To evaluate the in vivo behaviour of a new bone cement loaded with antibiotics, in a rabbit bone infection model.
Material and methodsSixteen New Zealand rabbits divided into 4 groups were used, depending on the cement (commercial or experimental) and the antibiotic (vancomycin or linezolid) used to control a bone infection caused by Staphylococcus aureus. The commercial cement is Palacos® R and the experimental cement has been achieved by adding PLGA to the solid phase of Palacos® R cement. A novel histological staging method based on bone histoarchitecture has been used. This staging allows us a global vision of bone repair capacity, in the presence of modified cement, and also allows us to correlate the damage generated with the functionality of the tissue.
ResultsThe degree of bone destructuration found depended on the type of cement and antibiotic, and was higher in the groups with commercial cement than in the experimental group (p<.01) and in the groups with linezolid with respect to vancomycin (p=.04) The percentage of macrophages varied exclusively depending on the antibiotic used, and was higher in the vancomycin groups (p=.04).
DiscussionThe development of new formulations of bone cement that release more, and more prolonged, new generation antibiotics such as linezolid, present an in vivo behaviour superior to commercial cement, respecting the bone structure. This behaviour would have a clinical implication in fighting infections by increasingly resistant germs in the treatment of prosthetic infection.
Valorar el comportamiento histológico, en un modelo animal de conejo, de un nuevo cemento óseo modificado, el cual aumenta la liberación local de antibiótico, en la infección ósea.
Material y métodosSe han utilizado 16 conejos Nueva Zelanda divididos en 4 grupos, en función del cemento (comercial o experimental) y del antibiótico (vancomicina o linezolid) empleados para controlar una infección ósea por Staphylococcus aureus. El cemento comercial es Palacos® R y el cemento experimental se ha conseguido añadiendo PLGA a la fase sólida del cemento Palacos® R. Se ha empleado un método de estadificación histológica novedoso, basado en la histoarquitectura ósea. Esta estadificación nos permite tener una visión global de la capacidad de reparación ósea, en presencia del cemento modificado, así como correlacionar el daño generado con la funcionalidad del tejido.
ResultadosEl grado de desestructuración ósea encontrado depende del tipo de cemento y del antibiótico, siendo mayor en los grupos con cemento comercial respecto al experimental (p<0,01) y en los grupos con linezolid respecto a vancomicina (p=0,04). El porcentaje de macrófagos varía exclusivamente en función del antibiótico utilizado, siendo mayor en los grupos con vancomicina respecto a linezolid (p=0,04).
DiscusiónEl desarrollo de nuevas formulaciones de cemento óseo que liberan mayor cantidad, y de forma más prolongada, de antibióticos de nueva generación como el linezolid presentan un comportamiento in vivo superior al cemento comercial, respetando más la estructura ósea. Este comportamiento tendría una implicación clínica para combatir las infecciones por gérmenes cada vez más resistentes y prevenir la colonización de los espaciadores de cemento usados habitualmente en el tratamiento de la infección protésica.
Infection is a major complication of prosthetic surgery. Despite its low prevalence (around .5%–3%),1 infection has become a devastating complication due to the peculiarities of biofilm formation2 making eradication difficult. In Spain, the Catalan registry of 2009 reported a 3% incidence of infection in hip prosthesis, and 3.3% incidence in knee prostheses.3
Several strategies have been described for treating prosthetic infections, fundamentally depending on their time of onset.4 For chronic infections, two-stage prosthesis revision surgery has been the method most employed, using a bone cement spacer between the first and second stage, with better rates of infection eradication.5–8
This bone cement, mostly comprising methyl polymethacrylate, is loaded with antibiotics that allow local concentrations very much higher than those achieved intravenously. This release depends, among other factors, on the composition and physicochemical features of the cement,9,10 which are higher in the first days, and subsequently maintained for between a few hours to several weeks, often below the minimum inhibitory concentrations.11,12 This has been associated with the development of antibiotic resistances (50% resistance to gentamycin), with the colonisation of the spacers, and therefore with therapeutic failure.
Around 20% of the Staphylococcus aureus and 80% of the Staphylococcus epidermidis isolated in prosthetic infections are methicillin-resistant.13 Vancomycin is the antibiotic of choice for treating methicillin-resistant staphylococcus.14 The growing appearance of multi-resistant bacteria has necessitated the use of new antibiotics to include in bone cement. Daptomycin and linezolid are the 2 main alternatives to vancomycin in cases of bacterial resistance.15 Both antibiotics have demonstrated good release kinetics and antibacterial properties in in vitro cement.16
The use in recent years of biodegradable compounds in cement to increase antibiotic release managed to increase the release of vancomycin, daptomycin and linezolid in vitro, and to significantly improve temporal release kinetics. This was achieved by incorporating biodegradable microparticles of polylactic-co-glycolic acid (PLGA) in the solid phase of Palacos® R cement.16 When cements formulated with these microparticles are used a biphasic release can be appreciated, rather than the linear release up until the third week that occurs with commercial cements, exponentially increasing between days 35 and 40 (sixth-seventh week).
