In our environment, it is increasingly necessary to perform an activity based on scientific evidence and the field of prosthetic surgery should be governed by the same principles. The national arthroplasty registries allow us to obtain a large amount of data in order to evaluate this technique. The aim of our study is to analyse the scientific evidence that supports the primary total knee arthroplasties implanted in Catalonian public hospitals, based on the Arthoplasty Registry of Catalonia (RACat).
Material and methodsA review of the literature was carried out on knee prostheses (cruciate retaining, posterior stabilised, constricted and rotational) recorded in RACat between the period 2005–2013 in the following databases: Orthopedic Data Evaluation Panel, PubMed, TripDatabase and Google Scholar. The prostheses implanted in fewer than 10 units (1358 prostheses corresponding to 62 models) were excluded.
Results41,947 prostheses (96.86%) were analysed out of 43,305 implanted, corresponding to 74 different models. In 13 models (n=4,715) (11.24%) no clinical evidence to support their use was found. In the remaining 36 models (n=13,609) (32.45%), level IV studies were the most predominant evidence.
ConclusionsThere was a significant number of implanted prostheses (11.24%) for which no clinical evidence was found. The number of models should be noted, 36 out of 110, with fewer than 10 units implanted. The use of arthroplasty registries has proved an extremely useful tool that allows us to analyse and draw conclusions in order to improve the efficiency of this surgical technique.
En nuestro medio es cada vez más necesario realizar una actividad basada en evidencia científica y el campo de la cirugía protésica deberían regirse por los mismos principios. Los registros nacionales de artroplastias permiten evaluar los resultados de esta práctica. El objetivo de nuestro trabajo es analizar la evidencia científica que respalda los modelos de artroplastia total de rodilla implantados en los hospitales públicos catalanes sobre la base del Registro de Artroplastias de Cataluña (RACat).
Material y métodosSe realizó una revisión de la literatura de las prótesis de rodilla (conserva cruzado posterior, estabilizada posterior, constreñida y rotacional) registradas en el RACat entre 2005 y 2013 en las siguientes bases de datos: Orthopaedic Data Evaluation Panel, PubMed, TripDatabase y Google Académico. Se excluyeron aquellas prótesis implantadas en número inferior a 10 unidades (1.358 prótesis correspondientes a 62 modelos).
ResultadosDe las 43.305 prótesis implantadas, se analizaron 41.947 (96,86%), correspondientes a 74 modelos diferentes. En 13 modelos (n = 4.715) (11,24%) no se encontraron evidencias clínicas que respaldasen su uso. En los 36 modelos restantes (n = 13.609) (32,45%) predominaban los estudios de nivel iv, con una baja evidencia.
ConclusionesExiste un número significativo de prótesis implantadas (11,24%) en las cuales no se ha encontrado evidencia científica. Cabe destacar el número de modelos, 36 de un total de 110, con implantación inferior a 10 unidades. La implantación de un registro de artroplastias se ha revelado como una herramienta extremadamente útil que permite analizar y extraer conclusiones que permitan mejorar la eficiencia de esta técnica quirúrgica.
In the current climate of continuous technological innovations and advances, there are an increasing number of prosthetic implants available to us. In light of this, it is appropriate that we should have information based on scientific evidence on the different models available to us, to promote clinical practice based on evidence-based medicine.
The arthroplasty registries were started through the scientific societies as a method to monitor and assess the various existing prosthetic models after they have been marketed.1–10 Although they cannot replace the methodological rigour of clinical trials, they offer information on long term effectiveness and safety and through their results enable efficiency and quality of care to be improved.11,12
The Arthroplasty Knee Registry of Catalonia (RACat) was set up in 2005 thanks to the common interest of the Catalan Health Service (CatSalut), the Catalan Society of Orthopaedic Surgery and Traumatology (SCCOT) and the Agency for Health Quality and Assessment of Catalonia (AQuAS). The AQuAS was the body in charge of completing the project. Hospitals use a CatSalut computer application to send data on patients, prostheses, surgical interventions and procedures to RACat. Initially, for reasons of plausibility, only hip and knee prostheses were included. This information was provided by 53 of the 61 centres of the Integral Public Healthcare System of Catalonia (SISCAT) that cover more than 85% of public activity.13–15
Countries like the United Kingdom, have set up complementary organisations in addition to an arthroplasty registry under their national health system, such as the Orthopaedic Data Evaluation Panel (ODEP)16 that serves as a benchmark to evaluate data in monitoring various prostheses, many of which have been implanted in our country. We can find recent studies in the literature that use the ODEP platform to demonstrate the level of evidence for all the prostheses planted in the UK. Two clear examples are the systematic total hip prosthesis reviews performed by Kynaston-Pearson et al.,17 and by Chaverri-Fierro et al.,18 in particular, since they used the RACat database.
