Vitamin D deficiency or insufficiency is a clinical problem particularly prevalent in elderly patients with low-energy fractures, particularly hip fractures, but has also been associated with stress fractures and high energy fractures.
There is much evidence that supports the need to maintain adequate levels of vitamin D in the blood in order to; reduce the number of fragility fractures, furthering the consolidation of these, improve neuromuscular function of patients, prevent falls, prevent surgical infections, or improve the length of arthroplasties.
However, it is rare for the orthopaedic surgeon to request the values of vitamin D in these patients and give the appropriate treatment.
It is recommended to maintain levels higher than 30–40ng/ml (75–100nmol/l) and increase vitamin D intake, in almost all cases, from 800 to 1000IU/day to achieve these levels.
La deficiencia (insuficiencia o deficiencia) de vitamina D es un problema clínico especialmente prevalente en ancianos con fracturas de baja energía, sobre todo de cadera, aunque también se ha relacionado con fracturas de estrés y de alta energía.
Son muchas las evidencias que apoyan la necesidad de mantener unos niveles adecuados de vitamina D en sangre para reducir el número de fracturas por fragilidad, favorecer la consolidación de las mismas, mejorar la función neuromuscular de los pacientes, evitar las caídas, prevenir las infecciones quirúrgicas o mejorar la duración de las artroplastias.
Sin embargo, no es habitual que el cirujano ortopédico y traumatólogo considere determinar los valores de vitamina D en este tipo de pacientes e instaurar el tratamiento adecuado.
Se recomienda mantener niveles superiores a 30–40ng/ml (75–100nmol/l) de vitamina D y la ingesta, en casi todos los casos, de 800 a 1.000UI/día de vitamina D para alcanzar estos niveles.
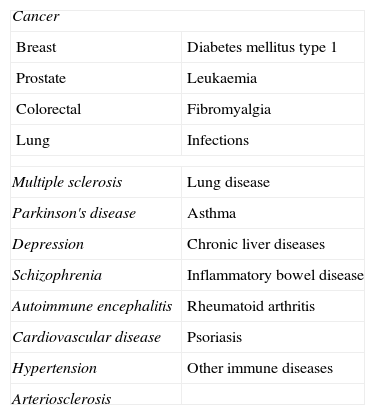
In the last few years there has been a noticeable increase in interest in vitamin D, not only because of its importance in bone-mineral metabolism, but also because of the extraosseous effects about which more and more is being found out regarding this hormonal system (Table 1).
Extraosseous processes vitamin D has been linked to.
| Cancer | |
| Breast | Diabetes mellitus type 1 |
| Prostate | Leukaemia |
| Colorectal | Fibromyalgia |
| Lung | Infections |
| Multiple sclerosis | Lung disease |
| Parkinson's disease | Asthma |
| Depression | Chronic liver diseases |
| Schizophrenia | Inflammatory bowel disease |
| Autoimmune encephalitis | Rheumatoid arthritis |
| Cardiovascular disease | Psoriasis |
| Hypertension | Other immune diseases |
| Arteriosclerosis | |
The fact that low vitamin D serum values have been found in different population groups, both healthy as well as patient populations, has raised the alarm regarding the importance of a deficiency in this vitamin.
From the standpoint of Orthopaedic Surgery and Traumatology (OST), vitamin D deficiency has been linked to poorer neuromuscular co-ordination, a greater incidence of falls, higher incidence of high and low energy fractures, and a higher rate of skeletal and extra-skeletal complications related with their treatment.
For all these reasons, we feel it especially timely to appraise the importance this deficiency has for professionals in this speciality, establishing the fundamental diagnostic and therapeutic criteria for this type of condition.
