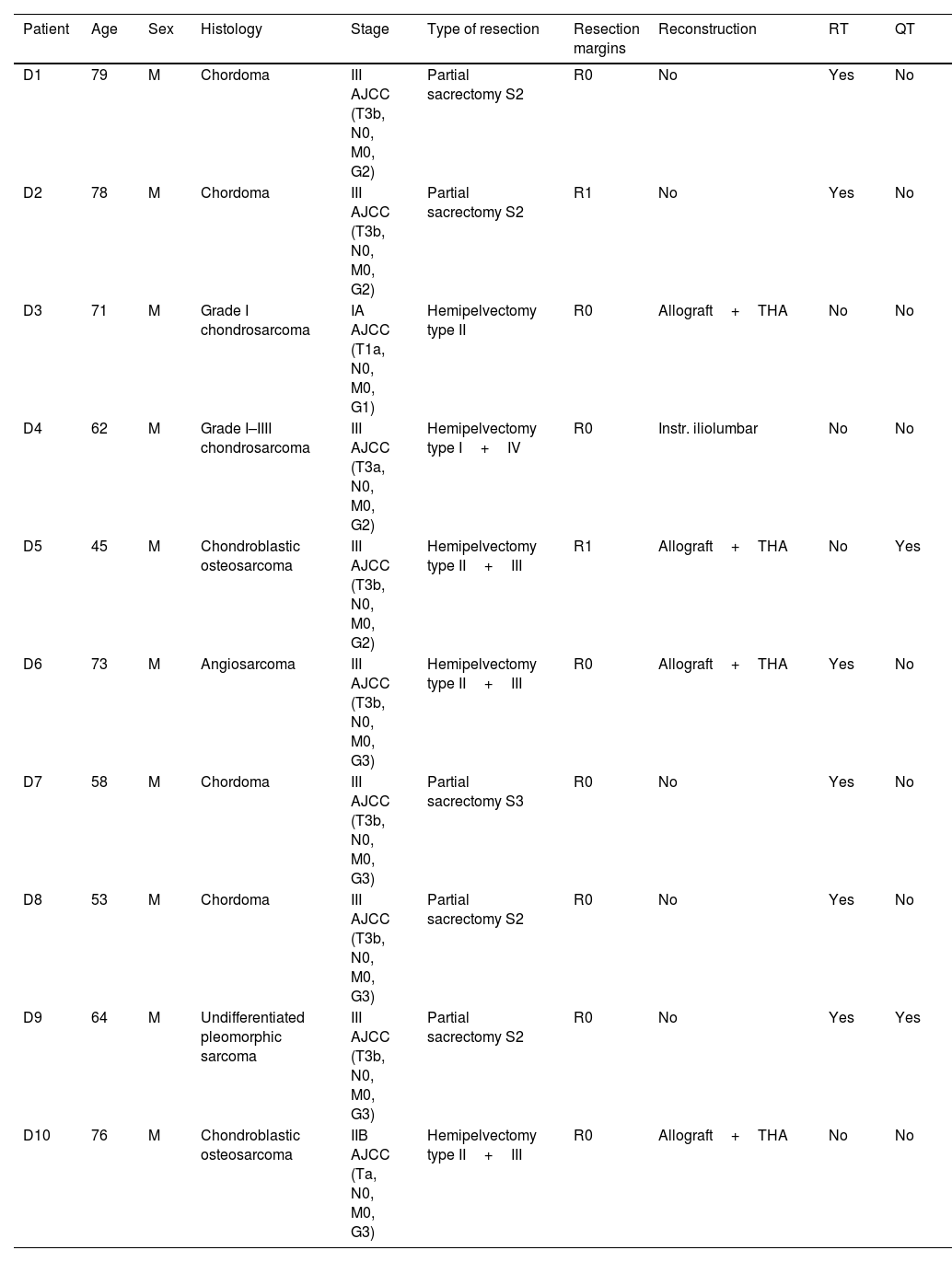
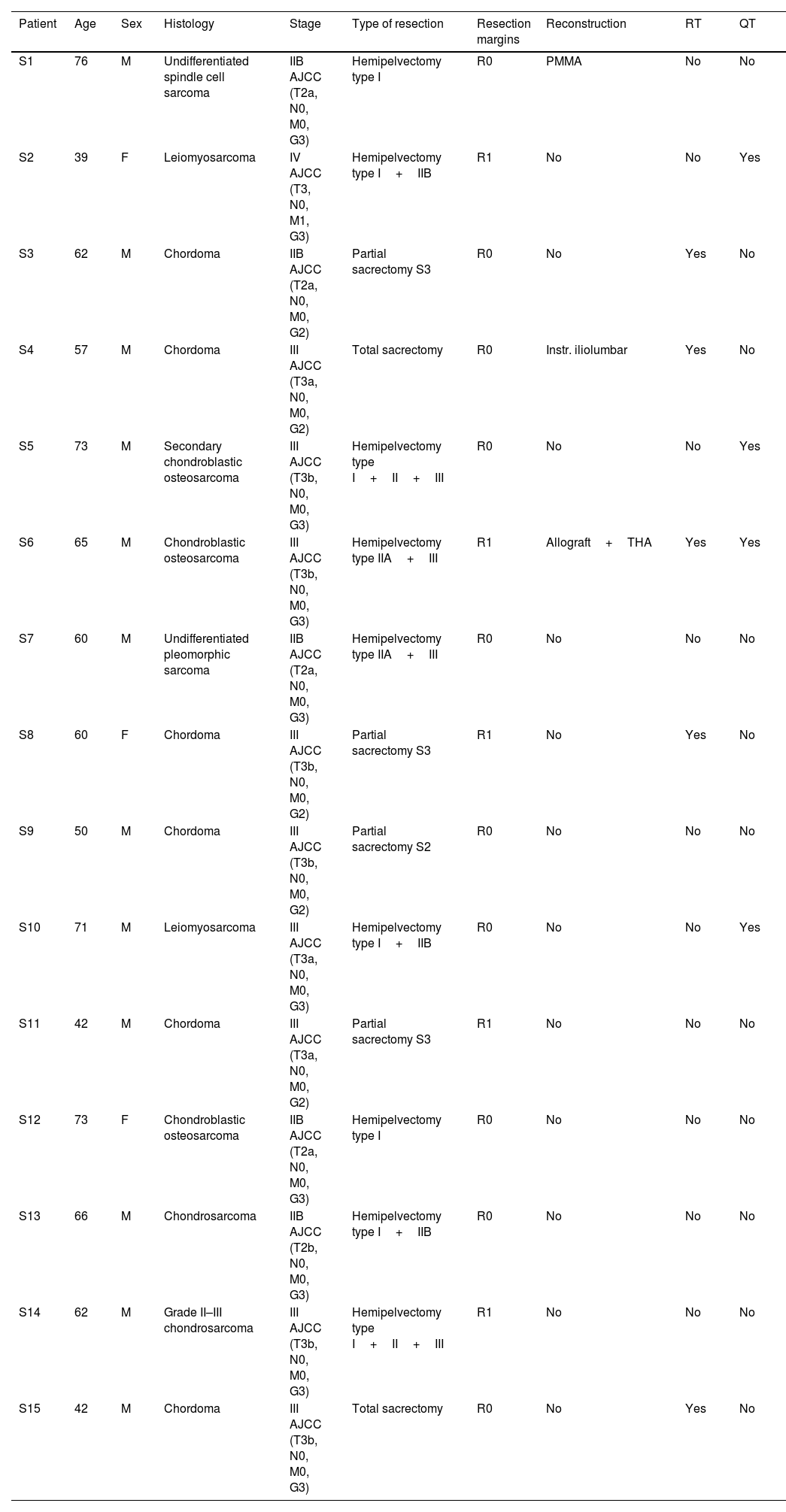
Demographic and clinical data were collected from all patients, including histological type, tumour stage, type of resection, associated reconstruction and adjuvant treatment (Tables 1 and 2). The resection margins obtained in each group were evaluated using the international UICC system: R0, R1 and R2. We defined R0 margins or free margins as those with at least 1mm of healthy tissue around the tumour; R1 or marginal resection as that with <1mm of healthy tissue and R2 or intralesional as that with macroscopically affected margins. Patients were staged according to the American Joint Committee on Cancer (AJCC) 8th edition classification.