The objective of this study was to analyse the scientific evidence behind primary knee arthroplasty (PKA) implantation in Catalonia's public hospitals.
Material and methodsSource of informationFrom the information available from the RACat19 models were identified that were implanted in total knee arthroplasty procedures performed in Catalonia between 2005 and 2013. Cruciate retaining, posterior stabilised, constricted and rotating primary total knee prostheses were chosen. Revision, unicompartmental, patellofemoral and tumour prostheses were excluded due to their low incidence. We also excluded implants placed in amounts of 10 units or fewer throughout the study period, since we consider that such a low amount lacks significance.
Search strategyFirst, the ODEP platform was used to identify the models with classified evidence. It operates based on the classification of implants by the number of years of follow-up (3, 5, 7 or 10) and on the quality of available scientific evidence (level A: strong evidence, level B: reasonable evidence, and level C: weak evidence). For the models not registered within the ODEP, a review of the studies published in the literature (national and international) was undertaken using internationally renowned search engines: Pubmed, Tripdatabase and Google Scholar.
The search terms and strategy were “prosthesis name” AND “knee”. The “prosthesis name” used was the commercial name that appears in the RACat, checked on the manufacturer's official website. In the event of any discrepancies, we used both names to perform the search.
Articles found in Spanish, Catalan, English, Italian and French were reviewed and those of potential relevance were chosen. We defined evidence as the publications that evaluated the clinical effectiveness of a particular implant. We excluded in vitro studies or other experimental laboratory techniques, as well as those performed on animals. Subsequently, a level of evidence was assigned to the articles found based on the classification of the Centre for Evidence-Based Medicine, Oxford.20
Two researchers performed the literature search and critical reading separately, then shared the data.
Analysis strategyFinally, the implants were put into order according to level of evidence, breaking them down overall and per category. Furthermore, those for which we found no scientific evidence in the search engines used were identified and classified. The descriptive analysis was performed using frequency and percentage tables. Office Excel 2013 was used for all the analyses.
ResultsOverall resultsDuring the years 2005–2013 the RACat was notified of the implantation of 43,305 prostheses corresponding to 136 models. Applying the abovementioned exclusion criteria, 41,947 prostheses were analysed, corresponding to 74 different models; 36 models (n=140) were implanted in 10 units or fewer, corresponding to 0.33% of the total implanted (Table 1).
Total sample of prostheses implanted and evaluated.
| Total prostheses implanted | 43,305 prostheses (100%) (136 models) |
| Excluded (unicompartimental, femorotibial, tumour, patellofemoral, revision and ≤10 units in number) | 1358 prostheses (3.14%) (62 models) |
| Total prostheses evaluated | 41,947 prostheses (96.86%) (74 models) |
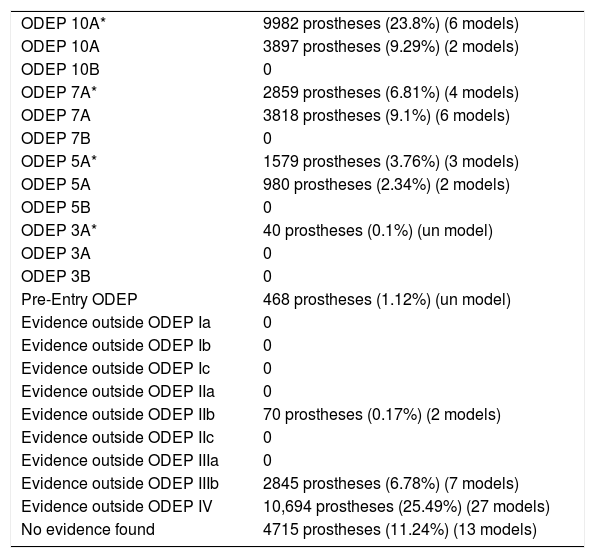
In 13 models (n=4715), 11.24%, no scientific evidence was found on the databases used; 25 models (n=23,623) were classified using the ODEP platform. In the remaining 36 models (n=13,612), the evidence varied according to the number of patients and the years of follow-up, studies with a level of evidence IV predominated (Tables 2 and 3).
