The purpose of this work is perform a biomechanical comparison of anatomic reconstruction of the anterior talofibular ligament (ATFL) with the intact ATFL.
Materials and methodsWe studied 18 fresh cadaveric ankles with intact ATFL. Each specimen was clinically assessed with the anterior drawer (AD) and varus tilt (VT) tests and the angular movement in the three spatial planes (axial, coronal and sagittal) was measured with an arthrometer using a sensor located in the talus.
ResultsStatistically significant differences were found in the axial plane, between the intact ATFL versus the sectioned ATFL for AD test with p = 0.012, and for VT test with p = 0.013. Regarding the coronal plane, we also observed a statistically significant difference for VT test with p = 0.016. In the sagittal plane, there are no statistically significant differences in both maneuvers.
No statistically significant differences were found when comparing the biomechanics of anatomic ligament reconstruction versus the intact ATFL.
ConclusionAutograft anatomic reconstruction of the ATFL showed biomechanical properties similar to those of the native ATFL, at the zero moment in a cadaveric model.
El objetivo de nuestro trabajo es valorar si la estabilidad biomecánica que presenta la reconstrucción anatómica del ligamento talofibular anterior (LTFA) es similar a la que exhibe LTFA sano.
Material y métodoRealizamos un estudio biomecánico en cadáver con una muestra de 18 tobillos. Tras la aplicación de las maniobras de cajón anterior (CA) y estrés en varo (EV), medimos con un artrómetro, el movimiento angular en los tres planos espaciales (axial, coronal y sagital) registrado por un sensor localizado en el astrágalo.
ResultadosExisten diferencias estadísticamente significativas en el plano axial entre el comportamiento biomecánico del LTFA intacto y del LTFA seccionado para la maniobra de CA con p = 0.012 y para la maniobra EV con p = 0.013. Por lo que respecta al plano coronal también objetivamos una diferencia estadísticamente significativa con la maniobra EV con p = 0.016. En el plano sagital no existen diferencias estadísticamente significativas con ninguna de las maniobras.
Para finalizar, no existen diferencias estadísticamente significativas, si comparamos la reconstrucción anatómica con injerto del LTFA frente al LTFA intacto con ninguna de las maniobras.
ConclusiónLa reconstrucción anatómica con injerto del LTFA permite reproducir la estabilidad biomecánica del LTFA íntegro, en un modelo cadavérico a tiempo cero.
Sprains are the most common ankle lesion, accounting for 80% of all the injuries to this joint. The lateral ligament complex is affected in 77% of sprains.1 The most frequent injury mechanism is the combination of inversion and plantar flexion. The anterior talofibular ligament (ATFL) functions as the main ankle stabiliser in this position. Additionally, this ligament is the weakest within the lateral ligament complex of the ankle, and it is therefore the one that is injured the most often.2 The initial treatment of ankle sprains is conservative, including a complete rehabilitation program with exercises to strengthen the peroneal muscles and increase appropriate proprioception.3 In spite of correctly applying conservative treatment, failure rates of from 20% to 40% have been described in cases, with the development of chronic lateral instability.4,5
Surgery is indicated when conservative treatment lasting at least six months fails.3,6,7 The first option for surgery is direct or anatomical repair of the ligaments, and this is currently considered to be the “gold standard”.8,9 This technique was described in 1966 by Broström,10 and subsequently several modifications of this technique have been described.11 When the remaining ligament is poor quality, or in patients in which it will not offer good long-term results, the most recommendable technique would be anatomical reconstruction with a graft.12–15
Our work aims to compare the biomechanical stability of anatomical reconstruction with an ATFL graft with leaving the intact ligament, in a series of fresh frozen cadavers. Our working hypothesis is that the anatomical reconstruction of the ATFL makes it possible to reproduce the biomechanical stability of a healthy ATFL, at zero time.
Material and methodCadaveric specimen handlingOur sample is composed of 18 fresh frozen cadaveric ankles with no morphological alterations, deformities or scars. They were obtained according to the Body Donation Centre program of Complutense University, Madrid.
All of the specimens had been frozen for no longer than two years and thawed according to existing guides. In each specimen the tibia together with the fibula was sectioned under the knee joint, keeping at least 20 cm length.