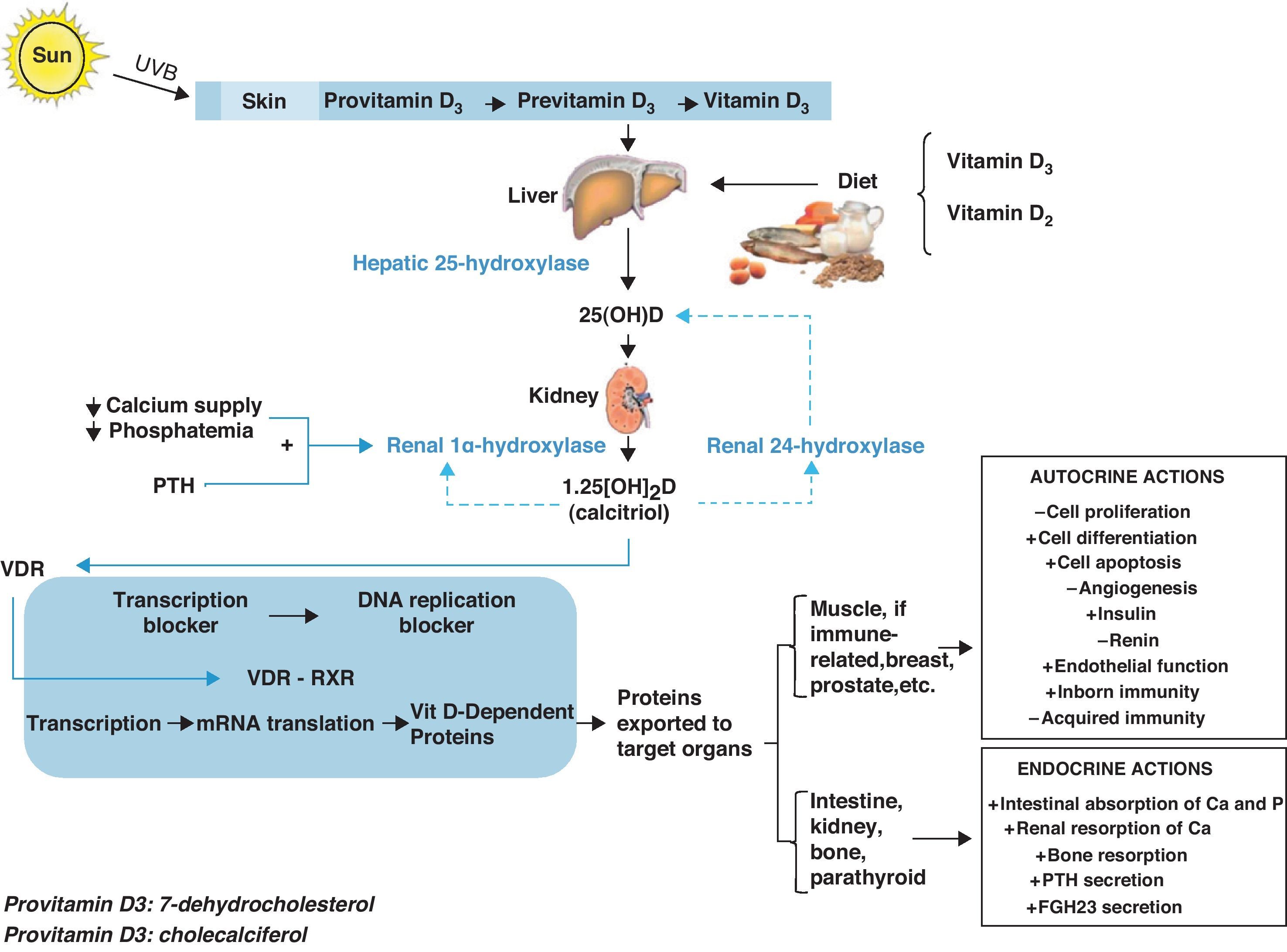
Vitamin D metabolismVitamin D is currently considered to be a hormonal complex capable of regulating the formation of calcium and the absorption of calcium and phosphorus in the intestine.1–4 Whether synthesized in the skin by means of a certain type of ultraviolet B rays (supplying 60–85%) or coming directly from the diet (supplying 15–40%), vitamin D circulates throughout the bloodstream bound to a specific protein transporter (vitamin D binding protein or [DBP]). Thus, the D hormone is transported to the different peripheral target tissues and organs, where it is stored in their fat deposits, and from there pushed to the cells that need it.