Overall classification of the evidence.
| ODEP 10A* | 9982 prostheses (23.8%) (6 models) |
| ODEP 10A | 3897 prostheses (9.29%) (2 models) |
| ODEP 10B | 0 |
| ODEP 7A* | 2859 prostheses (6.81%) (4 models) |
| ODEP 7A | 3818 prostheses (9.1%) (6 models) |
| ODEP 7B | 0 |
| ODEP 5A* | 1579 prostheses (3.76%) (3 models) |
| ODEP 5A | 980 prostheses (2.34%) (2 models) |
| ODEP 5B | 0 |
| ODEP 3A* | 40 prostheses (0.1%) (un model) |
| ODEP 3A | 0 |
| ODEP 3B | 0 |
| Pre-Entry ODEP | 468 prostheses (1.12%) (un model) |
| Evidence outside ODEP Ia | 0 |
| Evidence outside ODEP Ib | 0 |
| Evidence outside ODEP Ic | 0 |
| Evidence outside ODEP IIa | 0 |
| Evidence outside ODEP IIb | 70 prostheses (0.17%) (2 models) |
| Evidence outside ODEP IIc | 0 |
| Evidence outside ODEP IIIa | 0 |
| Evidence outside ODEP IIIb | 2845 prostheses (6.78%) (7 models) |
| Evidence outside ODEP IV | 10,694 prostheses (25.49%) (27 models) |
| No evidence found | 4715 prostheses (11.24%) (13 models) |
During 2005–2013 the RACat was notified of the implantation of 20,709 prostheses (34 models) that retained the posterior cruciate. Applying the abovementioned exclusion criteria, 20,694 prostheses were analysed, 99.93%, corresponding to 30 different models; 4 models (n=15) were implanted in 10 units or fewer, corresponding to 0.07% of the total implanted.
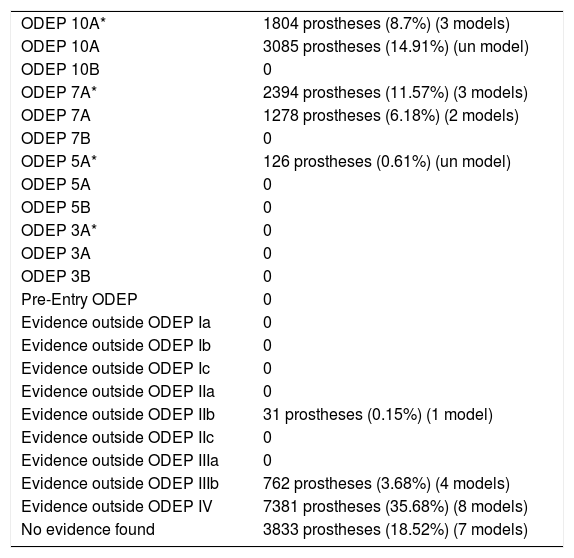
In 7 models (n=3833), 18.52%, no scientific evidence was found on the databases used; 10 models (n=8687), 41.98%, were classified using the ODEP platform. In the remaining 13 models (n=8174), in 39.5%, the evidence varied according to the number of patients and the years of follow-up, studies with a level of evidence IV predominated (Tables 4–6).
Classification of the evidence for CR type implants.
| ODEP 10A* | 1804 prostheses (8.7%) (3 models) |
| ODEP 10A | 3085 prostheses (14.91%) (un model) |
| ODEP 10B | 0 |
| ODEP 7A* | 2394 prostheses (11.57%) (3 models) |
| ODEP 7A | 1278 prostheses (6.18%) (2 models) |
| ODEP 7B | 0 |
| ODEP 5A* | 126 prostheses (0.61%) (un model) |
| ODEP 5A | 0 |
| ODEP 5B | 0 |
| ODEP 3A* | 0 |
| ODEP 3A | 0 |
| ODEP 3B | 0 |
| Pre-Entry ODEP | 0 |
| Evidence outside ODEP Ia | 0 |
| Evidence outside ODEP Ib | 0 |
| Evidence outside ODEP Ic | 0 |
| Evidence outside ODEP IIa | 0 |
| Evidence outside ODEP IIb | 31 prostheses (0.15%) (1 model) |
| Evidence outside ODEP IIc | 0 |
| Evidence outside ODEP IIIa | 0 |
| Evidence outside ODEP IIIb | 762 prostheses (3.68%) (4 models) |
| Evidence outside ODEP IV | 7381 prostheses (35.68%) (8 models) |
| No evidence found | 3833 prostheses (18.52%) (7 models) |
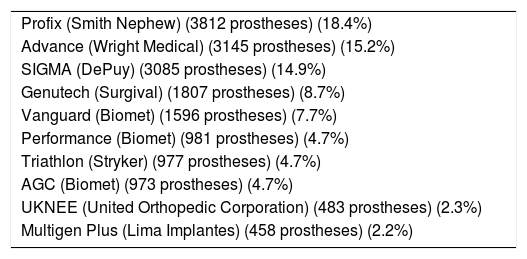
List of the 10 models of CR prosthesis implanted most in Catalonia, according to data notified to the RACat.