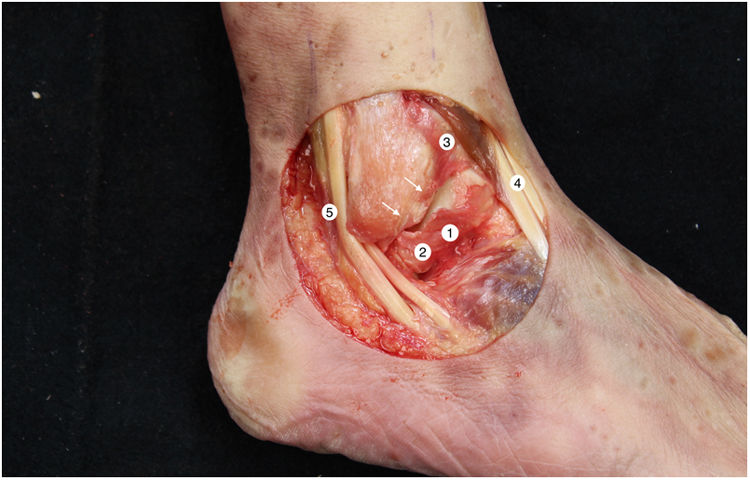
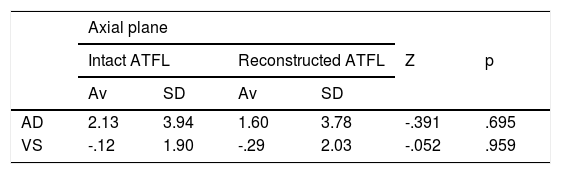
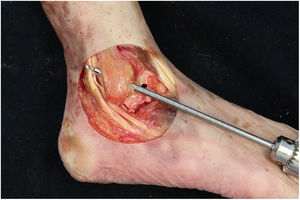
The tibiotalar joint was exposed by raising an anterolateral flap, resecting the skin and subcutaneous tissue, together with part of the anterior capsule, to obtain direct access to the neck and body of the astragalus, the complete external ligament and the tendons of the extensor digitorum longus and the peroneus tertius. We then immediately proceeded to dissect and identify the ATFL. The lateral ligament complex and the medial and syndesmosis ligaments were maintained intact (Fig. 1).
Right ankle lateral anatomy. 1: superior fasciculus of the ATFL, 2: inferior fasciculus of the ATFL, 3: distal anteroinferior tibiofibular ligament, 4: third peroneus + long toe extensor, 5: peroneus tendons. The arrows indicate the anatomical mark left by the ATFL in the peroneus after it was cut. We must recognise this detail in the anatomical reconstruction technique.
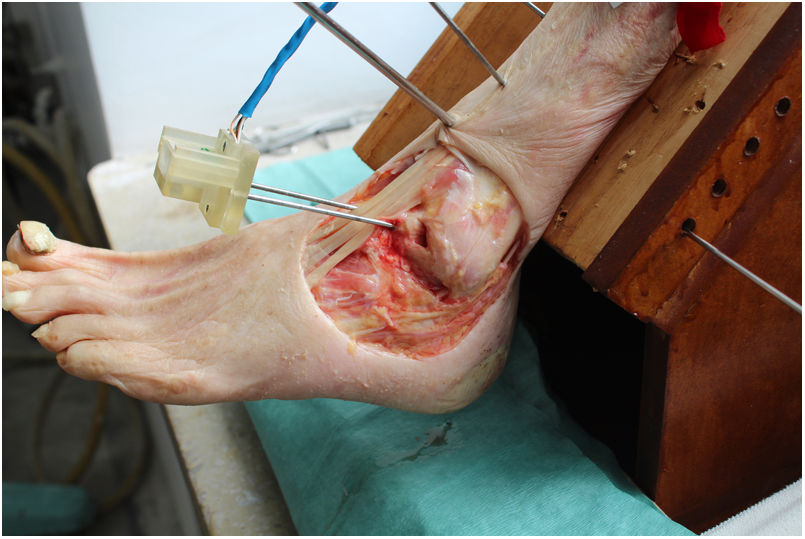
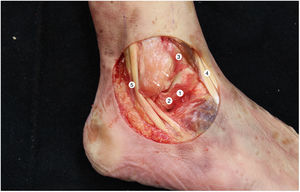
We used an arthrometer that was specifically designed to measure angular displacements in the three anatomical planes (axial, coronal and sagittal) of a sensor located on the astragalus. This instrument made it possible to immobilise the tibia, so that only the complex composed of the talocrural, subtalar and midfoot joints was able to move (Fig. 2).
The device consists of a gyroscope and triaxial accelerometer which records through a computer developed for the use of an Arduino Mega 2560 microcontroller, with the aid of an Mpu6050, an inertial measurement unit (IMU).16 By using a fusion algorithm it was possible to acquire and interpret the data received by the IMU. The software enabled analysis of the angular displacement of the astragalus in real time and simultaneously in three planes using the Tait-Bryan angles.17
Working protocolThe tibia was rigidly affixed by five Kirschner needles (KN) in different planes to a wooden support that was specifically designed for this project, ensuring that there was no mobility between the tibia and the support. The support kept the leg at 45° to the horizontal plane.
Two more KN were inserted into the neck of the astragalus in an anteroposterior direction, along its longitudinal axis. The IMU was then aligned and fixed parallel to the axis, ensuring that the astragalus and the IMU were rigidly connected.