The aim of this paper was to evaluate the histological behaviour of this cement loaded with vancomycin or linezolid (to-date not tested in animal models) in an experimental model of osteomyelitis in rabbits, using a new system of histological staging as the measurement method based on bone histoarchitecture, which gave us an overall view of bone repair capacity, and to correlate the damage generated with tissue functionality.
Material and methodsA total of 16 New Zealand white rabbits were used. The animals were managed in compliance with the current international animal-testing regulations (609/86/EEC and ETS 123) in the Animal Experimentation Centre of the University of Alcalá, with Research Ethics Committee suitability report CEI UAH 2011017.
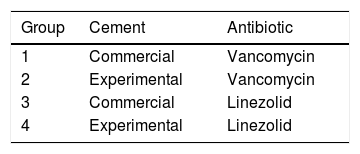
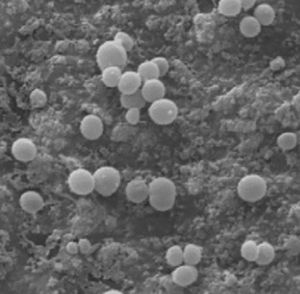
The selected strain of multi-resistant S. aureus was prepared in an inoculum adjusted to 4, according to the McFarland scale (bioMérieux, Marcy-l’Etoile, France), in a 3ml saline suspension, equivalent to 1.2×109CFU/ml. A hydroxyapatite-coated titanium rod was inserted into each tube containing this suspension for 24h in order to contaminate it. We observed the bacteria adhering to the hydroxyapatite-coated rod using a scanning electron microscope (Fig. 1). This rod was the contaminated implant that we inserted into the rabbit's bone tissue.
Two bone cements were used: Palacos® R (Heraeus Medical) as the commercial cement, and the partial substitution of 45% of the solid phase of Palacos® R with PLGA microspheres as the experimental cement, presenting curing parameters (as laid down in ISO-5833),22 with a reduction in the setting temperature of the cements prepared with PLGA microparticles and/or antibiotics. The mechanical properties of these formulations are lower than those required by ISO-5833 for cements used in joint prosthesis fixation17 due to the use of PLGA microparticles, as described in previous studies.16
The animals were divided into 4 study groups: the commercial cement groups (1 and 3) and the experimental cement groups (2 and 4), according to the type of cement (commercial or experimental) and on the antibiotic (vancomycin or linezolid) used. Table 1 shows the nomenclature and composition of each group.
The anaesthesia protocol included the intramuscular administration of ketamine chlorhydrate 70mg/kg, diazepam 1.5mg/kg, and chlorpromazine 1.5mg/kg.
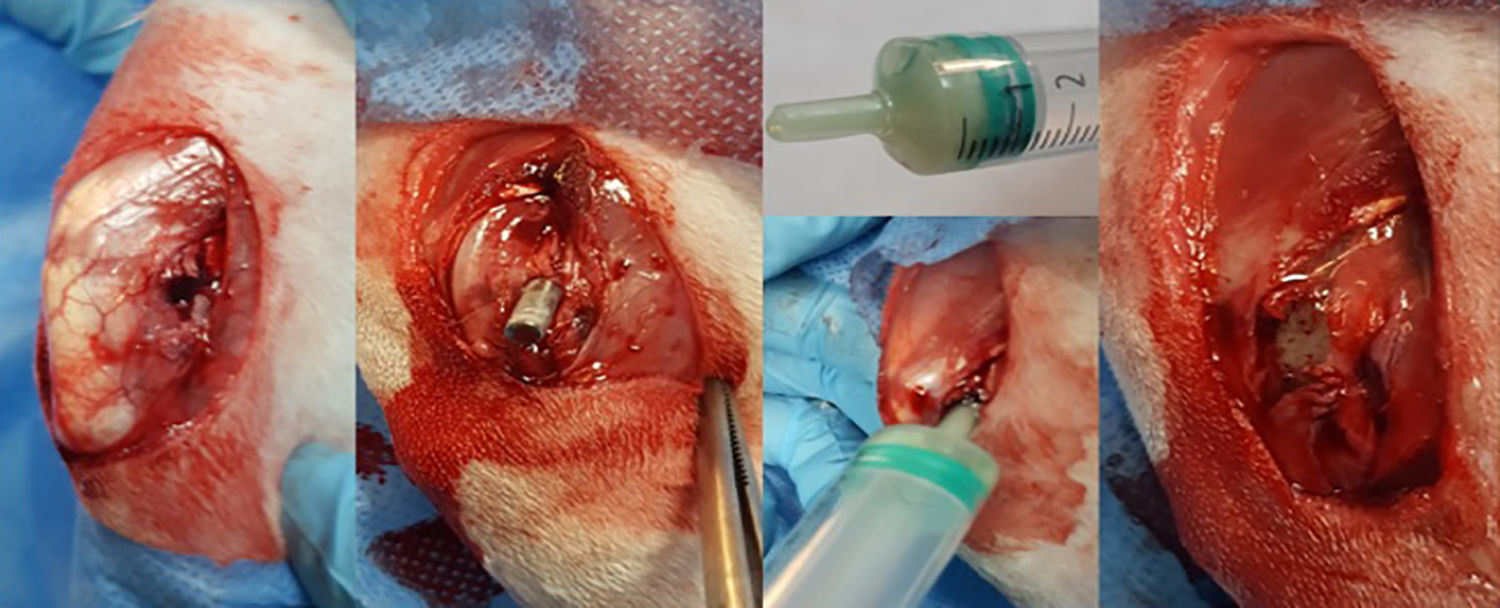
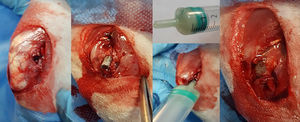
After shaving and disinfecting their back leg with alcoholic chlorhexidine, the animal was placed in the lateral position and sterile draped, leaving only the distal third of the thigh and knee exposed. An ascending parapatellar skin incision was made in search of the longitudinal and lateral axis of the thigh, to 2.5–3cm. The intermuscular plane between the tensor muscle of the fascia lata and the femoral biceps muscle was then located which, after it was cut open, exposed the external face of the external femoral condyle. Once the condyle had been exposed, an opening was made in the metaphyso-epiphyseal region manually using a 3mm cylindrical section punch. Once the external cortical bone had been perforated perpendicularly, we proceeded to place the axis obliquely in a diaphyseal direction resulting in an oblong opening of about 5mm that crossed the metaphyso-epiphyseal region of spongy bone, until reaching the diaphyseal medullary canal (Fig. 2).