| Profix (Smith Nephew) (3812 prostheses) (18.4%) |
| Advance (Wright Medical) (3145 prostheses) (15.2%) |
| SIGMA (DePuy) (3085 prostheses) (14.9%) |
| Genutech (Surgival) (1807 prostheses) (8.7%) |
| Vanguard (Biomet) (1596 prostheses) (7.7%) |
| Performance (Biomet) (981 prostheses) (4.7%) |
| Triathlon (Stryker) (977 prostheses) (4.7%) |
| AGC (Biomet) (973 prostheses) (4.7%) |
| UKNEE (United Orthopedic Corporation) (483 prostheses) (2.3%) |
| Multigen Plus (Lima Implantes) (458 prostheses) (2.2%) |
From 2005 to 2013 the RACat was notified of the implantation of 20,488 posterior stabilised prosthesis (47 models). Applying the abovementioned exclusion criteria, 20,444 prostheses were applied, 99.79%, corresponding to 34 different models; 13 models (n=44) were implanted in 10 units or fewer, corresponding to 0.21% of the total implanted.
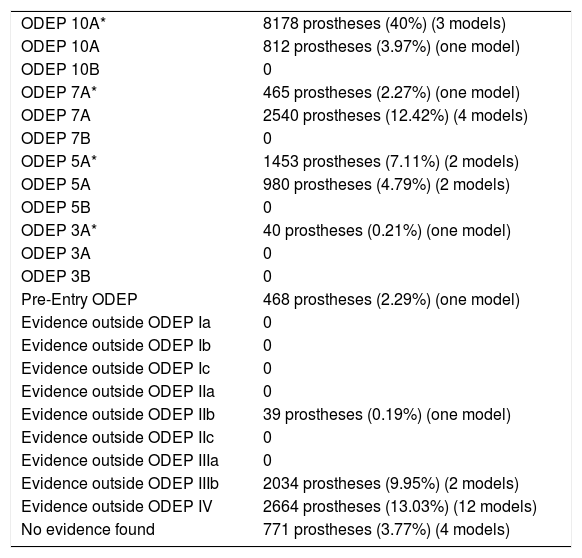
In 4 models (n=771), 3.77%, no scientific evidence was found on the databases used; 15 models (n=14,936), 73.06%, were classified using the ODEP platform. In the remaining 15 models (n=4737), 23.17%, the evidence varied according to the number of patients and the years of follow-up, the studies with a level of evidence ODEP 10A* predominated (Tables 7–9).
Classification of the evidence for PS implants.
| ODEP 10A* | 8178 prostheses (40%) (3 models) |
| ODEP 10A | 812 prostheses (3.97%) (one model) |
| ODEP 10B | 0 |
| ODEP 7A* | 465 prostheses (2.27%) (one model) |
| ODEP 7A | 2540 prostheses (12.42%) (4 models) |
| ODEP 7B | 0 |
| ODEP 5A* | 1453 prostheses (7.11%) (2 models) |
| ODEP 5A | 980 prostheses (4.79%) (2 models) |
| ODEP 5B | 0 |
| ODEP 3A* | 40 prostheses (0.21%) (one model) |
| ODEP 3A | 0 |
| ODEP 3B | 0 |
| Pre-Entry ODEP | 468 prostheses (2.29%) (one model) |
| Evidence outside ODEP Ia | 0 |
| Evidence outside ODEP Ib | 0 |
| Evidence outside ODEP Ic | 0 |
| Evidence outside ODEP IIa | 0 |
| Evidence outside ODEP IIb | 39 prostheses (0.19%) (one model) |
| Evidence outside ODEP IIc | 0 |
| Evidence outside ODEP IIIa | 0 |
| Evidence outside ODEP IIIb | 2034 prostheses (9.95%) (2 models) |
| Evidence outside ODEP IV | 2664 prostheses (13.03%) (12 models) |
| No evidence found | 771 prostheses (3.77%) (4 models) |
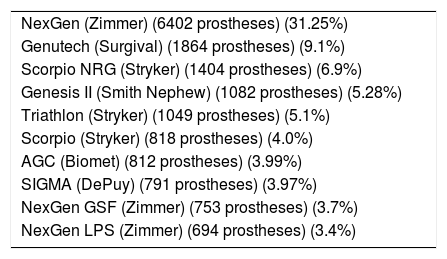
List of the 10 models of PS knee prostheses most implanted in Catalonia, according to data notified to the RACat.