To evaluate stability we use the anterior drawer (AD) and varus stress (VS) manoeuvres, in a similar way to clinical practice.18 The force is applied manually, always by the same researcher and following the same order in performing the stability manoeuvres. Each manoeuvre is repeated three times, and the computer displays the average of the three measurements.
The system was calibrated in the three planes, keeping the ankle in neutral position (plantar flexion: 0 degrees). The same measure was repeated with the intact ankle after allowing the foot to fall to its natural resting position. In the axial plane we define the positive values (x > 0) as external rotation movement, and negative values are defined as internal rotation (x < 0). In the coronal plane we consider positive values to correspond to the movement of inversion, while negative values correspond to eversion. Lastly, in the sagittal plane we define positive values to be plantar flexion and negative values as dorsal flexion. Thus following the performance of each AD and VS manoeuvre we obtain the angular displacement described by the sensor located on the astragalus, in each one of the anatomical planes.
We evaluate the stability of the ankle joint in three situations: with intact ATFL (1), with a simulated lesion of the ATFL by sectioning the ligament (2) and after anatomical reconstruction of the ATFL with a tendon graft (3).
Our working protocol with each cadaveric specimen is as follows:
Firstly, with the external ligament complex intact, we perform the AD and VS manoeuvres, recording the angular mobility of the astragalus in the three anatomical planes.
We then cut the ATFL and repeat both manoeuvres.
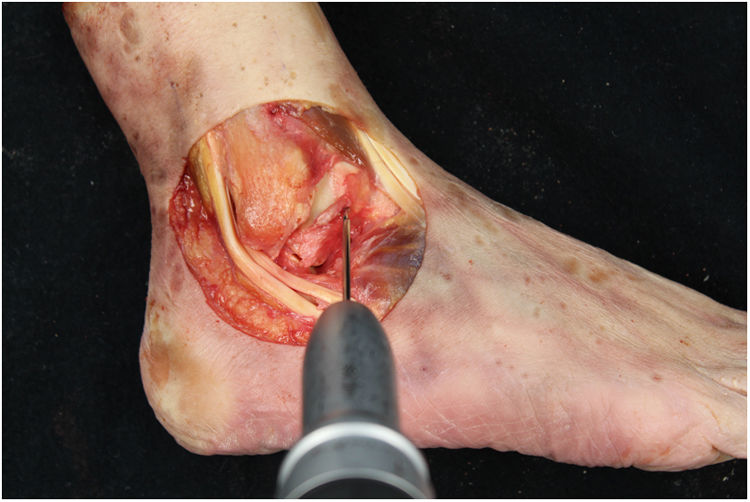
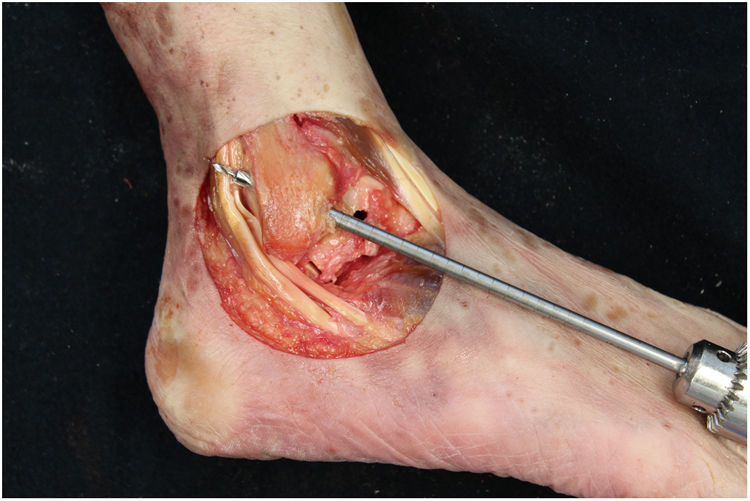
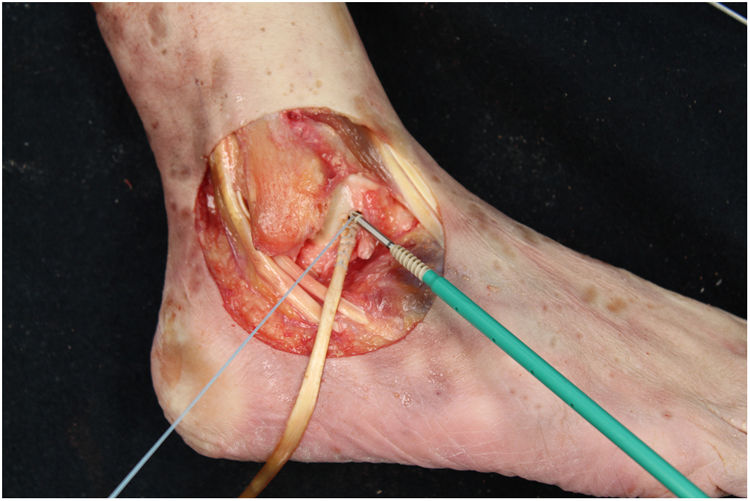
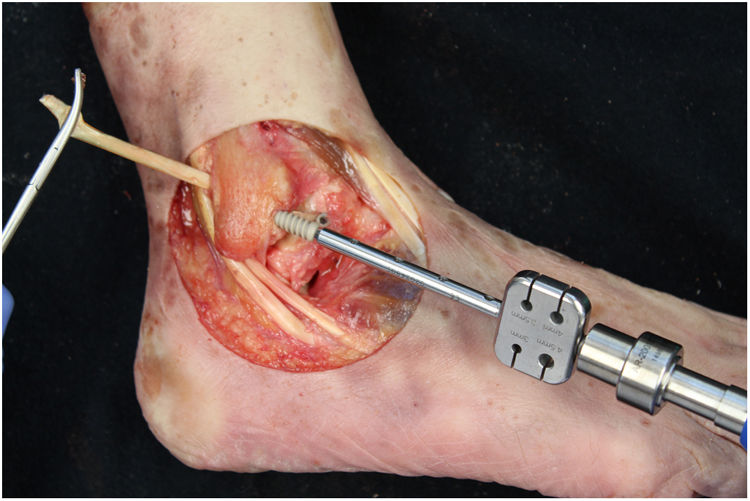
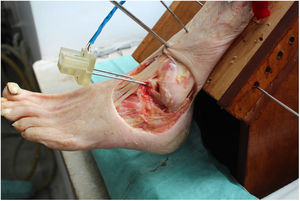
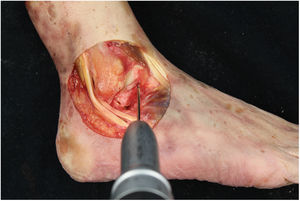