With the opening in the bone ready to receive the rod, we uncovered the tube containing the hydroxyapatite-coated titanium rod that had been in the suspension prepared with methicillin resistant S. aureus for 24h. Using a holder we inserted the rod into the opening in the bone until it was completely buried within the bone.
After we had inserted the rod, the bone cement was prepared. The two components of the cement (solid phase and liquid phase) had been in separate containers under sterile conditions until the time came to prepare the cement. The solid phase contained an antibiotic powder mixture.
The mixture was made by pouring both phases into a 5ml syringe with no embolus, and vigorously whisking until the product was homogenised with the aid of a 14G spinal needle for 1min. When the mixture became doughy in consistency, the embolus was placed in the syringe and the bone cement inserted by plunging the tip of the syringe into the prepared opening in the femoral condyle, until the cement was distributed inside the bone, surrounding the rod and completely covering the opening (Fig. 2). While the cement cured, the wound was thoroughly flushed and then closed – first the muscle plane and then the skin. The rabbit was then placed in its cage where it awoke spontaneously and was allowed free movement.
Three weeks after the first operation, the animal was sacrificed and the anatomical specimen obtained for analysis. This sample was placed in a sterile container and completely submerged in a fixing and decalcifying solution (Osteosoft®) for a minimum of 60 days.
The samples were then processed for histological study as follows: the specimen was placed in paraffin, cut into 5μm sections and haematoxylin–eosin and Gram stained. Immunohistochemical techniques using the monoclonal RAM-11 antibody (DakoCytomation, Glostrup, Denmark) were also completed for a quantitative analysis of the macrophagic response of the tissue.
We used our own very simple staging model for the bone histoarchitecture, based on the integrity and preservation of the architecture of the territory affected, differentiating metaphyseal bone from physeal cartilage, and from epiphyseal bone. This scale is from 1 to 9. In grades 1–3 there is mild destructuration of the bone, with progressive involvement of the metaphyseal bone and unaffected cartilage. In grades 7–9 there is severe destructuration of the bone, with total loss of cartilage and progressive disorganisation of the epiphyseal bone. During the assessment we applied the histoarchitectural staging model by 2 blinded assessors to 4 samples per rabbit, obtaining means per rabbit (n=4), and per group (n=4). The degree of concordance between both was studied using Lin's concordance correlation coefficient, and we obtained as a valid value of analysis, if they did not coincide, the mean value obtained between the 2 observers.