On its own, vitamin D is not an active product; instead, it must go through various hydroxylations to reach its biologically active form. The first of these takes place in the liver, where, thanks to hepatic 25-hydroxylase, it is transformed into 25-hydroxycholecalciferol (25[OH]D). The second hydroxylation, in the kidney, is thanks to the action of renal 1α-hydroxylase, which turns it into 1,25 dihydroxyvitamin D3 (1,25[OH]2D), also known as 1,25 dihydroxycholecalciferol or calcitriol, its active metabolite.
In the target tissues and organs, 1,25[OH]2D binds very specifically to certain nuclear receptors, members of the superfamily of nuclear hormone receptors, called vitamin D receptors (NR1l, calcitriol receptor, vitamin D receptor, or VDR),5 which act by activating or inhibiting transcription of the various genes responsible for the synthesis of vitamin D-dependent proteins. VDR has been proved to act by modulating the effects that the active form of vitamin D has on the retinoid X receptor (RXR).6,7
This mechanism of action accounts for the tremendous variety of endocrine actions (related with circulating 1,25[OH]2D derived from the kidney), autocrine actions (linked to the 1,25[OH]2D synthesized locally in target tissues and organs), and paracrine actions that calcitriol has in the body (Fig. 1).
Better insight into vitamin D's various mechanisms of action and its molecular bases has been transcendental in estimating how important it is to attain and maintain adequate levels of 1,25[OH]2D for the proper functioning of any number of biological processes,8–14 which has in some way revolutionized how the treatment of their pathophysiological alterations are approached.15–20
Vitamin D and low energy fracturesFractures resulting from a fall from a height equal to or less than a person's own height, or those that occur in the absence of clear, prior trauma are known as low energy or fragility fractures.
Low vitamin D levels have been related to a higher prevalence of this type of fracture (especially fragility fractures of the hip), largely due to the secondary hyperparathyroidism they induce, although, as we will analyze later on, they might also be related to decreased neuromuscular tone and control, and, hence, to the increased risk of falls that vitamin D deficiency leads to.
From the pathophysiological standpoint, the persistent increase in parathyroid hormone (PTH) levels acts as a powerful stimulator of bone resorption, which determines, on the one hand, a gradual decrease of the amount of bone formed and, on the other, a thinning of all its structural elements, with the consequent lessening of bone resistance all this entails.
Rates of hypovitaminosis D in patients with hip fracture vary depending on the series: 36% in Finland,21,22 40–68% in the United Kingdom,23–25 50–78% in the US,26,27 62–90% in Japan,28,29 67–91% in Spain,30,31 and 96.7% in India.32 Nevertheless, despite this high prevalence, hypovitaminosis D is notoriously underdiagnosed in all these patients, possibly due to different factors,33 among which, the lack of consideration of this condition as an aetiopathogenic agent is undoubtedly noteworthy.
In addition to low energy hip fractures, insufficient vitamin D levels have also been observed in patients with vertebral and non-vertebral fractures excluding hip fractures.22,30–32
In spite of the clear relationship that exists between low energy fractures and vitamin D deficiency, there continues to be a degree of controversy in the literature with respect to its preventive effect. Different meta-analyses34,35 appear to show that the administration of vitamin D by itself is unlikely to prevent fragility fractures, although it does appear to lower the risk of hip fractures, particularly in institutionalized patients, when it is administered together with calcium supplements.