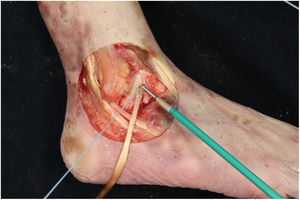
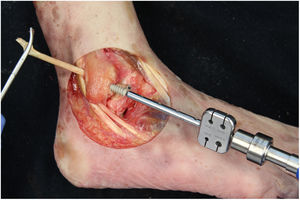
We perform the technique of anatomical reconstruction with plasty of the ATFL. We use the extensor hallucis longus (EHL) tendon from the same cadaver as the donor. The graft measured approximately 4.5 mm in diameter and was at least 100 mm long. We embedded high strength suture in each end (FiberWire, Arthrex Inc., Naples FL, U.S.A.) and subjected it to a tension of 88 N during 10 minutes. It is necessary to identify the marks left by the ligament residues, as these are the references for the anatomic reconstruction (Fig. 1). We drill a 5 mm diameter and 25 mm deep hemitunnel in the astragalus body, in the mark of the talar insertion of the ATFL. We make a second 5 mm diameter tunnel at an angle of 50° starting in the anterior tibia cortex and ending in the proximal posterior cortex in the mark of the fibular insertion of the ATFL (Fig. 3). We insert the plasty into the astragalus hemitunnel and fix it with a 4.75 mm anchorage (SwiveLock Arthrex Inc.) (Fig. 4). We pass the opposite end through the fibular tunnel and stabilise it with a 5.5 × 20 mm Bio-tenodesis screw (BioComposite Tenodesis Screw, Arthrex Inc.) (Fig. 5). During fixation it is important to keep the ankle in a position of slight eversion with neutral dorsiflexion. It is advisable to make each tunnel 0.5 mm larger than the plasty diameter, to facilitate the passage of the same. We repeat both manoeuvres (AD and VS) after the reconstruction technique has been applied (Fig. 6).
All statistical analysis was undertaken using version 24.0 of IBM SPSS (SPSS Inc. Armonk, New York). To evaluate our hypothesis we used Wilcoxon’s non-parametric test to compare the response of the healthy ATFL with the response following the reconstruction technique to the AD and VS manoeuvres in the three anatomical planes. The P value was considered to be significant when it equal to or lower than 0.05.