The macrophagic response was quantitatively assessed using the RAM-11+ cell count in 15 fields per rabbit, obtaining means per rabbit and per group.
The normality of the means obtained was checked using statistical and graphic tests. Given that the distribution was not normal, the bivariate analyses were performed using the Mann–Whitney U test and the multivariate analyses using regressions on the median (quantile regressions). All these tests were performed using STATA 14 (Texas, United States).
ResultsThe use of histological sections enabled us to study the bone territory affected by the presence of the rod and the cement after 3 weeks.
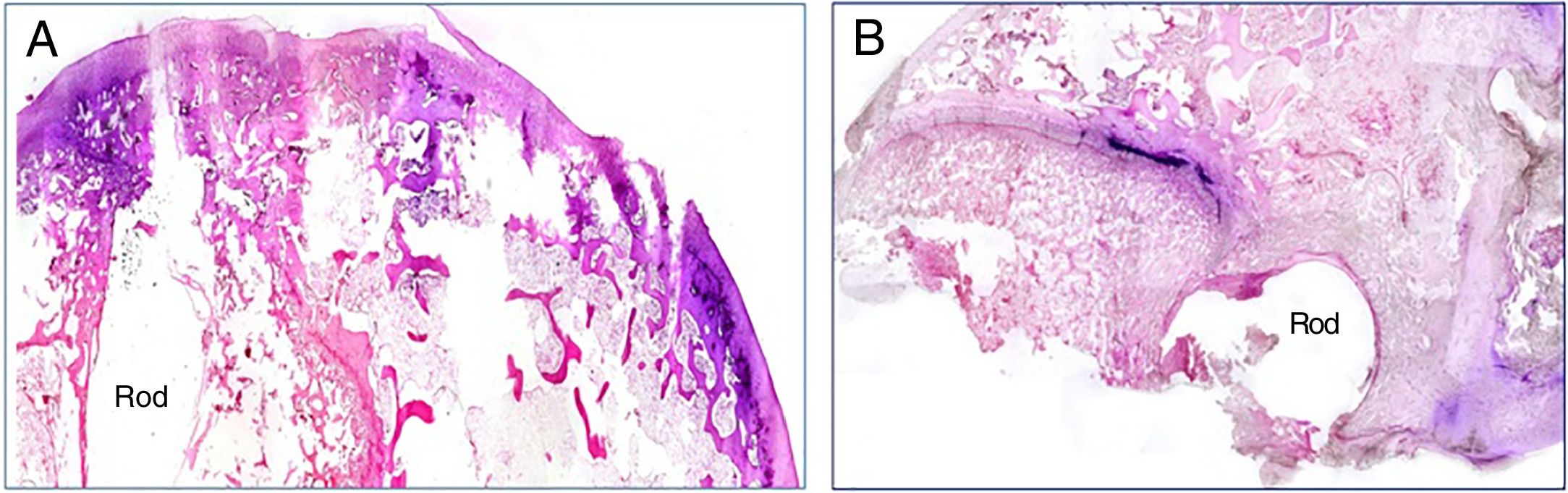
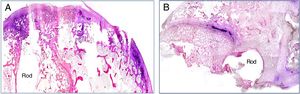
The presence of infection was assessed by Gram stain in all the histological specimen study groups. Bacterial spread was maximal in the animals implanted with a contaminated rod with the commercial cement, and minimal in the animals in which the experimental cement was used. In these cases, the bacterial presence was restricted to the vicinity of the implant (Fig. 3A and B).