Demographic and clinical data of the 3D group.
| Patient | Age | Sex | Histology | Stage | Type of resection | Resection margins | Reconstruction | RT | QT |
|---|---|---|---|---|---|---|---|---|---|
| D1 | 79 | M | Chordoma | III AJCC (T3b, N0, M0, G2) | Partial sacrectomy S2 | R0 | No | Yes | No |
| D2 | 78 | M | Chordoma | III AJCC (T3b, N0, M0, G2) | Partial sacrectomy S2 | R1 | No | Yes | No |
| D3 | 71 | M | Grade I chondrosarcoma | IA AJCC (T1a, N0, M0, G1) | Hemipelvectomy type II | R0 | Allograft+THA | No | No |
| D4 | 62 | M | Grade I–IIII chondrosarcoma | III AJCC (T3a, N0, M0, G2) | Hemipelvectomy type I+IV | R0 | Instr. iliolumbar | No | No |
| D5 | 45 | M | Chondroblastic osteosarcoma | III AJCC (T3b, N0, M0, G2) | Hemipelvectomy type II+III | R1 | Allograft+THA | No | Yes |
| D6 | 73 | M | Angiosarcoma | III AJCC (T3b, N0, M0, G3) | Hemipelvectomy type II+III | R0 | Allograft+THA | Yes | No |
| D7 | 58 | M | Chordoma | III AJCC (T3b, N0, M0, G3) | Partial sacrectomy S3 | R0 | No | Yes | No |
| D8 | 53 | M | Chordoma | III AJCC (T3b, N0, M0, G3) | Partial sacrectomy S2 | R0 | No | Yes | No |
| D9 | 64 | M | Undifferentiated pleomorphic sarcoma | III AJCC (T3b, N0, M0, G3) | Partial sacrectomy S2 | R0 | No | Yes | Yes |
| D10 | 76 | M | Chondroblastic osteosarcoma | IIB AJCC (Ta, N0, M0, G3) | Hemipelvectomy type II+III | R0 | Allograft+THA | No | No |
CT: chemotherapy; F: female; Instr.: instrumentation; M: male; RT: radiotherapy; THA: total hip arthroplasty.
Demographic and clinical data of the control group.
| Patient | Age | Sex | Histology | Stage | Type of resection | Resection margins | Reconstruction | RT | QT |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 76 | M | Undifferentiated spindle cell sarcoma | IIB AJCC (T2a, N0, M0, G3) | Hemipelvectomy type I | R0 | PMMA | No | No |
| S2 | 39 | F | Leiomyosarcoma | IV AJCC (T3, N0, M1, G3) | Hemipelvectomy type I+IIB | R1 | No | No | Yes |
| S3 | 62 | M | Chordoma | IIB AJCC (T2a, N0, M0, G2) | Partial sacrectomy S3 | R0 | No | Yes | No |
| S4 | 57 | M | Chordoma | III AJCC (T3a, N0, M0, G2) | Total sacrectomy | R0 | Instr. iliolumbar | Yes | No |
| S5 | 73 | M | Secondary chondroblastic osteosarcoma | III AJCC (T3b, N0, M0, G3) | Hemipelvectomy type I+II+III | R0 | No | No | Yes |
| S6 | 65 | M | Chondroblastic osteosarcoma | III AJCC (T3b, N0, M0, G3) | Hemipelvectomy type IIA+III | R1 | Allograft+THA | Yes | Yes |
| S7 | 60 | M | Undifferentiated pleomorphic sarcoma | IIB AJCC (T2a, N0, M0, G3) | Hemipelvectomy type IIA+III | R0 | No | No | No |
| S8 | 60 | F | Chordoma | III AJCC (T3b, N0, M0, G2) | Partial sacrectomy S3 | R1 | No | Yes | No |
| S9 | 50 | M | Chordoma | III AJCC (T3b, N0, M0, G2) | Partial sacrectomy S2 | R0 | No | No | No |
| S10 | 71 | M | Leiomyosarcoma | III AJCC (T3a, N0, M0, G3) | Hemipelvectomy type I+IIB | R0 | No | No | Yes |
| S11 | 42 | M | Chordoma | III AJCC (T3a, N0, M0, G2) | Partial sacrectomy S3 | R1 | No | No | No |
| S12 | 73 | F | Chondroblastic osteosarcoma | IIB AJCC (T2a, N0, M0, G3) | Hemipelvectomy type I | R0 | No | No | No |
| S13 | 66 | M | Chondrosarcoma | IIB AJCC (T2b, N0, M0, G3) | Hemipelvectomy type I+IIB | R0 | No | No | No |
| S14 | 62 | M | Grade II–III chondrosarcoma | III AJCC (T3b, N0, M0, G3) | Hemipelvectomy type I+II+III | R1 | No | No | No |
| S15 | 42 | M | Chordoma | III AJCC (T3b, N0, M0, G3) | Total sacrectomy | R0 | No | Yes | No |
CT: chemotherapy; F: female; Instr.: instrumentation; M: male; PMMA: polymethylmethacrylate; RT: radiotherapy; THA: total hip arthroplasty.