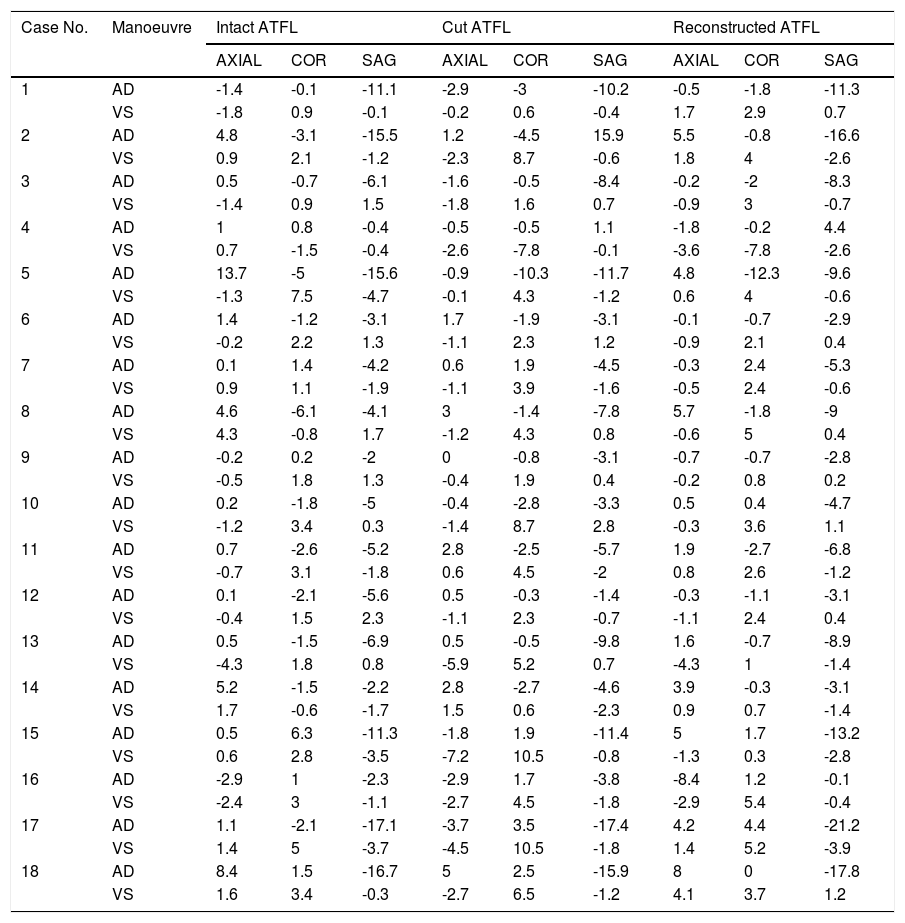
ResultsThe angular movement recorded by the sensor located on the astragalus after the application of the AD and VS manoeuvres is shown in descriptive statistics in Table 1, for each cadaveric specimen and in the three anatomical planes. This is done for each scenario (the complete ATFL, the cut ATFL and the anatomical reconstruction of the ATFL).
Descriptive statistics of the angular movement recorded by the sensor located on the astragalus, after the application of the AD and VS manoeuvres in each cadaveric specimen, in the three anatomical planes and in each one of the said scenarios (Intact ATFL, cut ATFL and anatomical reconstruction of the ATFL).
| Case No. | Manoeuvre | Intact ATFL | Cut ATFL | Reconstructed ATFL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AXIAL | COR | SAG | AXIAL | COR | SAG | AXIAL | COR | SAG | ||
| 1 | AD | -1.4 | -0.1 | -11.1 | -2.9 | -3 | -10.2 | -0.5 | -1.8 | -11.3 |
| VS | -1.8 | 0.9 | -0.1 | -0.2 | 0.6 | -0.4 | 1.7 | 2.9 | 0.7 | |
| 2 | AD | 4.8 | -3.1 | -15.5 | 1.2 | -4.5 | 15.9 | 5.5 | -0.8 | -16.6 |
| VS | 0.9 | 2.1 | -1.2 | -2.3 | 8.7 | -0.6 | 1.8 | 4 | -2.6 | |
| 3 | AD | 0.5 | -0.7 | -6.1 | -1.6 | -0.5 | -8.4 | -0.2 | -2 | -8.3 |
| VS | -1.4 | 0.9 | 1.5 | -1.8 | 1.6 | 0.7 | -0.9 | 3 | -0.7 | |
| 4 | AD | 1 | 0.8 | -0.4 | -0.5 | -0.5 | 1.1 | -1.8 | -0.2 | 4.4 |
| VS | 0.7 | -1.5 | -0.4 | -2.6 | -7.8 | -0.1 | -3.6 | -7.8 | -2.6 | |
| 5 | AD | 13.7 | -5 | -15.6 | -0.9 | -10.3 | -11.7 | 4.8 | -12.3 | -9.6 |
| VS | -1.3 | 7.5 | -4.7 | -0.1 | 4.3 | -1.2 | 0.6 | 4 | -0.6 | |
| 6 | AD | 1.4 | -1.2 | -3.1 | 1.7 | -1.9 | -3.1 | -0.1 | -0.7 | -2.9 |
| VS | -0.2 | 2.2 | 1.3 | -1.1 | 2.3 | 1.2 | -0.9 | 2.1 | 0.4 | |
| 7 | AD | 0.1 | 1.4 | -4.2 | 0.6 | 1.9 | -4.5 | -0.3 | 2.4 | -5.3 |
| VS | 0.9 | 1.1 | -1.9 | -1.1 | 3.9 | -1.6 | -0.5 | 2.4 | -0.6 | |
| 8 | AD | 4.6 | -6.1 | -4.1 | 3 | -1.4 | -7.8 | 5.7 | -1.8 | -9 |
| VS | 4.3 | -0.8 | 1.7 | -1.2 | 4.3 | 0.8 | -0.6 | 5 | 0.4 | |
| 9 | AD | -0.2 | 0.2 | -2 | 0 | -0.8 | -3.1 | -0.7 | -0.7 | -2.8 |
| VS | -0.5 | 1.8 | 1.3 | -0.4 | 1.9 | 0.4 | -0.2 | 0.8 | 0.2 | |
| 10 | AD | 0.2 | -1.8 | -5 | -0.4 | -2.8 | -3.3 | 0.5 | 0.4 | -4.7 |
