Reported prevalence for reamputation in diabetic foot is diverse, risk factors are not clear for minor amputations. This study aims to determine the prevalence for reamputation in diabetic foot from minor amputations and to evaluate associated factors for such outcome.
MethodsCross sectional study developed in 2 hospitals. Patients hospitalized for diabetic foot ulcer requiring a minor amputation were included. A descriptive analysis of all variables is presented, as well as prevalence ratios (PR) and a multivariate logistic regression.
ResultsThe prevalence was of 48% for 15 years. Toes were the most frequent minor amputation that required reamputation and above the knee amputation was the most frequent reamputation level (45%). Variables whose PR was associated to reamputation risk were: smoking history (PR 1.32, CI 95%: 1.02–1.67, p=0.03), vascular occlusion in doppler (PR 1.47, CI 95%: 1.11–1.73, p=0.01), revascularization (PR 1.73, CI 95%: 1.31–2.14, p=0.00002), Wagner>3 (PR 1.75, CI 95%: 1.16–1.84, p=0.01) and leucocytosis>11,000 (PR 1.39, CI 95%: 1.07–1.68, p=0.01).
Leucocytosis>11,000, Wagner>3, vascular occlusion in doppler and revascularization were the variables that best predicted the outcome. Furthermore, leucocytosis was the best variable for predicting reamputation (OR 2.4, CI 95%: 1.1–5.6, p=0.04).
ConclusionsReamputation prevalence was 48%. The toes were the minor amputation more frequently requiring reamputation and above the knee was the most frequent reamputation level. Risk for reamputation was associated with variables related to vascular compromise and infection.
La prevalencia de reamputación en pie diabético es variada; los factores de riesgo para reamputación no son claros en amputaciones menores. El objetivo de este estudio es determinar la prevalencia de reamputación en pacientes con pie diabético a partir de amputaciones menores iniciales y evaluar los factores asociados a dicho desenlace.
MétodosEstudio de corte transversal en 2 hospitales. Se incluyó a pacientes hospitalizados para manejo de úlcera por pie diabético con amputación menor. Se realizó un análisis descriptivo de todas las variables, se calcularon razones de prevalencia (RP) y se realizó un análisis de regresión logística multivariada.
ResultadosLa prevalencia fue del 48% en 15 años. El nivel inicial más frecuente de amputación fueron los dedos del pie. La reamputación más frecuente fue supracondílea (45%). Las variables con RP asociadas a riesgo de reamputación fueron: antecedente de tabaquismo (RP 1,32; IC 95%: 1,02-1,67; p=0,03), oclusión arterial en el doppler (RP 1,47; IC 95%: 1,11-1,73; p=0,01), revascularización (RP 1,73; IC 95%: 1,31-2,14; p=0,00002), Wagner >3 (RP 1,75; IC 95%: 1,16-1,84; p=0,01) y leucocitosis> 11.000 (RP 1,39; IC 95%: 1,07-1,68; p=0,01).
Leucocitosis> 11.000, Wagner> 3, oclusión arterial en doppler y revascularización fueron las variables que mejor predicen la reamputación según la regresión logística. La leucocitosis es la que mejor predice este desenlace (OR 2,4; IC 95%: 1,1-5,6; p=0,04).
ConclusionesLa prevalencia de reamputación fue del 48%. La amputación menor que con mayor frecuencia requirió reamputación fueron los dedos del pie y el nivel de reamputación elegido con mayor frecuencia fue el supracondíleo. El riesgo de reamputación se asoció con variables asociadas a daño vascular e infección.
One of the most frequent complications of diabetes mellitus is diabetic foot, and in particular, the development of diabetic foot ulceration (DFU), the risk of which during the course of the disease is 25%.1–6 It is estimated that about 25% of these lesions will not heal during the course of the disease and will require amputation.1,2,5 In turn, these DFUs often present episodes of infection that will require hospital management. The infectious process increases the need for hospitalisation 50 times and even increases the risk of amputation 155 times compared to patients with non-infected ulcers.7–9
It is described in the literature that 50% of patients with minor amputation may require a new amputation (reamputation) within 1–5 years from the first amputation.10–19 The amputation level should aim to control the focus of infection and preserve the greatest possible functional abilities.1,11,12,20–24 The literature reports a prevalence of reamputation in patients with DFU with a previous minor amputation of between 26.7% and 60.7% in the first 5 years.1,2,4,8,25 Defining an ideal initial level is complicated and lies in finding a balance between decreasing the probability of reintervention and achieving the most distal amputation level possible to favour the rehabilitation process.