Complications were recorded in each case, local or distant recurrence if any, and time to recurrence or death. With this, we calculated the local, distant and overall disease-free survival of each patient. Nonparametric statistics such as the Mann–Whitney U test and Fisher's exact test were used to compare the variables between the two groups. In addition, Kaplan–Meier curves were created for the variables disease-free survival until local and distant recurrence and overall survival, applying the log-rank test for analysis.
Clinical–radiological data were collected from the review of medical records and imaging studies included in the Picture Archiving and Communication System (PACS). All statistical tests were performed using SPSS® v.20 software (IBM, Armonk, NY, USA).
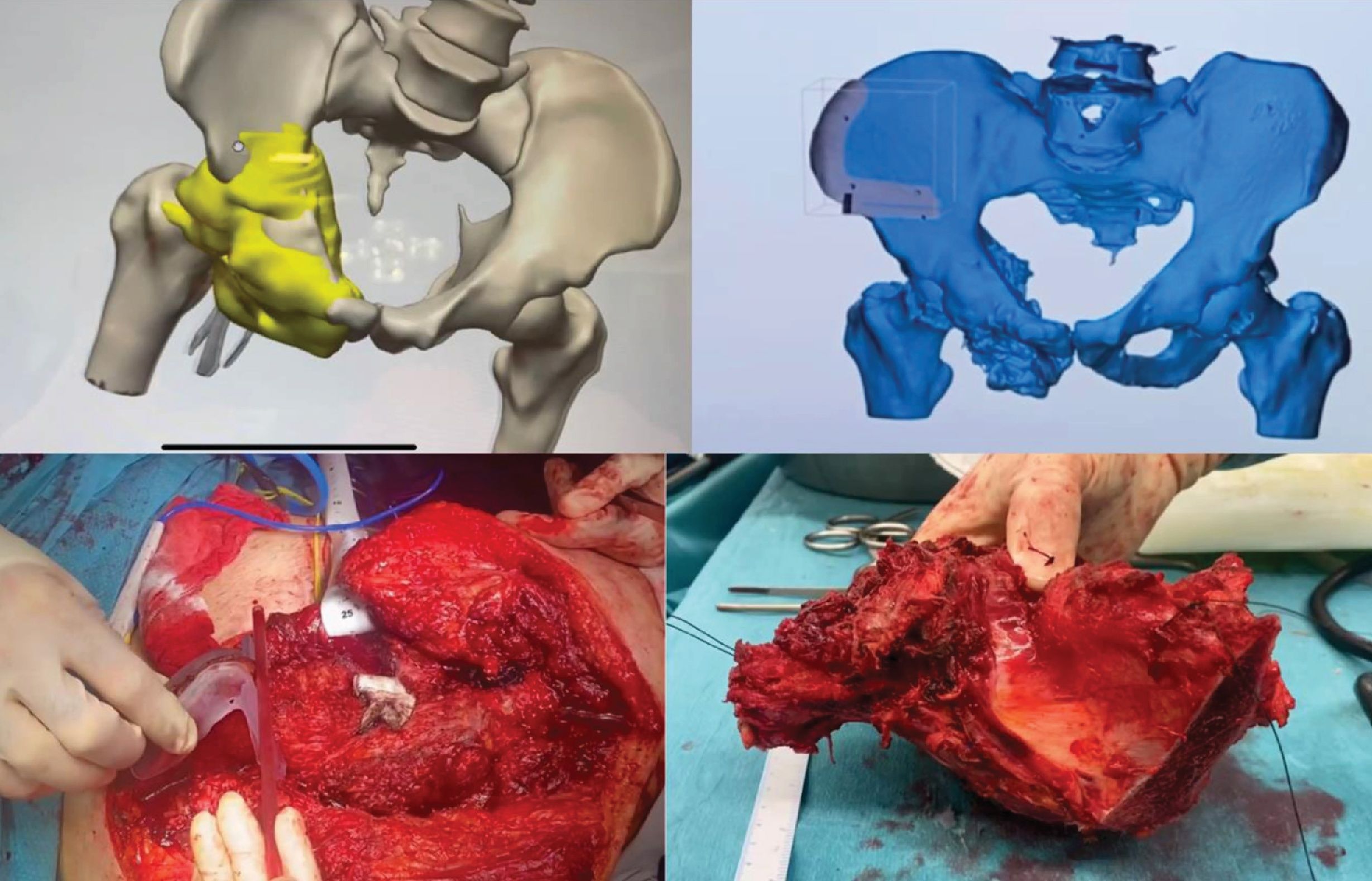
Planning, design and printing of the PSIsThe images obtained from batch studies of the affected area (computed tomography and/or nuclear magnetic resonance) were processed in 3D Slicer, a segmentation software that is able to provide a file in STL format with a three-dimensional replica of both the pelvis and the tumour (Fig. 1A). On this three-dimensional replica, the specific cutting guides for each case were designed using a specific software: Meshmixer® (Autodesk Inc., USA) (Fig. 1B). This generated a new file in STL format with a three-dimensional replica of these templates.