Bischoff-Ferrari et al.35 after analyzing 12 randomized, placebo-controlled clinical trials for non-vertebral fractures (n=42,279 individuals) and 8 randomized clinical trials for hip fractures (n=40,886 individuals) in which vitamin D with or without calcium was compared with calcium or placebo, revealed that the prevention of non-vertebral fractures and hip fractures with vitamin D supplements is dose-dependent. In their study, the higher doses of vitamin D (>400IU) reduced the incidence of non-vertebral fractures in both participants living in the community (−29%), as well as institutionalized patients (−15%) and its effect was independent of the additional calcium supplements. Vitamin D's anti-fracture effect was greater in patients over the age of 70 years, as well as in those who presented low levels of vitamin D at the beginning of the study, and as long as there was due compliance with treatment.
Therefore, and due to the cost-benefit the reduction of this type of fracture implies, authors such as van den Bergh et al.36 propose that all patients with osteoporotic-type fragility fractures should start treatment with 800IU of vitamin D per day. In the same regard, it is worth pointing out that the anti-fracture response of anti-osteoporotic drugs is lower when vitamin D repletion is insufficient.
Vitamin D and stress fracturesNot long ago, interest arose in finding out whether stress fractures appear pathophysiologically in bone that has been structurally altered by the effect of low levels of vitamin D.
Thus, in a cohort study carried out in 600 women with stress fractures of the tibia and fibula, Burgi et al.37 found an inverse relation between the gradient of 25[OH]D and the risk of stress fracture. The risk turned out to be twice as high in women with serum levels of 25[OH]D<20ng/mL versus those who had values of ≥40ng/mL. However, Välimäki et al.38 in a prospective study on the appearance of stress fracture and its aetiopathogenic factors conducted in 179 young people who were carrying out their military service found that stress fracture is associated with high levels of PTH without concomitant vitamin D insufficiency (p=0.022), despite which they, too, recommended taking vitamin D supplements to prevent vitamin D deficiency and lower serum PTH levels.
These findings appear to point toward the existence of a possible genetic predisposition to the appearance of this type of fracture, related or not to vitamin D deficiency. Thus, it has been proved that certain polymorphisms of the vitamin D receptor (VDR) can be linked to the increased risk of stress fracture in military personnel.39,40
Insofar as the administration of vitamin D as a preventive measure in this type of fracture is concerned, Lappe et al.41 showed that the administration of high daily doses of calcium (2g) and vitamin D (800IU) to female recruits for 8 weeks determined a reduction of 20% in the incidence of stress fracture (p<0.003). Along the same lines, Burgi et al.37 contemplate the administration of high doses of vitamin D until target serum concentrations of 25[OH]D>40ng/ml are reached for the prevention of stress fractures. This initial evidence led Tenforde et al.42 to pose the need to conduct new controlled studies that show a lower incidence of stress fracture following preventive treatment with vitamin D.
Vitamin D and high energy fracturesAlthough there is clear scientific evidence that vitamin D insufficiency/deficiency is detected more often in patients with low energy fractures, there are doubts when it comes to agreeing that the incidence of this condition is similarly elevated in patients with high energy fractures.
Regardless of the doubts posed by the fact that this condition has been found in relatively high percentages (25%) of young males with high energy fractures, there are still few studies positing a relationship between high energy fractures and vitamin D insufficiency/deficiency.43,44
In their analysis of risk factors for osteoporosis (including the presence of vitamin D insufficiency/deficiency) in patients over the age of 50 years with vertebral or non-vertebral fractures and densitometric osteoporosis of the spine, Dumitrescu et al.43 failed to find significant differences between fractures associated with high or low energy trauma, suggesting higher or lower prevalence rates of osteoporosis may appear in both types of fracture.
Nevertheless, Steele et al.44 by means of a retrospective analysis of 44 clinical history of patients of between 19 and 95 years of age with non-vertebral fractures, have shown that, in general, vitamin D insufficiency is more common in women than in men (75% vs. 40%), a proportion that is even more conspicuous in the case of high energy fractures (80% vs. 25%). The percentage of vitamin D insufficiency in males with high energy fractures was therefore lower than in females, although in the males, these fractures occurred in significantly younger patients. The authors conclude their work recommending that 25[OH]D levels be assessed in all patients with of low or high energy fractures and that said levels be corrected in accordance with the recommendations of the American Society for Bone and Mineral Research (ASBMR) and the National Osteoporosis Foundation (NOF).