There is no consensus regarding decision-making for the management of this condition, especially regarding achieving a balance between a safe and optimal level from a functional point of view. In the literature, reports of algorithms and surgical procedures that aim to achieve an amputation as distal as possible are increasingly frequent. Unfortunately, this type of amputation is not without potential complications, which revolve around a higher risk of requiring more proximal reamputations due to uncontrolled infections.6,10–12,23 However, one principle seems to prevail: minor amputations preserve functionality better, but more proximal amputations have a greater chance of healing.11,12,14,21,23
Most of the studies available are descriptive in nature and report an association between several variables and the possibility of failure of minor amputations (failure defined as requiring reamputation). Severe peripheral arterial disease, male sex, advanced age, haemodialysis, initial heel injury, and severe infection have been proposed as poor prognostic factors.1,14,26–30 Despite this, there is still much controversy surrounding the issue and it seems that there is no “ideal patient” for this type of procedure. It is for this reason that this study was carried out to establish the prevalence of reamputation from minor amputations.
MethodsA cross-sectional, retrospective, multicentre study was conducted to identify the prevalence of reamputation in minor amputations of the lower limbs. Patients treated by the trauma services of the participating hospitals for the management of DFU were included. An institutional database was analysed between January 2006 and December 2021, taking as inclusion criteria patients >18 years of age with a diagnosis of type II diabetes mellitus who had been hospitalised for the management of DFU and who, as a result of this hospitalisation, had required a minor amputation of the lower limbs. Patients who did not have a follow-up of >90 days and those who had not had metabolic, infectious, or vascular studies prior to the initial amputation were excluded.
Amputation levels were defined as follows: major amputation is above the ankle joint (Syme, transtibial, knee disarticulation, supracondylar, hip disarticulation) and minor amputation is below the ankle joint, including the toes (Chopart, Lisfranc, transmetatarsal). Revascularisation procedures carried out by vascular surgery correspond to either implantation of a stent or the performance of a bypass.
A descriptive analysis of all continuous variables was performed, using means, ranges and standard deviation. Regarding categorical variables, percentages and absolute values were used. Prevalence ratios (PR) were calculated with their respective 95% confidence intervals (95% CI) according to the variables studied. Additionally, the χ2 test was used to evaluate the frequency of adverse events between groups.
A logistic regression analysis was then performed to estimate the probability of outcome according to the variables studied. Variables that were statistically significant in a previous bivariate analysis or that had clinical relevance described in the literature were included in the model. Using a likelihood ratio test using an ANOVA and evaluating the AIC (Akaike Information Criteria) of each model, the most parsimonious model was chosen.
The data used correspond to the time when the patient was taken for the initial amputation as a reference for the analysis. In the case of reporting a transmetatarsal amputation, this was included in the Lisfranc group. Likewise, when the initial amputation affected the toes, it was considered reamputation if 2 or more toes were reamputated.
The corresponding analyses were performed with the R programme and the standards of the STROBE initiative were followed for the development of the study. The project was previously endorsed by the ethics committees of the participating institutions.