Planning and design of the 3D guides. (A) Three-dimensional model of the pelvis and tumour with the 3D-printed guide highlighted in yellow. (B) Visualisation of the 3D guide attached to the pelvis in a three-dimensional model. (C) Intraoperative image showing the placement of the 3D guide in the patient. (D) The resected specimen, including the tumour, is shown for postoperative verification.
The particular anatomical reliefs were taken into account for the precise coupling of the guide to the bone, together with the adjustment of the cutting plane to the location of the tumour. These files in STL format are recognised by the software used for 3D printing. In these software programmes, different parameters are programmed to improve the printing quality and the hardness and resistance of the models and guides. In preparing the printing of the bioreplicas of both the pelvis and the tumour, the Cura software (Ultimaker®) was used and the printing was done in thermoplastic material (polylactic acid) with a 3D printer model Ultimaker 2 (Ultimaker®) that prints by fused filament deposition. In preparing the guides, the PreForm software (Formlabs®) was used and the printing was completed in biocompatible resin (BioMed Clear Resin, Formlabs®) with a 3D printer model Form2 (Formlabs®) that prints by stereolithography.
The average printing time of the pelvis replicas with the tumour was 10h, while the guides could be obtained in less than 2h. The sterilisation of the pieces was done at a low temperature with ethylene oxide at 55°C to avoid deformation of the material. The design of the guides enables them to fit only in one position on the bone relief to increase their precision. Their provisional fixation was carried out with osteosynthesis material, to provide stability during the use of the oscillating saw for the osteotomy (Fig. 1C).
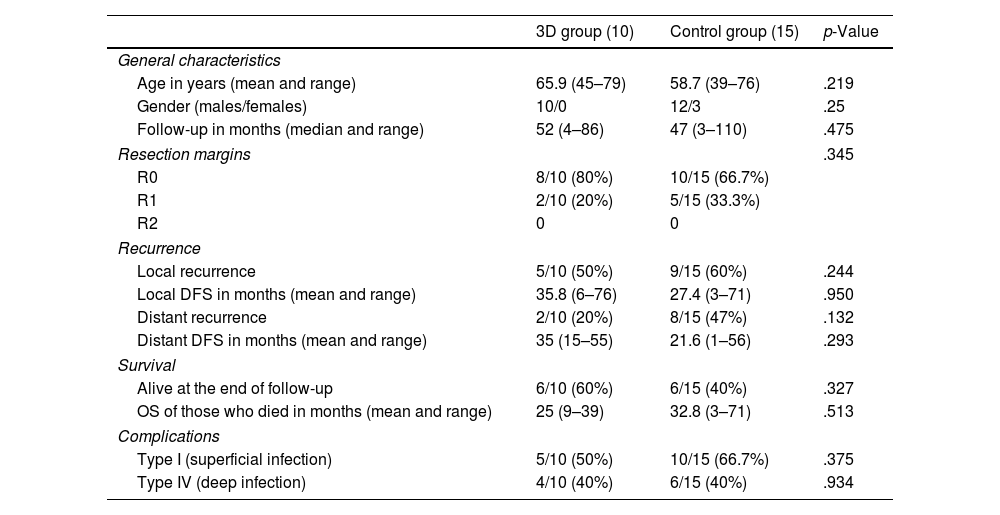
ResultsThe mean patient age was 65.9 years (range: 45–79) in the 3D group and 58.7 years (range: 39–76) in the conventional group. All were men in the 3D group, compared with 80% in the control group. The median follow-up was 52 months (range: 4–86) in the 3D group and 47 months (range: 3–110) in the control group (Table 3).