Vitamin D, neuromuscular tone, strength, and controlNeuromuscular tone, strength, and control are constrained, among other factors, by age, diet, and physical exercise, but also possibly by the existence of a relationship among them and specific vitamin D receptors found in striated muscle.45
In this regard, several alleles have been identified (ApaI, BSMIyTaqI)46–49 related in one way or another with muscle strength in studies carried out in both young females and women over the age of 70. Likewise, the diminished muscle tone that takes place with age has been seen to be due to a lower expression of VDR.50 Both circumstances might account for the fact that regardless of their degree of clinical repercussions, the differences in muscle strength linked to vitamin D deficiency are as high as 34%.51
Nonetheless, very recently, the theory of muscle tone and strength modulation as a function of specific vitamin D receptors in striated muscle has come under fire, because they are undetectable in skeletal muscle.52
From a clinical perspective, values of 20ng/mL of 25[OH]D have been seen to be associated with increased body sway when walking,53 as well as a greater shift of the centre of gravity (waddling or duck-like gait), as well as low propioception of the extremity, which implies altered gait control. This alteration might be accounted for by the possible effects of vitamin D on the different components involved in regulating gait, including the muscle component, the neurofeedback component, and the central neurological component.54
Serum 25[OH]D concentrations <10–12ng/mL lead to a clear decrease in muscle strength,53,55–57 2.57 times more than that of patients who have significantly higher vitamin D values.57
Serum values of 25[OH]D<8ng/mL are commonly linked to the presence of frank myopathy, characterized by an important loss of muscle mass (sarcopenia)57,58 with the Folkl allele of the VDR. Myopathy owing to vitamin D deficiency is proximally located and tends to affect the lower limbs, is characterized by muscle weakness, altered gait with increased body sway, difficulty in standing up and going up stairs, and diffuse muscle pain, symptoms sometimes accompanied by paresthesia, joint pain and/or other equally non-specific symptoms that are often the cause of mistakes in clinical diagnosis (rheumatic disease, polymyalgia, psychoneurotic disorders, fibromyalgia, and malignant diseases, etc.).51
The electrophysiological study of this myopathy usually reveals decreased duration, low-amplitude polyphasic motor unit action potentials, without evidence of abnormal transmission in the motor nerve or in the neuromuscular junction. The histological study confirms myopathy and identifies selective atrophy of the fast twitch muscle fibres (type II), which would explain the slower and weaker muscle contraction, as well as the delay in the relaxation phase following muscle contraction.59 As a result of this, the fast twitch muscle response needed to compensate imbalances is altered and this, because it affects the proximal musculature of the legs, tends to entail an increased risk of falls.
All these neuromyopathic alterations are accounted for by the direct pathophysiological mechanism linked to VDR, but also, in part, by other indirect mechanisms, such as the reduction of serum calcium and phosphate secondary to hypovitaminosis D, which would also explain the fact that the relaxation phase in muscle contraction is prolonged. Likewise, in the cases of vitamin D insufficiency/deficiency associated with secondary hyperparathyroidism, the increase of PTH can lead to muscle atrophy and weakness all by itself as a result of the increased rate of intracellular calcium and the decrease in the amount of contractile proteins produced.60
In contrast, serum levels of vitamin D ≥20ng/mL are associated with better functioning of the lower extremity, greater muscle strength,61 and fewer falls, with a positive correlation between 25[OH]D values and the absence of falls.55,56,62–64 This is undoubtedly why vitamin D supplementation at doses of 700–1000IU have been proved to lower the risk of falls by 19% in older people, whereas doses of less than 700IU do not.62 In this same regard, a recent meta-analysis conducted by Bischoff-Ferrari et al.65 has shown that treatment with vitamin D, with or without calcium supplements, reduces the rate of falls by 22%. A secondary analysis of the subgroup of individuals who took vitamin D (700IU/day) with calcium (500mg/day) revealed that falls where decreased by 46% in women, although not in men; this decrease was as high as 65% among less active women.66 In the light of these results, Cameron points out that the vitamin D supplements certainly lower the rate of falls, but not the risk of suffering them.67
The beneficial effect of vitamin D on neuromuscular tone, strength, and function might have to do with a direct effect on the number and size of type muscle fibres on the one hand, and, on the other hand, with protein C kinase activation that fosters increased intracellular calcium reserves that are necessary for proper muscle contraction.