| VS | -1.2 | 3.4 | 0.3 | -1.4 | 8.7 | 2.8 | -0.3 | 3.6 | 1.1 | |
| 11 | AD | 0.7 | -2.6 | -5.2 | 2.8 | -2.5 | -5.7 | 1.9 | -2.7 | -6.8 |
| VS | -0.7 | 3.1 | -1.8 | 0.6 | 4.5 | -2 | 0.8 | 2.6 | -1.2 | |
| 12 | AD | 0.1 | -2.1 | -5.6 | 0.5 | -0.3 | -1.4 | -0.3 | -1.1 | -3.1 |
| VS | -0.4 | 1.5 | 2.3 | -1.1 | 2.3 | -0.7 | -1.1 | 2.4 | 0.4 | |
| 13 | AD | 0.5 | -1.5 | -6.9 | 0.5 | -0.5 | -9.8 | 1.6 | -0.7 | -8.9 |
| VS | -4.3 | 1.8 | 0.8 | -5.9 | 5.2 | 0.7 | -4.3 | 1 | -1.4 | |
| 14 | AD | 5.2 | -1.5 | -2.2 | 2.8 | -2.7 | -4.6 | 3.9 | -0.3 | -3.1 |
| VS | 1.7 | -0.6 | -1.7 | 1.5 | 0.6 | -2.3 | 0.9 | 0.7 | -1.4 | |
| 15 | AD | 0.5 | 6.3 | -11.3 | -1.8 | 1.9 | -11.4 | 5 | 1.7 | -13.2 |
| VS | 0.6 | 2.8 | -3.5 | -7.2 | 10.5 | -0.8 | -1.3 | 0.3 | -2.8 | |
| 16 | AD | -2.9 | 1 | -2.3 | -2.9 | 1.7 | -3.8 | -8.4 | 1.2 | -0.1 |
| VS | -2.4 | 3 | -1.1 | -2.7 | 4.5 | -1.8 | -2.9 | 5.4 | -0.4 | |
| 17 | AD | 1.1 | -2.1 | -17.1 | -3.7 | 3.5 | -17.4 | 4.2 | 4.4 | -21.2 |
| VS | 1.4 | 5 | -3.7 | -4.5 | 10.5 | -1.8 | 1.4 | 5.2 | -3.9 | |
| 18 | AD | 8.4 | 1.5 | -16.7 | 5 | 2.5 | -15.9 | 8 | 0 | -17.8 |
| VS | 1.6 | 3.4 | -0.3 | -2.7 | 6.5 | -1.2 | 4.1 | 3.7 | 1.2 | |
AD: Anterior drawer; VS: Varus stress; COR: Coronal; SAG: Sagittal.
With the complete ATFL, after the AD manoeuvre the astragalus performed an average angular movement of 2.13° in external rotation, 0.92° eversion and 7.47° dorsal flexion. In contrast, with the cut ATFL the average displacement was 0.19° external rotation, 1.12° eversion and 5.84° dorsal flexion. With the intact ATFL and after the application of VS, the average movement recorded was 0.12° internal rotation, 1.12° eversion and 0.62° dorsal flexion. In contrast with the cut ATFL, the average movement recorded was 1.90° internal rotation, 4.06° inversion and 0.44° dorsal flexion.
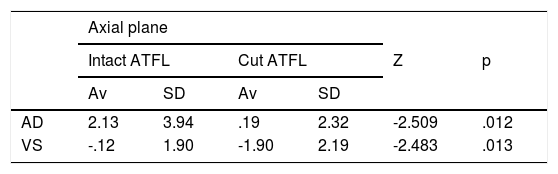
There are statistically significant differences between the biomechanical behaviour of the intact and cut ligament after the AD manoeuvre in the axial plane (P = .012), and after the VS manoeuvre in the axial and coronal planes (P = .013, P = .016, respectively) (Table 2).