Comparison of them main study findings between both groups.
| 3D group (10) | Control group (15) | p-Value | |
|---|---|---|---|
| General characteristics | |||
| Age in years (mean and range) | 65.9 (45–79) | 58.7 (39–76) | .219 |
| Gender (males/females) | 10/0 | 12/3 | .25 |
| Follow-up in months (median and range) | 52 (4–86) | 47 (3–110) | .475 |
| Resection margins | .345 | ||
| R0 | 8/10 (80%) | 10/15 (66.7%) | |
| R1 | 2/10 (20%) | 5/15 (33.3%) | |
| R2 | 0 | 0 | |
| Recurrence | |||
| Local recurrence | 5/10 (50%) | 9/15 (60%) | .244 |
| Local DFS in months (mean and range) | 35.8 (6–76) | 27.4 (3–71) | .950 |
| Distant recurrence | 2/10 (20%) | 8/15 (47%) | .132 |
| Distant DFS in months (mean and range) | 35 (15–55) | 21.6 (1–56) | .293 |
| Survival | |||
| Alive at the end of follow-up | 6/10 (60%) | 6/15 (40%) | .327 |
| OS of those who died in months (mean and range) | 25 (9–39) | 32.8 (3–71) | .513 |
| Complications | |||
| Type I (superficial infection) | 5/10 (50%) | 10/15 (66.7%) | .375 |
| Type IV (deep infection) | 4/10 (40%) | 6/15 (40%) | .934 |
OS: overall survival; DFS: disease-free survival.
Both groups presented similar histologies, with chordoma (40% in both) being the most prevalent tumour, followed by osteosarcoma (20% in both), chondrosarcoma (20% in the 3D group and 13.3% in the control group), undifferentiated pleomorphic sarcomas (10% and 13.3%) and other less frequent histologies (leiomyosarcoma, angiosarcoma).
Most patients in the 3D group presented stage III at diagnosis (80%). Of the remaining two, one had a low-grade chondrosarcoma limited to periacetabular zone II (stage IA) and another had osteosarcoma in zone I, stage IIB. In the conventional group, 60% began with stage III, 33.3% with stage IIB and one case with stage IV due to a single renal metastasis of leiomyosarcoma.
In those with sacral involvement, total sacrectomy was performed if the lesion included S1; or partial at the level of S2 or S3 otherwise. Fifty per cent of the patients in the 3D group underwent partial sacrectomy vs. 26.7% in the conventional partial sacrectomy group and 13.3% total sacrectomy. The type of internal hemipelvectomy was planned based on the Enneking zones in which the tumour was located, the most frequent being type II+III in the 3D group (30%) and type I+IIB in the conventional group (20%).
Regarding adjuvant therapy, 6 patients in the 3D group received adjuvant radiotherapy (RT) (60%), preoperatively in 4 of them and postoperatively in 2, associated in all cases with intraoperative radiotherapy (IORT). In the control group, the percentage was similar (53.3%): 3 patients received RT preoperatively (associated with IORT in 2 of the 3 cases), 2 with postoperative RT after a dose of IORT and 2 only IORT as a single dose. Regarding chemotherapy (CT), it was prescribed in 2 patients in the 3D group (20%) with a diagnosis of pelvic osteosarcoma and in 4 patients in the control group (26.7%): neoadjuvant in one case of osteosarcoma and adjuvant in one chondroblastic osteosarcoma (initially diagnosed by chondrosarcoma biopsy) and in the 2 cases of leiomyosarcoma.
No significant differences were observed between the number of complications and the type between the study group and the control group, the most frequent being infections. Deep infections (type IV) occurred in 40% in both groups (p=.934). There were no significant differences in the incidence of superficial infection (associated or not with deep infection), which was 50% in the 3D group and 66.7% in the control group. A lower percentage of superficial infection (associated or not with deep infection) was observed in the 3D guide group (50%) compared to the control group (66.7%), although these differences were not statistically significant (p=.375).
Resection marginsA higher percentage of free margins (R0) was observed in the 3D group (80%) than in the control group (66.7%), differences that were not statistically significant (p=.345) (Table 3). In addition, one of the R1 margins in the 3D group was planned to reduce morbidity in a 78-year-old patient with a history of bladder and colon carcinoma with tumour involvement of the left S1 root. The same trend was observed in the comparison between subgroups (hemipelvectomies and sacrectomies). Twenty per cent of hemipelvectomies in the 3D group presented R1 margins vs. 33.3% in the conventional group (p=.298). Similarly, regarding sacrectomies, only 1 patient out of 5 in the 3D group had marginal resection (20%) vs. 2 out of 6 in the conventional group (33.3%) (p=.234).