Vitamin D and bone callusDespite the important role vitamin D has in the appearance and treatment of osteoporosis, there is a paucity of works that have focused on the influence on its repair and consolidation, both from a clinical, as well as a basic research perspective.
Melhus et al.68 in an experimental study carried out in vitamin D depleted ovariectomized rats, showed that the mechanical properties of the fracture callus are conserved, which suggest that unaltered callus formation can, in and of itself, compensate for the potentially significant effects of the lack of vitamin D and oestrogens. Along this line, Delgado-Martínez et al.69 indicate that vitamin D deficiency does not worsen consolidation, with the overall mechanical resistance of the callus depending only in part on the prior calcitriol levels.
However, Lindgren et al.70 in their study showed that vitamin D deficiency blocks the formation of mature bone during the repair phase of the fracture, whereas the deposit of osteoid remains unaltered.
Whatever the effects of vitamin D deficiency on the fracture callus, the benefits of vitamin D administration and its metabolites on healing fractures have been firmly demonstrated in experimental models with healthy animals,70,71 vitamin D deficient animals,72–75 and animals with osteoporotic fractures.76 Most of them have evaluated the effects of 1,25[OH]2D3 and 24,25[OH]2D3 and fewer, the action of 1-hydroxycholecalciferol, 25-OH-D,69,77 or that of 1α,25-dihydroxy-2β(3-hydroxypropoxy) vitamin D3 (ED-71).78,79
Although there was initially concern that, following administration of 1,25[OH]2D, the synthesis of type I collagen would be inhibited and there would be a decrease in the levels of procollagen mRNA,80 Omeroglu et al.71 demonstrated that a single high dose of vitamin D3 increases the amount of collagen present in the fracture callus and accelerates the organization of collagen fibres, while also increasing the proliferation and differentiation of osteoprogenitor cells in the callus. It is worth noting that, in this study, a higher degree of vascularization was seen in the fracture calluses in the group treated with vitamin D3 in the early stages of healing of the fracture, an angiogenic effect that was also reported by Hulth et al.81
More specifically, and in subsequent experimental studies, cells derived from the periostium of old rats have been seen to respond very poorly to 1,25[OH]2D3,82 with cancellous bone being where the proliferative response to vitamin D is largely seen. With respect to the combined administration of 1,25[OH]2D and 24,25[OH]2D, Dekel et al.73 have shown that in chickens with vitamin D deficiency this association provides better mechanical properties of the fracture callus. Complementing the previous study, Lidor et al.74,75 demonstrated that vitamin D3 metabolite concentrations increase in the callus and in the proximal epiphysis of the fractured bone, finding a significant correlation between 24,25[OH]2D levels, phosphatase alkaline activity in the first 7 days following the fracture and cartilaginous bone callus formation. In contrast, 1,25[OH]2D was correlated with the degree of mineralization and remodelling of the callus. When Yamane et al.79 they evaluated the effect of the metabolite ED71 (1α,25[OH]2D3), a product analogous to vitamin D that is a powerful inhibitor of bone resorption in situations of oestrogenic depletion,83 they proved in an experimental model of bone distraction that this vitamin D substitute is capable of increasing the volume of the bone callus in the early stages, facilitating the formation and apposition of dense, cortical bone.