| NexGen (Zimmer) (6402 prostheses) (31.25%) |
| Genutech (Surgival) (1864 prostheses) (9.1%) |
| Scorpio NRG (Stryker) (1404 prostheses) (6.9%) |
| Genesis II (Smith Nephew) (1082 prostheses) (5.28%) |
| Triathlon (Stryker) (1049 prostheses) (5.1%) |
| Scorpio (Stryker) (818 prostheses) (4.0%) |
| AGC (Biomet) (812 prostheses) (3.99%) |
| SIGMA (DePuy) (791 prostheses) (3.97%) |
| NexGen GSF (Zimmer) (753 prostheses) (3.7%) |
| NexGen LPS (Zimmer) (694 prostheses) (3.4%) |
From 2005 to 2013 the RACat was notified of the implantation of 375 constricted prostheses. Applying the abovementioned exclusion criteria, 344 prostheses were analysed, 89.1%, corresponding to 5 different models; 10 models (n=41) were implanted in 10 units or fewer, corresponding to 10.9% of the total implanted.
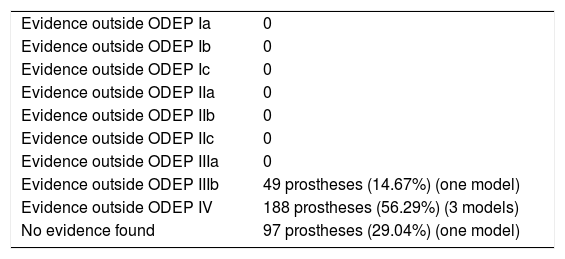
In 1 model (n=97), 29.04%, no scientific evidence was found on the databases used. In the 4 remaining models (n=237), 70.96%, the evidence varied according to the number of patients and the years of follow-up, the studies with a level of evidence IV predominated (Table 10).
Classification of the evidence for constricted implants.
| Evidence outside ODEP Ia | 0 |
| Evidence outside ODEP Ib | 0 |
| Evidence outside ODEP Ic | 0 |
| Evidence outside ODEP IIa | 0 |
| Evidence outside ODEP IIb | 0 |
| Evidence outside ODEP IIc | 0 |
| Evidence outside ODEP IIIa | 0 |
| Evidence outside ODEP IIIb | 49 prostheses (14.67%) (one model) |
| Evidence outside ODEP IV | 188 prostheses (56.29%) (3 models) |
| No evidence found | 97 prostheses (29.04%) (one model) |
From 2005 to 2013 the RACat was notified of the implantation of 515 rotating prostheses. Applying the abovementioned exclusion criteria, 475 prostheses were analysed, 92.23%, corresponding to 5 different models; 9 models (n=40) implanted in 10 units or fewer, corresponding to 7.7% of the total implanted.
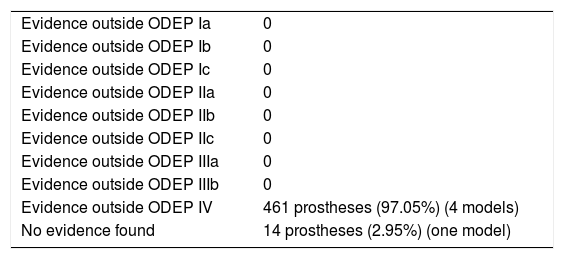
In one model (n=14), 2.95%, no scientific evidence was found on the databases used. In the 4 remaining models (n=461), 97.05%, the evidence varied according to the number of patients and years of follow-up, the studies with a level of evidence IV predominated (Table 11).