Local and distance recurrenceThe local recurrence rate in the group in which PSIs were used was 50%, while in the control group it was 60% (p=.244) (Table 3). Among the patients who suffered local recurrence, a higher local disease-free survival (LDFS) was observed in the 3D group (35.8 months, range: 6–76) than in the control group (27.4 months, range: 3–71).
Regarding distant recurrence, the 3D group had a rate of 20% vs. 47% in the control group (p=.132) (Table 3). The distant DFS was also higher in the PSI group (35 months, range: 15–55) than in the control group (21.6 months, range: 1–56).
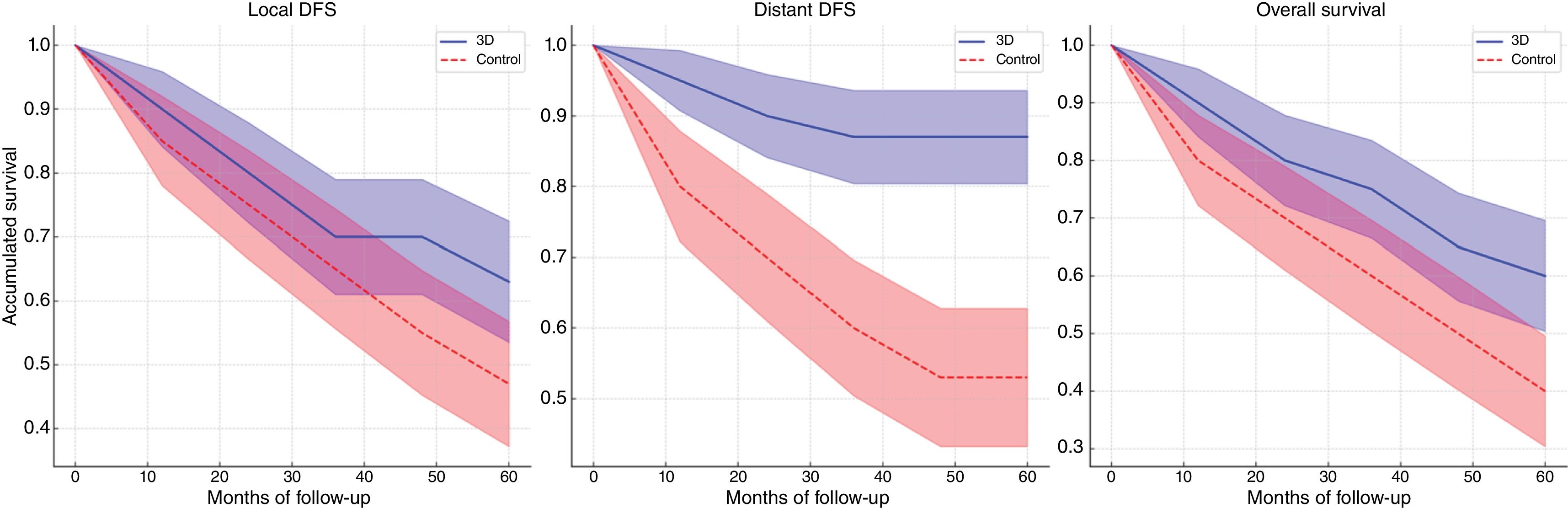
Survival analysisAt 5 years of follow-up, 63% of patients in the 3D group were free of local disease vs. 47% in the control group (log-rank p=.950) (Fig. 2A). The same is true for distant DFS, with 87% of patients in the 3D group remaining free of distant metastasis at 5 years vs. only 53% in the control group (log-rank p=.293) (Fig. 2B).
At the end of follow-up, a higher survival rate was observed in the 3D group (60%) vs. the control group (40%) (p=.327). In fact, at 5 years, 60% of patients in the 3D group were still alive vs. 47% in the conventional group (Fig. 2C) (log-rank p=.513).
DiscussionAmong the possible applications of 3D printing technology in musculoskeletal oncology are patient-specific instruments (PSIs) obtained through 3D planning and printing models,17 especially useful in the resection of tumours in complex locations such as the pelvic area.18 Obtaining adequate surgical margins is an independent prognostic factor for local recurrence (p=.001) and overall survival (p=.025)19 in these patients.
In recent years, several case series have been published in which this type of guide has been used as an aid in the resection of pelvic tumours with favourable results. However, most of them are descriptive studies with relatively short follow-ups: median of 7, 8,20 2410 and 26 months.11 We know that local recurrences occur mainly during the first 3 years after diagnosis and are extremely rare after the first 5 years,21 so we consider a minimum follow-up of 36 months to be adequate for the analysis of the impact of these tools’ use on the local recurrence rate. Our median follow-up was 52 months in the 3D group and 47 in the control group.