From the point of view of clinical research, several authors84,85 appear to have shown the active role played by 24,25[OH]2D in the mineralization of the callus during the process of fracture repair, by finding a significant increase in plasma levels of this metabolite 6 weeks after the fracture versus baseline levels (p<0.05), with increases accompanied by gradual increments of up to 40% of 24–renal hydroxylase.85 Likewise, Jingushi et al.86 demonstrated that the plasma concentration of 1,25[OH]2D falls rapidly starting on the third day following the fracture and that it continues to fall until the tenth day, possibly due to increased absorption by the fracture callus. This increase in the absorption of vitamin D metabolites by the fracture callus enabled Fu et al.76 to suggest that this is the mechanism by which 1,25[OH]2D promotes healing of osteoporotic fractures, by acting on the local bone cells and their receptors. In their work, the authors also assessed the effect of 1,25[OH]2D on the microstructural and biomechanical properties of the bone callus, showing that the maximum resistance of the callus on the flexion test was greater in the 1,25[OH]2D3 group in comparison with the control group. In the light of the results obtained in these pre-clinical and clinical studies, various types of treatment interventions with vitamin D and its metabolites have been proposed to improve the formation of fracture callus, recommending a general prescription of 1,25[OH]2D,76 a combination of 1,25[OH]2D and 24,25[OH]2D, or vitamin D3 and vitamin K187 for patients with fragility fracture in an attempt not only to optimize the consolidation process and the biomechanical result of the callus, but also to enhance osteoporotic bone quality overall. In this same regard, Lidor et al.75 go so far as to promote the local application of 24,25[OH]2D in fragility fractures to accelerate healing and to prevent pseudo-osteoarthritis.
Vitamin D, osteoarthritis, arthroplasty, and prosthetic looseningSeveral works have evaluated the role of vitamin insufficiency/deficiency in osteoarthritis of the hip or knee, in joint replacement surgery and in the process of prosthetic integration and loosening, both experimentally and clinically.
Low 25[OH]D levels have been linked to joint pain88 and to developing osteoarthritis of the knee or hip,89–92 above all, in patients under the age of 60 years92; however, no association was found between the VDR TaqI VDR, BSMI, and ApaI and susceptibility to osteoarthritis in a meta-analysis that included 3372 subjects.93 This possible association with the osteodegenerative disease led Heidari et al. to suggest the advisability of determining the levels of this vitamin in the first stages of the disease.92
In joint reconstruction surgery, and possibly due to the high degree of aggression to tissue, substantial loss of bone mass, and the subsequent inflammatory reaction, it has been shown that, starting on the second day after surgery, there is a significant increase in protein C-reactive concentrations (p<0.001) and a decrease that is also significant, of 25[OH]D (≈40%) (p<0.001), a reduction that is even more striking 3 months following surgery (20–30%; p<0.01).94
If we add to this the fact that many of the patients who have undergone arthroplasty due to osteoarthritis present osteoporosis, vitamin D deficiency27,90,94–96 and secondary hyperparathyroidism,94 it would be logical to think that implant survival would be altered due to the abnormally high rate of bone catabolism. In this regard, Nawabi et al.97 have found significantly higher survival results in patients whose levels of vitamin D are adequate, finding a positive correlation between their serum levels and the post-operative implant evolution.
The most widely accepted hypothesis to explain aseptic loosening of arthroplasties98 proposes that the wear particles induce a chronic inflammatory reaction as a result of the direct stimulation of monocytes and macrophages,99–102 which generates greater local release of enzymes and inflammation mediating substances. This enzymatic cascade, by means of RANK-RANKL (receptor activator of nuclear factor kappa-B, receptor activator of nuclear factor kappa-B ligand) would give rise to greater activation of osteoclasts, thereby producing an increase of bone resorption in the bone–implant interface103 that would lead to prosthetic failure due to loosening.