Comparison of the intact ATFL vs cut ATFL.
| Axial plane | ||||||
|---|---|---|---|---|---|---|
| Intact ATFL | Cut ATFL | Z | p | |||
| Av | SD | Av | SD | |||
| AD | 2.13 | 3.94 | .19 | 2.32 | -2.509 | .012 |
| VS | -.12 | 1.90 | -1.90 | 2.19 | -2.483 | .013 |
| Coronal plane | ||||||
|---|---|---|---|---|---|---|
| Intact ATFL | Cut ATFL | Z | p | |||
| Av | SD | Av | SD | |||
| AD | -.92 | 2.74 | -1.12 | 3.16 | -.567 | .571 |
| VS | 2.09 | 2.12 | 4.06 | 4.31 | -2.417 | .016 |
| Sagittal plane | ||||||
|---|---|---|---|---|---|---|
| Intact ATFL | Cut ATFL | Z | p | |||
| Av | SD | Av | SD | |||
| AD | -7.47 | 5.58 | -5.84 | 7.34 | -.047 | .962 |
| VS | -.62 | 2.00 | -.44 | 1.34 | -.218 | .827 |
There are statistically significant differences between the intact ATFL and the cut ATFL in the anterior drawer (AD) and varus stress (VS) manoeuvres in the axial plane (Av: average, SD: standard deviation).
There are statistically significant differences between the intact ATFL and the cut ATFL in the varus stress (VS) manoeuvre and in the coronal plane (Av: Average, SD: Standard Deviation).
We found no statistically significant differences between the intact ATFL and the cut ATFL with the anterior drawer (AD) and varus stress (VS) manoeuvres in the sagittal plane (Av: Average, SD: Standard Deviation).
We then evaluate the stability produced by the anatomical reconstruction technique. After the AD manoeuvre, the astragalus had an average angular movement of 1.60° external rotation; 0.83° eversion and 0.779° dorsal flexion. After the application of VS, the average movement recorded is 0.29° internal rotation; 2.29° inversion and 0.767° dorsal flexion. If we compare the biomechanical behaviour of the healthy ATFL with the anatomical reconstruction and plasty, we find no statistically significant differences in the three spatial planes with any of the manoeuvres (Table 3).
Comparison of the intact ATFL and the ATFL reconstruction with plasty.
| Axial plane | ||||||
|---|---|---|---|---|---|---|
| Intact ATFL | Reconstructed ATFL | Z | p | |||
| Av | SD | Av | SD | |||
| AD | 2.13 | 3.94 | 1.60 | 3.78 | -.391 | .695 |
| VS | -.12 | 1.90 | -.29 | 2.03 | -.052 | .959 |
| Coronal plane | ||||||
|---|---|---|---|---|---|---|
| Intact ATFL | Reconstructed ATFL | Z | p | |||
| Av | SD | Av | SD | |||
| AD | -.92 | 2.74 | -.83 | 3.34 | -.240 | .811 |
| VS | 2.09 | 2.12 | 2.29 | 2.95 | -.719 | .472 |
| Sagittal plane | ||||||
|---|---|---|---|---|---|---|
| Intact ATFL | Reconstructed ATFL | Z | p | |||
| Av | SD | Av | SD | |||
| AD | -7.47 | 5.58 | -.779 | 6.52 | -.893 | .372 |
| VS | -.62 | 2.00 | -.767 | 1.46 | -.741 | .459 |
We found no statistically significant differences between the intact ATFL and the reconstruction with plasty with the anterior drawer and varus stress (VS) manoeuvres in the axial plane (Av: Average, SD: Standard Deviation).
We found no statistically significant differences between the intact ATFL and the reconstruction with plasty with the anterior drawer (AD) and varus stress (VS) manoeuvres in the coronal plane (Av: Average, SD: Standard Deviation).
We found no statistically significant differences between the intact ATFL and the reconstruction with plasty with anterior drawer (AD) and varus stress (VS) manoeuvres in the sagittal plane (Av: Average, SD: Standard Deviation).
Direct anatomical repair is considered to be the gold standard in the surgical treatment of lateral ankle instability.6,9,15 This study provides biomechanical validation of anatomical ligament reconstruction using an ATFL graft. This is the technique that is indicated in those cases when direct repair is impossible, or when it will not give good results over the long term.19 It would be indicated in cases of the failure of direct repair or for those patients with clinical factors that predispose failure of the Broström technique: poor quality of the remaining ligament, instability that evolved over a long period of time (longer than 12 months), ligament hyperlaxity or a high level of demand (competitive sport or obesity).6,20,21
The technique we use dis vaguely based on the one described by Coughlin, Schenck et al.22 in 2001, as they propose augmentation by tendon transfer from the free gracilis to reconstruct the ATFL and the calcaneofibular ligament (CFL). This work presents a simpler technique, demonstrating that anatomic plasty reconstructs the stability of the ATFL. Additionally, augmentation would not be a valid technique in some indications for the reconstruction technique, such as cases of instability which evolve over the long term or failure of previous direct repair, in which the residual ligaments are usually either low quality or non-existent.