It is known that the use of PSIs provides greater precision in tumour resection in pelvic sarcomas,15 but only one study to date analyses the clinical impact in the medium term on local control. The study by Evrard et al. demonstrates a lower rate of local recurrence in the medium term (mean follow-up of 52 months) with the use of 3D guides (p=.035).12 From its results the Belgian group infers a probable correlation with a higher overall survival of patients in the 3D group, but fails to analyse this.
Our study is the first to analyse the clinical impact on the rate of distant recurrence and disease-free and overall survival of the use of 3D guides in pelvic sarcomas. The analysis performed in our study shows the achievement of better margins with the use of 3D guides compared to the control group: 80% free margins vs. 66.7%; p=.345. These results are consistent with those published by other groups: 90.9% free margins in the 3D group vs. 63.6% in the control group10; 88.8% vs. 68.4% (p=.479)12; 98% in the 3D case series of Dong et al.11
This higher rate of free margins in the 3D group is correlated in our analysis not only with better local control of the disease (local DFS of 63% vs. 47%, log-rank p=.950) but also with better distant control (distant DFS of 87% vs. 53%, log-rank p=.293). In short, the overall survival rates are substantially higher in the 3D group compared to the control at the end of follow-up: 60% vs. 40% (log-rank p=.513). It can be stated that the use of this type of guides improves the overall survival of these patients by approximately 20% over the standard 5-year overall survival of 40%19–47%22 for these types of tumours.
The data obtained from our analysis show a clinically relevant but are not statistically significant trend. This could be due to the modest sample size (N=25) that decreases the statistical power of the analysis to obtain statistical significance and to the heterogeneity of the histology of the pelvic sarcomas. In fact, local and distant recurrences are a multifactorial event. They are closely related to the quality of surgical margins,2 but depend not only on the resection precision but also on the histological tumour type, grade, size, location and the response to adjuvant therapies.22
A limitation to this study is its retrospective design. However, the low incidence of these tumours makes it difficult to recruit a considerable number of patients for a prospective randomised study. In addition, given the evidence in the literature regarding the improvement offered by that this type of guidelines,14,15 a randomised study with patients could be considered by many as ethically unacceptable.
These improvements with 3D guides are achieved with a similar rate of complications to the conventional technique, the most frequent being, as in other series, infection of the surgical bed in 40% of our patients (35.6%23–41%24).
It also appears that the use of 3D guides does not significantly increase surgical times, they actually decrease time compared to other tools such as navigation.13 In our case, it was not possible to compare surgical times between both groups because the data we had regarding the duration of surgery inconsistently included the reconstructive time (in those cases performed in the same surgical act), leading to a confounding bias. However, the work of Evrard et al. shows similar surgical times (p=.887).12
Unlike the Belgian group, in our case all the design, processing and printing of the guides was carried out in the hospital (point of care manufacturing), which optimised time and reduced production costs of the guides.25 The healthcare savings from avoiding a local recurrence would probably cover the cost of producing PSIs for many patients. However, this is only a hypothesis. A cost-effect study would be required to validate the efficiency of the healthcare system's use of these tools.
The conclusion of this study is that the use of 3D-printed patient-specific tools (PSIs) in the resection of primary pelvic tumours not only achieves a higher rate of free margins, but also shows a trend towards greater local, distant and global disease-free survival in these patients. Studies with a larger sample size and level of evidence would be necessary to validate the clinical trend observed from the results of this study.
Level of evidenceLevel of evidence III.
Ethical considerationsThis study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All subjects involved gave their written informed consent. In addition, the study was approved by the Clinical Research Ethics Committee (CEIC for its initials in Spanish) of the health centre.
FundingThis research was conducted thanks to the grant for Research Initiation Projects awarded by the SECOT Foundation in 2023 for the project “Digital transformation in orthopaedic oncology: artificial intelligence and augmented reality for personalized surgery” and the financing of European funds for the project TED2021-132200B-I00/TED2021-129392B-I00 – “Digital transformation in cancer treatment. Artificial intelligence, 3D printing and augmented reality for personalised medicine (IAR3D-ONC)”.
Conflict of interestsThe authors have no conflict of interests to declare.
Our special thanks to the bioengineering team of the UPAM-3D service of our centre for their availability, cooperation and dedication in the care and scientific work.