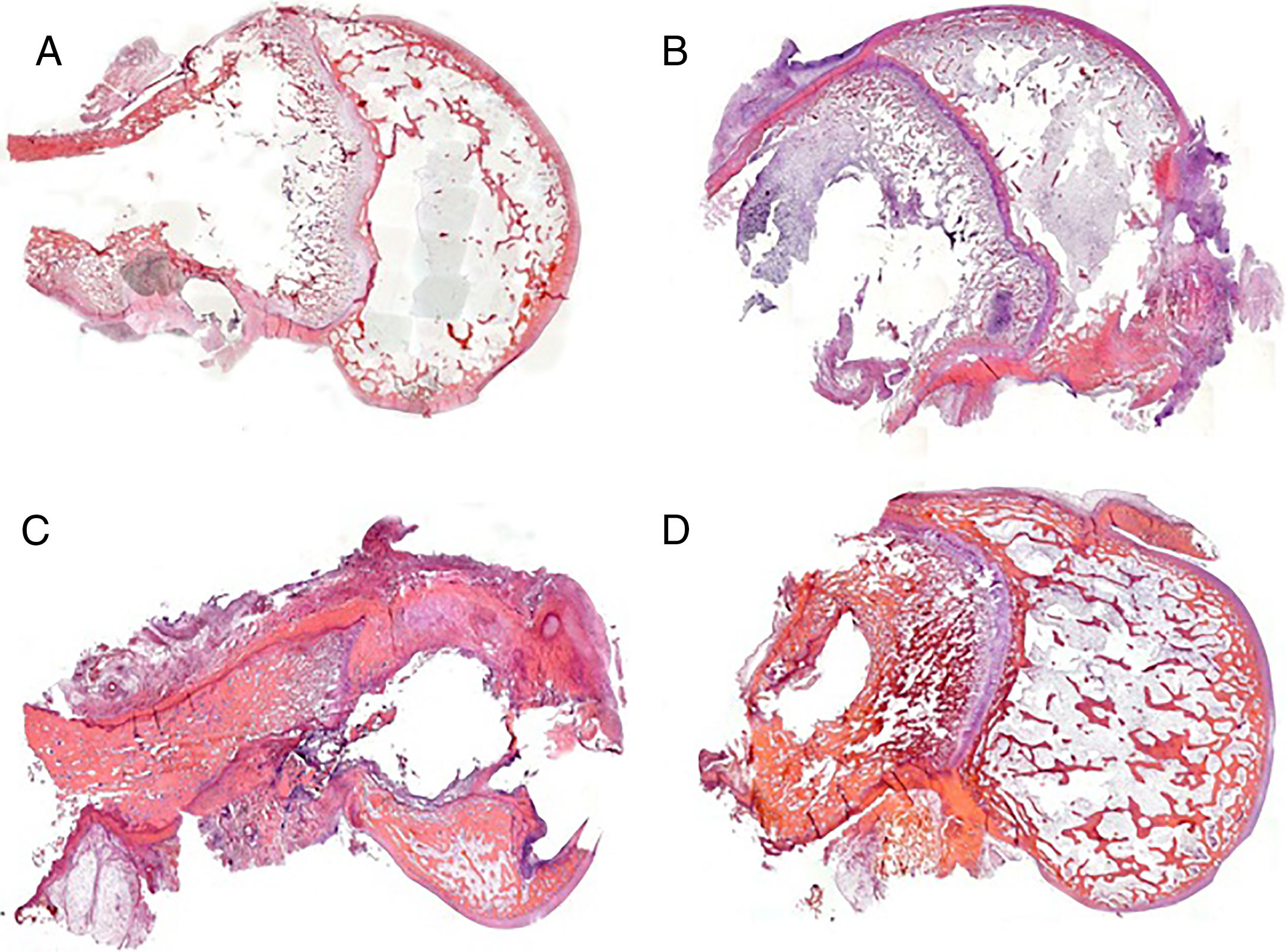
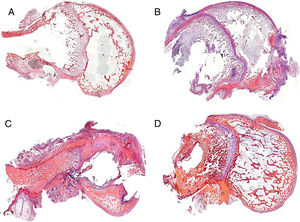
We were able to observe better preservation of the tissue structures in the groups where the experimental cement was used in the bone-rod interface (groups 2 and 4) compared to those with the commercial cement (groups 1 and 3) (Fig. 4). This fact might be related to the greater spread of infection in the groups with commercial cement compared to the groups with the experimental cement.
In the group with commercial cement and vancomycin (group 1) there was little tissue response, observing the imprint of the rod and the remains of the cement in the diaphyseal medullary area. In the areas where the metaphyseal cartilage had been affected, there was no response, and in the areas with affected epiphyseal bone, destructuration was obvious, and classified as moderate in type (Fig. 4A). In the group treated with linezolid, the histological pictures show a high degree of tissue destructuration. The samples in this group, after the decalcification process, barely maintained anatomical integrity and it was very difficult to process them histologically. The histoarchitectural destructuration included areas of necrosis and fibrosis. The cortical bone tissue was clearly altered, and was lacunar in its medial portion, only respecting a fine layer of compact bone in the outer rim. The remains of adhering cement showed good adhesion to the inner rim. The remaining structures involved due to the presence of the rod (cartilage and epiphysis) were barely preserved. This was classified as severe destructuration in this case (Fig. 4C).
In the groups with experimental cement and with both types of antibiotic the rod imprint, although obvious, enabled some bone regeneration at medullary level, observed due to the presence of newly formed trabecular bone. There were occasional areas of altered cartilage. This type of histoarchitecture preservation was classified as mild destructuration (Fig. 4B and D).