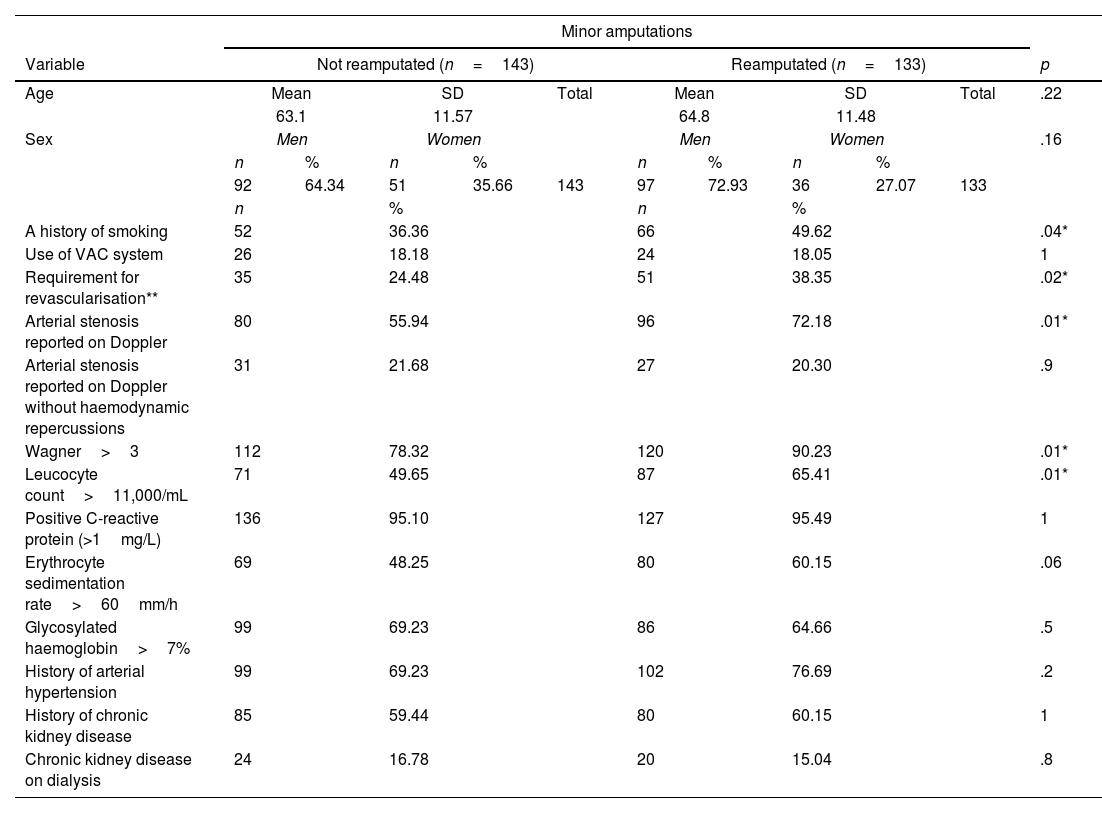
ResultsBetween the two institutions 276 amputations were recorded. Of these 133 required reamputations during the 15-year period. A 48% prevalence of reamputation was determined. The population characteristics are contained in Table 1. In the reamputee group, almost half of the patients had a history of smoking. Similarly, more than 70% of the patients had arterial stenosis reported on Doppler and more than 90% had Wagner 3 or higher (abscesses or necrosis), as well as positive C-reactive protein. Similarly, similar proportions of chronic kidney disease and dialysis were identified between the 2 groups.
Patient characteristics with and without reamputation of lower extremities.