But, what does vitamin D do in this process? It is known that monocytes and macrophages can differentiate into cells having their own cytochemical and functional characteristics of osteoclasts. This fact has been achieved in vitro using mouse splenic cells in the presence of 1,25[OH]2D and of osteoclast differentiation factor (ODF), RANKL and the macrophage colony-stimulating factor (M-CSF)104 or from macrophages taken from prosthetic loosenings in the presence of 1,25[OH]2D, without the need for M–CSF.105 In the light of these and other studies, Peersman et al.106 suggested the need to confirm the possible negative effects of oral vitamin D3 supplements in patients at risk for prosthetic loosening, above all in individuals with underlying inflammatory processes. In this sense, and from a clinical point of view, Nixon et al.107 determined vitamin D levels in 80 patients with total hip replacement with and without prosthetic loosening and found no significant differences between both groups (t-test; p=0.31), although 19% were vitamin D deficient (<25mmol/L) and 41% presented vitamin D insufficiency (<50mmol/L).
Vitamin D and infectionsVitamin D exerts antimicrobial effects through different mechanisms that have yet to be fully elucidated. In this regard, let us remember that vitamin D receptors are found in almost all tissues, and their circulating levels act directly on macrophages, facilitating neutrophilic motility and phagocytic action by stimulating antimicrobial stimulation, such as beta-defensin 2 and cathelicidin.108
One of the surgical complications that is both most serious and highly complicated to eradicate is infection due to Staphylococcus aureus (S. aureus) and methicillin-resistant S. aureus (MRSA), germs proven109 to contain a potent osteoclast activating stimulator and the main causes of osteomyelitis, arthritis, and septic arthroplasty loosening.
Olsen et al.110 appraised the possibility of vitamin D being a determining factor in nasal colonization by S. aureus. In their study, an increment in the level of vitamin D implied a reduction of colonizations by between 30 and 50%. An earlier study conducted to examine the association between serum levels of vitamin D and the nasal carrier status of MRSA in an institutionalized population in the USA (data from the National Health and Nutrition Examination Survey for the years 2001–2004) revealed that people who are vitamin D deficient had a statistically significant risk of 2.04 of MRSA carriage (95% CI: 1.09–3.84).111
Several research groups are trying to evaluate the possibility that there is a genetic conditioning for this relationship and that different polymorphisms of VDR are involved in it.112–114
ConclusionsVitamin D insufficiency (levels of 25[OH]D of between 20 and 30ng/mL [50 and 75nmol/L]) and deficiency (levels of 25[OH]D below 20ng/mL [<50nmol/L]) are a relevant clinical issue in adult patients who are candidates for orthopaedic and/or trauma surgery and is particularly prevalent in elderly individuals with a hip fracture.
Age (given that skin and renal synthesis of vitamin D decreases with age), a low degree of exposure to the sun, inadequate dietary supply (not compensated by skin synthesis), and functional disability prior to the fracture can act as triggers for this condition.
There is a large body of evidence that corroborates the need to maintain adequate levels of vitamin D in blood to lower the number of fragility fractures, enhance fracture consolidation, improve patients’ neuromuscular function, prevent surgical infections, etc., and yet it is unusual for vitamin D levels to be determined in this type of patient and for appropriate treatment to be initiated.
Hence, the scientific evidence currently available indicates that, irrespective of the fact that, in most cases, there is no adequate laboratory technique or that it is too expensive, determination of vitamin D levels is indicated in all patients presenting an osteoporotic fracture of the hip and in patients with high or low energy fractures and who present risk factors for osteoporosis.
There is a clear criterion largely in favour of advising vitamin D levels greater than 30–40ng/mL (75–100nmol/L) and recommending, in almost all cases, between 800 and 1000IU/day of vitamin D in order to achieve these levels,115 bearing in mind that treatment must be maintained over time, since the benefit attained by supplying vitamin D is lost in great part within 2 years of suspending treatment.116
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Mesa-Ramos M, et al. Aspectos de interés para el cirujano ortopédico y traumatólogo sobre la vitamina D. Rev esp cir ortop traumatol. 2012;56(2):164-173.