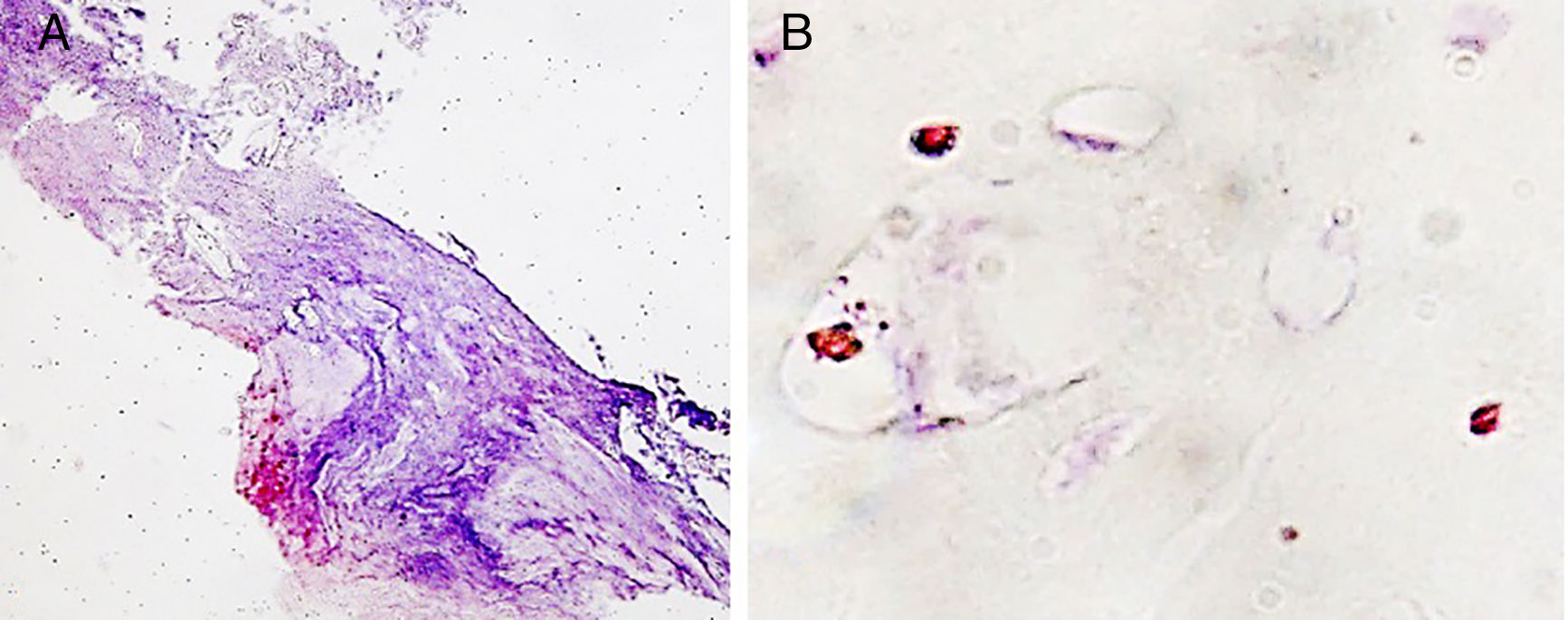
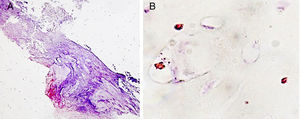
Finally, we assessed the macrophagic response. This behaved quantitatively similarly in all the study groups, but at the level of the location of these cells the pattern was clearly different. Only macrophages clustered in granulomatous areas in the fibrous capsule were visible, which was observed in the groups with the experimental cement, especially in the vancomycin group (Fig. 5A). The remaining macrophagic presence was seen in areas of cartilage, and dispersed in the vascular environment (Fig. 5B).
The means of the degree of bone destructuration of each group applying the histoarchitectural staging model, and of the percentage of RAM-11-labelled macrophages compared to total cells, are shown in Table 2.
Degree of destructuration and percentage of RAM-11 macrophages.
| Group | Degree of destructuration | Percentage of RAM-11 macrophages |
|---|---|---|
| Mean (standard deviation) | Mean (standard deviation) | |
| 1 | 5 (1.73), moderate | 24.32 (16.21) |
| 2 | 3 (2.64), mild | 22.52 (10.01) |
| 3 | 8 (0), severe | 21.33 (16.23) |
| 4 | 2.67 (1.15), mild | 19.71 (7.0) |
The grade of concordance in the bone destructuration measurements between the 2 professionals who studied all the samples was moderate-high, according to Lin's coefficient (Rho .91, with a 95% confidence interval [.8–.0]).
Comparing the bone destructuration, we saw in the bivariate analyses that the experimental cement altered the bone structure less than the commercial cement (p=.02), and no statistically significant differences were found between the 2 antibiotics (p=.57).