We use the EHL tendon in this study due to several reasons: its availability in the anatomical piece, its easy extraction and its diameter of approximately 4.5 mm. Debate is ongoing about whether to use autograft or homograft in the reconstruction of the crossed anterior ligament, and some works support the use of a homograft, with similar biomechanical results.23 The surgical technique described may be performed with both options, and the choice of one or the other will depend on the patient, availability in the tissue bank and the surgeon’s preferences. We require a graft of 4.5 mm–5 mm, which is the approximate thickness of healthy ATFL, and a length of at least 100 mm. In our case it was not possible to use the goose foot tendons, which are used the most often in other cadaveric or clinical studies, as we did not have the complete lower limb, so that we were obliged to use another anatomic piece with the corresponding disadvantages this involves.15,24
Fixation using absorbable interferential screws gives appropriate tendon-bone strength in the foot and ankle, with the advantage that they require a shorter plasty and less surgical time in comparison with traditional methods of fixation by tendon-tendon sutures.12,25
Few cadaveric biomechanical works study anatomical reconstruction techniques for cases of ankle instability. In 2004 Clanton et al.15 published a biomechanical study of anatomical reconstruction technique using semitendinosus tendon as the donor in six cadaveric specimens. The maximum load at failure of the reconstruction with graft did not differ significantly from that corresponding to the intact ATFL. Nor was the average rigidity of the reconstruction with graft significantly different from that of the intact ATFL. They therefore conclude, in agreement with our study, that the anatomical reconstruction of ATFL with graft has similar strength and rigidity to the initial intact ligament in a frozen cadaveric model.
We are aware that our study has some weaknesses. Firstly, we would like to mention the intrinsic limitations of a cadaveric study. We evaluate the anatomical reconstruction technique immediately, without taking into account the biological effect of the scarring and fibrosis processes that occur over time in vivo, and which contribute to the stabilisation of the ankle joint. In the reconstruction with plasty we do not consider fibrosis to be the most important factor for stabilisation, as it may be in repair techniques.
On the other hand, it was not possible to evaluate the dynamic effect of muscle stabilisation. We can only evaluate the intrinsic stability supplied by bone and ligament structures. We do not consider this aspect to be a weakness in itself, given that we sought to evaluate whether the stabilisation produced by reconstruction using plasty is similar to that of the healthy ligament, regardless of the active stabilisers.
Secondly, we have to mention the limitations which arise due to the measuring instrument. The arthrometer records the angular movement in the three anatomical planes of a sensor located on the astragalus after the AD and VS manoeuvres. The force used in the manual application of the said manoeuvres was not measured in an objective way. This error was minimised by the same researcher always performing the stability manoeuvres, three times for each test. Ankle stability evaluation was undertaken in a similar way to the physical examination that is used in everyday clinical practice to diagnose patients with ankle instability, with the advantage that we performed an objective quantification of angular movement with the arthrometer. Laurin stated that a physiological talar varus inclination is easy to demonstrate and does not require excess force, similar to manual and instrument strength tests.26
A strength of this work that should be underlined is that to our knowledge no other study has been published to date which evaluates the angular stability that arises from the anatomical reconstruction technique if the ATFL with a graft. Measuring stability by angular instead of lineal displacement is a new concept that was introduced by Guerra-Pinto et al.16,17 in a recent publication.
Moreover, this is an experimental cadaveric study with a sample size of 18 specimens and a rigorously protocol-governed design to minimise errors, using a specifically designed measuring instrument that has been used before in published studies of ankle biomechanics.
Different surgical techniques have been described in recent publications, many of them involving arthroscopic repair, augmentation and reconstruction using an autograft or homograft. The majority of these studies are series of cases - with no control group - although very few experimental works in cadavers support the use of the said techniques. This work provides a biomechanical validation of the reconstruction of the lateral complex with a graft, opening the door to new biomechanical cadaveric studies.
ConclusionAnatomical reconstruction of the ATFL using a graft reproduces the angular stability of the intact ATFL, in a cadaveric model and immediately.
Level of evidenceLevel of evidence IV.
FinancingThis study was financed thanks to the support of “Proyectos de Inicio a la Investigación de la Fundación SECOT”, announced in the year 2017.
Conflict of interestsThe author J. Vilá y Rico is an international Arthrex consultant.
We would like to thank the Department of Anatomy and Embryology staff of Complutense University, Madrid.
Arthrex España, for the generous donation of the material needed to undertake this research work.
Please cite this article as: Mellado-Romero MÁ, Guerra-Pinto F, Guimarães-Consciência J, Sánchez-Morata EJ, Vacas-Sánchez E, Arroyo-Hernández M, et al. Estudio biomecánico de la reconstrucción ligamentosa anatómica con autoinjerto en la inestabilidad lateral de tobillo. Rev Esp Cir Ortop Traumatol. 2020. https://doi.org/10.1016/j.recot.2020.06.010