| Minor amputations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Not reamputated (n=143) | Reamputated (n=133) | p | ||||||||
| Age | Mean | SD | Total | Mean | SD | Total | .22 | ||||
| 63.1 | 11.57 | 64.8 | 11.48 | ||||||||
| Sex | Men | Women | Men | Women | .16 | ||||||
| n | % | n | % | n | % | n | % | ||||
| 92 | 64.34 | 51 | 35.66 | 143 | 97 | 72.93 | 36 | 27.07 | 133 | ||
| n | % | n | % | ||||||||
| A history of smoking | 52 | 36.36 | 66 | 49.62 | .04* | ||||||
| Use of VAC system | 26 | 18.18 | 24 | 18.05 | 1 | ||||||
| Requirement for revascularisation** | 35 | 24.48 | 51 | 38.35 | .02* | ||||||
| Arterial stenosis reported on Doppler | 80 | 55.94 | 96 | 72.18 | .01* | ||||||
| Arterial stenosis reported on Doppler without haemodynamic repercussions | 31 | 21.68 | 27 | 20.30 | .9 | ||||||
| Wagner>3 | 112 | 78.32 | 120 | 90.23 | .01* | ||||||
| Leucocyte count>11,000/mL | 71 | 49.65 | 87 | 65.41 | .01* | ||||||
| Positive C-reactive protein (>1mg/L) | 136 | 95.10 | 127 | 95.49 | 1 | ||||||
| Erythrocyte sedimentation rate>60mm/h | 69 | 48.25 | 80 | 60.15 | .06 | ||||||
| Glycosylated haemoglobin>7% | 99 | 69.23 | 86 | 64.66 | .5 | ||||||
| History of arterial hypertension | 99 | 69.23 | 102 | 76.69 | .2 | ||||||
| History of chronic kidney disease | 85 | 59.44 | 80 | 60.15 | 1 | ||||||
| Chronic kidney disease on dialysis | 24 | 16.78 | 20 | 15.04 | .8 | ||||||
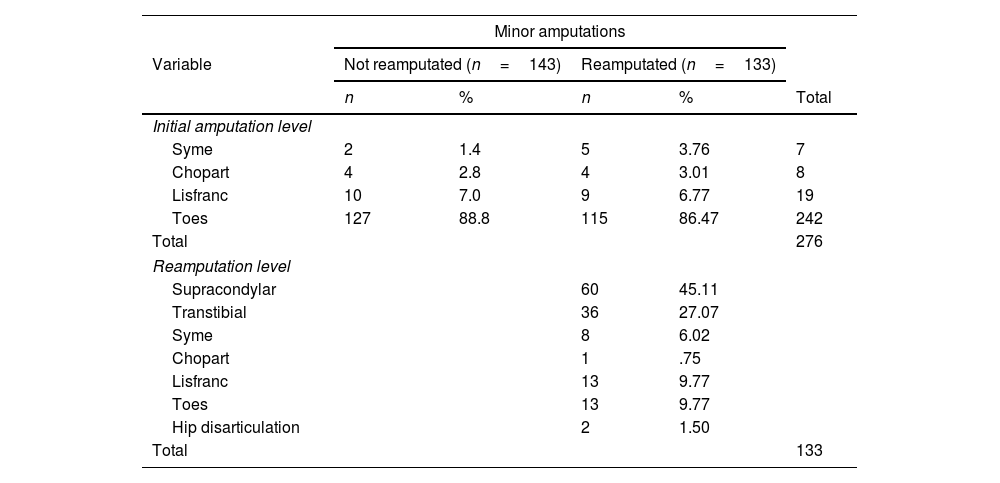
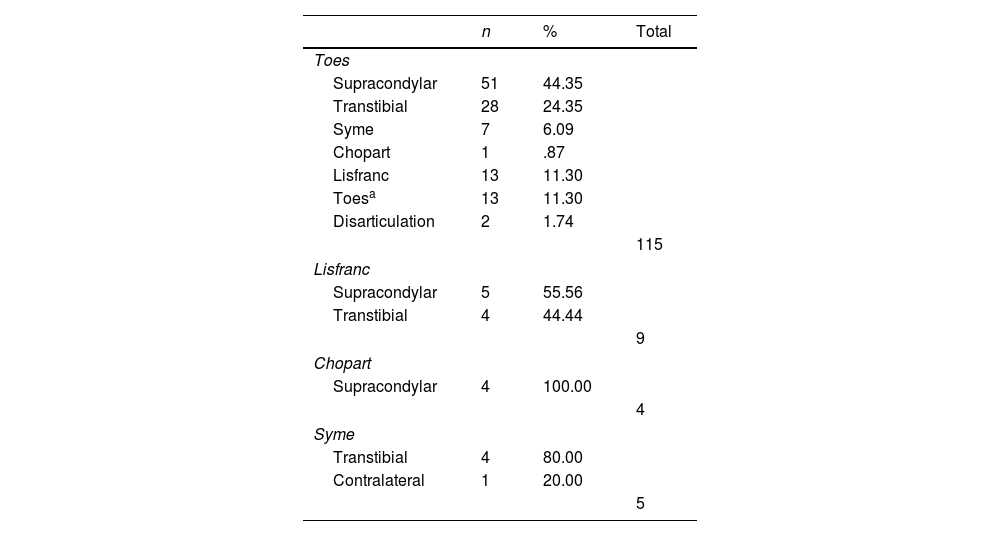
The amputation levels (initial and reamputation) are described in Table 2. In both groups, toe amputation predominated as the initial level. The most frequent reamputation was supracondylar (n=60, 45%). Table 3 describes the levels of reamputation according to the initial level.