Classification of the evidence for rotating implants.
| Evidence outside ODEP Ia | 0 |
| Evidence outside ODEP Ib | 0 |
| Evidence outside ODEP Ic | 0 |
| Evidence outside ODEP IIa | 0 |
| Evidence outside ODEP IIb | 0 |
| Evidence outside ODEP IIc | 0 |
| Evidence outside ODEP IIIa | 0 |
| Evidence outside ODEP IIIb | 0 |
| Evidence outside ODEP IV | 461 prostheses (97.05%) (4 models) |
| No evidence found | 14 prostheses (2.95%) (one model) |
The ODEP database is a useful tool that can help orthopaedic and traumatology specialists to make a rapid and simplified comparison of a multitude of prosthetic models in terms of viability and efficacy based on clinical criteria. However, according to our findings, only 56.31% of the primary knee prostheses implanted in Catalonia over the study period have been classified on this database. The posterior stabilised prostheses present the highest level of ODEP evidence (15 models, 73.06%), followed by the posterior cruciate-retaining prostheses (10 models, 41.98%). There is no ODEP evidence for the constricted and rotating prostheses.
It should be outlined that there was a low level of evidence (level IV) for the studies found outside the ODEP database with small sample sizes and short follow-up periods.
According to our review, no scientific evidence was found to support the use of 11.24% of the primary knee prostheses implanted in Catalonia between 2005 and 2013. Therefore these are similar results to the 2 abovementioned studies: Kynaston-Pearson et al.17 found no evidence for 7.8% of the total hip prosthesis models implanted in the United Kingdom in 2011, while Chaverri-Fierro et al.18 report a lack of scientific evidence for 13.56% of the sockets and 9.53% of the stems of primary total hip prostheses implanted in Catalonia between 2005 and 2013. Therefore, we highlight in this paper the prostheses with the best level of evidence that have demonstrated the best parameters in terms of safety, reliability and clinical efficacy.
Although we performed an extensive literature review, we must take into account the literature that is not indexed in the databases we used and consider the fact, therefore, that there might be relevant literature that we did not identify in our search. An example of this is the paper by Poolman et al.,21 where they collate the total hip prosthesis models implanted in Holland, and note that 25% are not on the ODEP database but are on other databases of more local scope.
Another element to highlight is the use of the commercial name of the implant as a search term in the different articles. There might be papers that refer to the generic name, for example “all-poly prosthesis” and therefore will not be found on the databases. Similarly, we found some errata among the names of the arthroplasty models in the RACat, because certain names did not coincide directly with the manufacturer's name on their official website, resulting in their duplication in the register. Therefore we believe that it is essential that surgeons transcribe the exact manufacturer's name to the RACat to prevent errors and enhance the operation of this very useful tool.
Another limitation would be the studies that are in the stage of publication or in initial stages with inconclusive results, since we have no access to them. Similarly, the language bias should not be forgotten, since we only reviewed the literature published in Spanish, Catalan, English, Italian and French.
The abovementioned limitations prevent us from being absolutely stringent, consequently we have to interpret our results based on these limitations. Thus, not having found any evidence for some implants does not mean that there is no evidence on other local databases, or that it cannot be found using different search criteria than those we used for our study.
Finally, we wish to highlight, on the one hand, the valuable work undertaken by the RACat, without which we would not have been able to complete this study, and on the other, the importance of implementing a national arthroplasty register that enables monitoring, evaluation and analyses such as this.
ConclusionsAlthough there are clinical studies that vouch for the use of most of the prostheses used, there are some prostheses (11.24%) implanted in Catalonia between 2005 and 2013 for which we found no scientific evidence. We must highlight the high number of models, 36 of a total of 110, that were implanted in 10 units or fewer over a period of 9 years, which corresponds to 0.32% of the total.
Implementing an arthroplasty register has been shown to be an extremely useful tool that enables conclusions to be analysed and extracted to improve the efficiency of this surgical procedure.
Level of evidenceLevel of evidence II.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their centre of work regarding patient data confidentiality.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Samaniego Alonso R, Gaviria Parada E, Pons Cabrafiga M, Espallargues Carreras M, Martinez Cruz O. Registro de artroplastias de rodilla de Cataluña: ¿qué evidencia científica respalda la implantación de nuestras prótesis? Rev Esp Cir Ortop Traumatol. 2018;62:290–296.