When we performed the multivariate analyses, bearing in mind both the type of cement and the type of antibiotic, the degree of destructuration found depended on both; it was greater in the groups with the commercial cement than in the experimental cement group (p<.01), and in the groups with linezolid rather than vancomycin (p=.04).
On analysing the percentage of RAM-11-labelled macrophages, all the groups had similar results, no differences were found with regard to the cement (p=.49) or the antibiotic (p=.08).
On performing the multivariate analyses, and taking into account the rabbit from which the analysed samples had come in addition to the cement and the antibiotic, we found that the percentage of macrophages varied exclusively according to the antibiotic used (p=.04), and was greater in the groups with vancomycin than in the groups with linezolid. We found no statistically significant interaction between the antibiotic and the cement.
Initially, although there were differences in the groups when the percentage of macrophages with RAM-11 (Table 2) was calculated, these were not statistically significant when the 2 types of cement used, commercial and experimental (p=.49), were compared, or when the 2 groups of antibiotics used, linezoid and vancomycin (p=.08) were compared.
However, when these results were analysed in a multivariate analysis, taking all the variables into account at once: the type of cement, the antibiotic and the rabbit on which the experiment had been performed, the percentage of macrophages related with the type of antibiotic in a statistically significant manner (p=.04), and was slightly higher in the vancomycin groups.
In the groups with bacterial contamination, the experimental cement preserved bone structure better, with similar macrophagic response. In the presence of antibiotics (vancomycin and linezolid), the experimental cement respected the structure better than the commercial cement, especially due to the high degree of destructuration in the commercial cement with linezolid group (with severe bone damage), with a similar degree of macrophagic response between vancomycin and linezolid (in favour of linezolid, with no statistically significant differences), and slightly less than the controls of the osteomyelitis model with no antibiotic.
DiscussionThe high rate of resistance to antibiotics frequently used in bone cement spacers for the treatment of prosthetic infections requires the development of new formulations. In this regard, we were able to gain an understanding of the histological behaviour of new modified bone cement, which increases the in situ release of the antibiotic used.
In order to increase antibiotic release, we set out to modify the composition of the solid phase of Palacos® R commercial cement, to which we added biodegradable PLGA microparticles. A polyhydroxy acid was used because of its degradation mechanism under physiological conditions. This mechanism is principally based on degradation in heterogeneous mass that is faster in the inner part than on the surface.18 A PLGA 50:50 copolymer was specifically chosen because it degrades faster than those richer in lactide.19 From a clinical perspective this is important in the treatment of prosthetic infections, since the interval between the first and the second phase can be approximately 8–12 weeks. Therefore, during this interval antibiotics are released at doses above the minimum inhibitory concentration against the causative germ.
The reduced curing temperature observed in the new cement formulation might be due to the effect produced by both the microparticles and the drugs when included in the solid phase, which results in an improvement in the dissipation of the heat which occurs during polymerisation of the monemer.
The use of an antibiotic has been widely studied in bone cement, essentially gentamycin. The combination of 2 antibiotics has also been studied, demonstrating, due to a synergic effect, a greater release of antibiotics from the bone cement due to a combination of the phenomenon of in mass and surface diffusion. However, to date, there is no ideal combination to cover all infections. In this regard, we chose a broad-spectrum antibiotic, linezolid (not used in any animal model to date), as the latest generation antibiotic with activity against resistant strains20,21 that has demonstrated good in vitro release,22 with excellent in vitro antimicrobial activity against S. aureus.16 The in vitro cellular toxicity of this antibiotic was accompanied by more aggressive behaviour on the bone histoarchitecture by the commercial cement compared to vancomycin, and less by the new cement formulation. The tissue fragility after the decalcification period corroborated this.
The mechanical properties of these formulations are inferior to those demanded by ISO-5833 for cements used in joint prosthesis fixation17 due to the use of PLGA microparticles. This would have a negative effect for their use in primary or revision surgery, since they would cause a mechanical complication of the prosthesis. Nevertheless, the aim of this paper was to use these cements as spacers in the treatment of prosthetic infections, in the interval between the first and second phase. These spacers do not need the mechanical properties required for the fixation of an implant, although they have sufficient resistance and stability.23
At present there is no ideal osteomyelitis experimental model, let alone for evaluating the results of antibiotic release by different biomaterials reproducing prosthetic infection. Rabbits are the research animals that are used most often due to their versatility and size, the New Zealand rabbit in particular. S. aureus is the bacterium most used in experimental models, 70% of strains are used with relevance to clinical practice, 30% of which are methicillin resistant.24 With regard to the method of inoculating the bacteria, the use of a foreign body improves the establishment of infection compared to an isolated bacterial suspension, particularly if the foreign body is introduced colonised,25 using an inoculum ranging between 103 and 108.