Distribution of amputations according to initial amputation level and reamputation level.
| Minor amputations | |||||
|---|---|---|---|---|---|
| Variable | Not reamputated (n=143) | Reamputated (n=133) | |||
| n | % | n | % | Total | |
| Initial amputation level | |||||
| Syme | 2 | 1.4 | 5 | 3.76 | 7 |
| Chopart | 4 | 2.8 | 4 | 3.01 | 8 |
| Lisfranc | 10 | 7.0 | 9 | 6.77 | 19 |
| Toes | 127 | 88.8 | 115 | 86.47 | 242 |
| Total | 276 | ||||
| Reamputation level | |||||
| Supracondylar | 60 | 45.11 | |||
| Transtibial | 36 | 27.07 | |||
| Syme | 8 | 6.02 | |||
| Chopart | 1 | .75 | |||
| Lisfranc | 13 | 9.77 | |||
| Toes | 13 | 9.77 | |||
| Hip disarticulation | 2 | 1.50 | |||
| Total | 133 | ||||
Reamputation was considered when it started with one joint and 2 or more were reamputated.
Distribution of reamputations according to initial amputation level.
| n | % | Total | |
|---|---|---|---|
| Toes | |||
| Supracondylar | 51 | 44.35 | |
| Transtibial | 28 | 24.35 | |
| Syme | 7 | 6.09 | |
| Chopart | 1 | .87 | |
| Lisfranc | 13 | 11.30 | |
| Toesa | 13 | 11.30 | |
| Disarticulation | 2 | 1.74 | |
| 115 | |||
| Lisfranc | |||
| Supracondylar | 5 | 55.56 | |
| Transtibial | 4 | 44.44 | |
| 9 | |||
| Chopart | |||
| Supracondylar | 4 | 100.00 | |
| 4 | |||
| Syme | |||
| Transtibial | 4 | 80.00 | |
| Contralateral | 1 | 20.00 | |
| 5 | |||
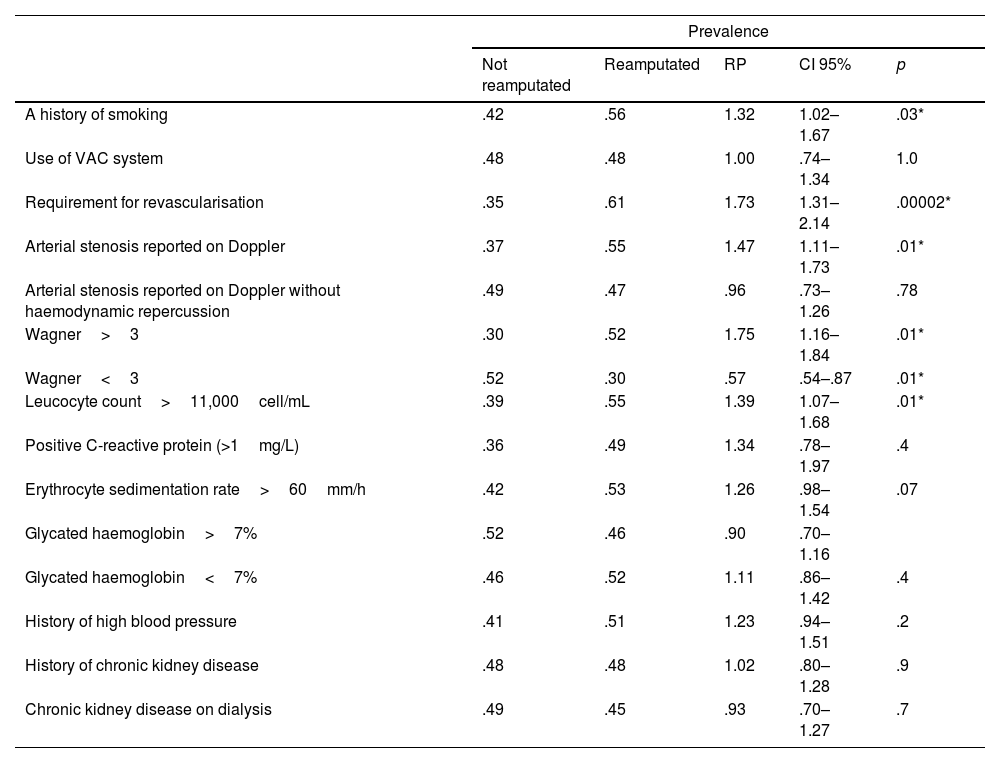
The PRs according to each of the variables are presented in Table 4. The variables with PRs associated with a higher risk of reamputation were: history of smoking (PR 1.32; 95% CI: 1.02–1.67; p=.03), arterial occlusion on Doppler (PR 1.47; 95% CI: 1.11–1.73; p=.01), revascularisation (PR 1.73; 95% CI: 1.31–2.14; p=.00002), Wagner>3 (PR 1.75; 95% CI: 1.16–1.84; p=.01) and leukocytosis>11,000 (PR 1.39; 95% CI: 1.07-1.68; p = .01).
Prevalence ratio according to different variables studied.
| Prevalence | |||||
|---|---|---|---|---|---|
| Not reamputated | Reamputated | RP | CI 95% | p | |
| A history of smoking | .42 | .56 | 1.32 | 1.02–1.67 | .03* |
| Use of VAC system | .48 | .48 | 1.00 | .74–1.34 | 1.0 |
| Requirement for revascularisation | .35 | .61 | 1.73 | 1.31–2.14 | .00002* |
| Arterial stenosis reported on Doppler | .37 | .55 | 1.47 | 1.11–1.73 | .01* |
| Arterial stenosis reported on Doppler without haemodynamic repercussion | .49 | .47 | .96 | .73–1.26 | .78 |
| Wagner>3 | .30 | .52 | 1.75 | 1.16–1.84 | .01* |
| Wagner<3 | .52 | .30 | .57 | .54–.87 | .01* |
| Leucocyte count>11,000cell/mL | .39 | .55 | 1.39 | 1.07–1.68 | .01* |
| Positive C-reactive protein (>1mg/L) | .36 | .49 | 1.34 | .78–1.97 | .4 |
| Erythrocyte sedimentation rate>60mm/h | .42 | .53 | 1.26 | .98–1.54 | .07 |
| Glycated haemoglobin>7% | .52 | .46 | .90 | .70–1.16 | |
| Glycated haemoglobin<7% | .46 | .52 | 1.11 | .86–1.42 | .4 |
| History of high blood pressure | .41 | .51 | 1.23 | .94–1.51 | .2 |
| History of chronic kidney disease | .48 | .48 | 1.02 | .80–1.28 | .9 |
| Chronic kidney disease on dialysis | .49 | .45 | .93 | .70–1.27 | .7 |
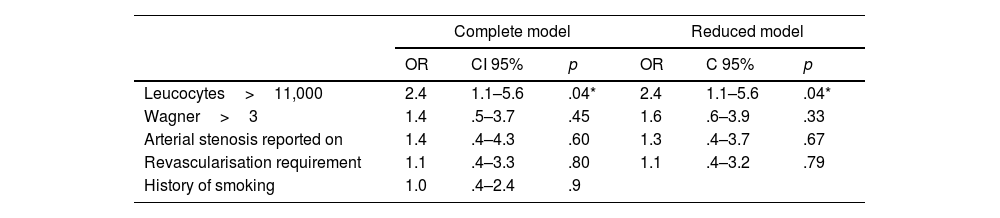
Bivariate analysis identified leukocytosis>11,000cells/mL, arterial occlusion on Doppler, requirement for revascularisation, Wagner>3, and a history of smoking as statistically significant enough to be included in the regression model. Both the full and reduced modelling (most parsimonious model) are presented in Table 5. The final model identifies leukocytosis>11,000cells/mL, Wagner>3, arterial occlusion on Doppler, and requirement for revascularisation as variables that can predict reamputation; however, leukocytosis is the best predictor of outcome (OR 2.4; 95% CI: 1.1–5.6; p=.04).