In assessing the efficacy of any treatment, the limitations for the comparison of results with the methods used traditionally due to their semiquantitative nature (either microbiological, histological or radiological) require a broad consensus to be reached on the gold standard method, which includes quantitative determinants in particular, to evaluate the results of treatment in experimental models of osteomyelitis in animals, and that replaces the traditional CFU counts or the PCR test.26
Assessing tissue damage and repair based on histoarchitecture provides a way of establishing tissue status, which varies widely according to the paper consulted. In this regard, the scales created by authors such as Smeltzer et al.27 or Petty et al.,28 and their many subsequent adaptations, have been greatly used, although both evaluate very specific aspects of bone histoarchitecture and therefore vary widely in terms of bacterial strain, inoculum and animal model used. The former scale (Smeltzer et al.) uses specific bone remodelling parameters, such as periosteal reaction, acute and chronic inflammatory response and the presence of necrosis. However, it is not easy to assess the parameters proposed by Smeltzer et al. since comparing bone response to bacterial infection in such an individual way has a high percentage of variability in the affected areas of bone, as we observed in our experimental model. Therefore, the entire affected area needs to be assessed, to be able to extrapolate this situation to the clinical setting or create an appropriate algorithm.
A classification that is less used, yet more objectifiable from a histological perspective, is that proposed by Petty et al., which clearly describes the alterations that can present in the different bone structures. However, this model does not take the reactive and reparatory process itself into account that we found at the time of the study, since it principally assesses the presence of inflammatory cells corresponding to processes of acute inflammation. In our model, this scale could not be evaluated, since the presence of inflammatory cells at 3 weeks was very low.
Based on previous experience, our model proposes a new histological scale that is capable of evaluating not only the abovementioned individual parameters, but also the preservation and remodelling of bone and cartilage histoarchitecture. A complete assessment of the tissue provides us an overall view of the bone repair capacity and enables the damage caused to be correlated with the functionality of the tissue. The structure of the scale enables less experienced assessors to visualise changes in tissue clearly and more simply. Furthermore, this staging applies to experimental models for evaluating all types of bone defects, even in studies of non-infected biomaterials, which is only possible if the defects and the surrounding newly formed bone are fully studied. Finally, and no less important, our model still allows assessment of the most reactive areas of tissue with the scales that have already been described in the literature, since greater enlargements enable us to evaluate tissue cellularity, encapsulation of the materials and newly formed bone at different points.
In this regard, the introduction of PLGA microspheres that modify the formula of commercial cements such as Palacos® R enabled us to control the aggressiveness of the infection in the tissue, demonstrating better preservation and remodelling of the architecture of the surrounding bone compared to the traditional bone cements. Again, the possibility of evaluating the complete defect histologically with the proposed scale enabled us to easily compare both types of cement and their effect on the bone.
The modification of commercial cement by including PLGA microparticles is highlighted in the study on the behaviour of the macrophagic cells, the results of which again showed that the quantitative assessment could be similar; however, their location showed us a different role for these types of cells. Thus it was observed that these cells appeared in clusters in the fibrous capsule in the groups that included PLGA, although they did not form granuloma, but remained as macrophages. In the cases with commercial cement, these cells, in addition to their natural niche (vascular lakes), were observed in the cartilage territories, the preferred place for the bacterial reservoir. This confirms bacterial spread in the case of commercial cement.
Therefore, although cultures of synovial fluid and periprosthetic tissues have been considered the gold standard, they can give false positives and false negatives,29 particularly in chronic infections.30 These results can be due to uneven bacterial spread and sample taking.
The method used in this experimental study provides a new overall view of the behaviour of infection and the role of antibiotics at 3 weeks, a step forward in this area, and proposes a new method for staging bone destructuration caused by infection and its interaction with the effect of the antibiotic itself. This proposal seeks to provide a new tool for the diagnosis and most appropriate treatment of patients with low-grade prosthetic infections, where the applicable tests are insufficient to provide a correct diagnosis.
In conclusion, we confirm that we have developed bone cement formulations with the introduction of PGLA microparticles, which improve antibiotic release and largely respect the bone structure surrounding them. This could have clinical implications for the treatment of prosthetic infections caused by germs through bone cement spacers that are resistant to conventional antibiotics.
Level of evidenceLevel of evidence i.
FundingThis study was financed by the Secot research grant 2016/0055, of 15,000€.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Azuara G, García-García J, Ibarra B, Parra-Ruiz FJ, Asúnsolo A, Ortega MA, et al. Estudio experimental de la aplicación de un nuevo cemento óseo cargado con antibióticos de amplio espectro para el tratamiento de la infección ósea. Rev Esp Cir Ortop Traumatol. 2019;63:95–103.