Logistic regression modelling, full and reduced.
The prevalence of reamputation in DFUs reported by our study corresponds to that described in the current literature, i.e., around 50% over a 15-year period.1,2,4,8,25 The literature has established that the probability of requiring a new ablative intervention is higher in minor amputations than in major ones. However, it is essential to try to preserve the greatest possible length of the limb in order to maintain the patient's function.1,11,12,20–23,31
When performing the descriptive analysis, a higher frequency of reamputation was found in patients whose initial amputation was the toes. It is striking that, frequently in the literature, this level is not considered as a “minor amputation” and the term is reserved for more proximal amputations.1,2,10,14,22,23 Some authors consider that omitting toe amputations leads to underreporting of reamputation procedures, which decreases the detection of the event and, therefore, its prevalence.32 Our results do not reflect an increase in prevalence.
Additionally, it was found that the reamputation level most frequently chosen was the supracondylar level. This indicates that choosing minor amputations as the initial level incurs the risk of requiring a new amputation at some point in the course of the disease that seeks a definitive, more proximal level. Discussion revolves around the fact that a more proximal level compromises functional walking ability, but can avoid readmissions and reinterventions that are costly for the health system if it were chosen as the initial level.11,12,14,21,23
Many studies focus on identifying risk factors to predict the need for reamputation. However, the variables identified are heterogeneous.1,14,26–28,30,32 In the studied population of minor amputations, the variables whose PR were associated with a higher frequency of reamputation were those related to vascular damage (history of smoking, arterial occlusion on Doppler and need for revascularisation) and infection (DFU with Wagner classification>3 and leukocytosis>11,000cells/mL). Although this is consistent with that reported by Thorud et al., Lin et al. and Rathnayake et al., in their systematic reviews, in our study we found there was no association between reamputation and the variables referring to disease status or metabolic control.23,29,31 This may have been due to the characteristics of the population assessed since, by including only hospitalised patients, they already had poor baseline metabolic control regardless of the outcome. Although this may constitute a limitation to our study, it is essential to clarify its scope and to warn about the applicability of its results.
Furthermore, through multivariate logistic regression analysis, it was found that the variable leukocytosis>11,000cells/mL is the one with the best predictive capacity for reamputation in the evaluated cohort. Although it is a highly nonspecific variable, it reflects that patients with a higher degree of infection had a high risk of failure in the healing of the minor amputation and, therefore, of treatment failure. This increases the debate regarding the best approach for the management of these patients, since the intervention of conditions such as arterial occlusion may not have an impact on the eventual need for reamputation. Although it seems that our findings advise against the use of vascular procedures that may prolong the time between amputations, this is not the authors’ intention. However, it is proposed that these procedures be reserved for patients with controlled infections to reduce the rate of therapeutic failures, unnecessary expenses and false patient expectations.
Due to the retrospective nature of the study, information and collection biases are inevitable, since a secondary analysis of an existing database was performed. In addition, it was not possible to include interesting variables, such as the patient's desire not to accept a proximal amputation as an initial level, a variable that has been little studied but has an important weight in decision-making. Finally, although it was not the objective of this study, external validation would be necessary to test the capacity of the model.
ConclusionsThe prevalence of amputations in our patients was 48% over a 15-year period, similar to that reported in the literature. Likewise, the minor amputation that most frequently required reamputation was the toes, and the most frequently chosen reamputation level was the supracondylar. Of the variables studied, the risk of reamputation was most frequently associated with a history of smoking, arterial occlusion on Doppler, the need for revascularisation, an ulcer with Wagner classification>3, and leukocytosis>11,000cells/mL. Finally, the variable with the best ability to predict the outcome was leukocytosis.
Level of evidenceLevel of evidence: iv.
Ethical considerationsAuthorisation was obtained from the corresponding ethical committees.
FundingNo funding was received.