The Dynesys® system is a non-fusion pedicular dynamic stabilization system. The aim of our study is to evaluate the clinical outcomes in patients with degenerative disc disease and/or stenosis, and to measure the prevalence of screw loosening and breakage after 4 years of follow up.
Material and methodsAll patients who underwent surgery with Dynesys® system in 2008 were reviewed. The surgery was performed in cases of low back pain of more than 6 months duration and a positive MRI for degenerative disc disease and/or stenosis.
ResultsA total of 22 patients (11 females, 11 males) with a mean age of 44.40±11 years were included, 20 patients (91%) underwent Dynesys® without any associated decompression maneuver. The evaluation of back and leg pain (0–10mm) showed a mean decrease of 2.4±2.06mm (P=.0001). The preoperative value of the Oswestry disability index was 52.36±16.56% (severe functional limitation). After surgery, this value was 34.27±17.87% (moderate functional limitation) (P=.001) with a decrease of 18.09±16.03% (P=.001). A total of 4 (18%) patients showed signs of loosening screws. One patient (4.5%) had a screw breakage.
ConclusionsSurgery with Dynesys® shows favorable long term clinical results, however the range of improvement in our series is lower than those reported in other studies. Comparative studies between Dynesys® and decompression need to be performed in order to isolate the benefit of the dynamic stabilization system. Implant-related complications are not uncommon.
El sistema de estabilización Dynesys® es un sistema pedicular de estabilización dinámica sin fusión. El objetivo de nuestro estudio es evaluar los resultados clínicos en pacientes con enfermedad degenerativa discal y/o estenosis, así como medir la prevalencia de aflojamiento de tornillos tras 4 años de seguimiento.
Material y métodosSe trata de un estudio de serie de casos retrospectivo donde fueron incluidos todos los pacientes intervenidos desde enero a diciembre de 2008 con Dynesys®. Se indicó la cirugía si presentaban dolor lumbar de más de 6 meses de evolución y una RM positiva para enfermedad degenerativa discal y/o estenosis.
ResultadosVeintidós pacientes (11 mujeres y 11 varones) con una edad media de 44,40±11 años fueron evaluados. Veinte pacientes (91%) recibieron el implante Dynesys® sin ninguna maniobra de descompresión asociada. La evaluación del dolor de espalda y piernas (0–10mm) registró una disminución media de 2,4±2,06mm (p=0,0001). El valor preoperatorio del índice de discapacidad de Oswestry fue de 52,36±16,56% (limitación funcional severa). Tras la cirugía este valor fue de 34,27±17,87% (limitación funcional moderada) con una disminución de 18,09±16,03% (p=0,001). Cuatro pacientes (18%) mostraron signos de aflojamiento de tornillos. Un paciente (4,5%) presentó rotura de tornillo.
ConclusionesLa cirugía con Dynesys® muestra resultados clínicos favorables, sin embargo el rango de mejoría en nuestra serie es menor a los comunicados por otros autores. Estudios comparativos entre Dynesys® y descompresión deberían realizarse para poder aislar el beneficio de la estabilización dinámica del obtenido por la descompresión. Las complicaciones relacionadas con el implante no son infrecuentes.
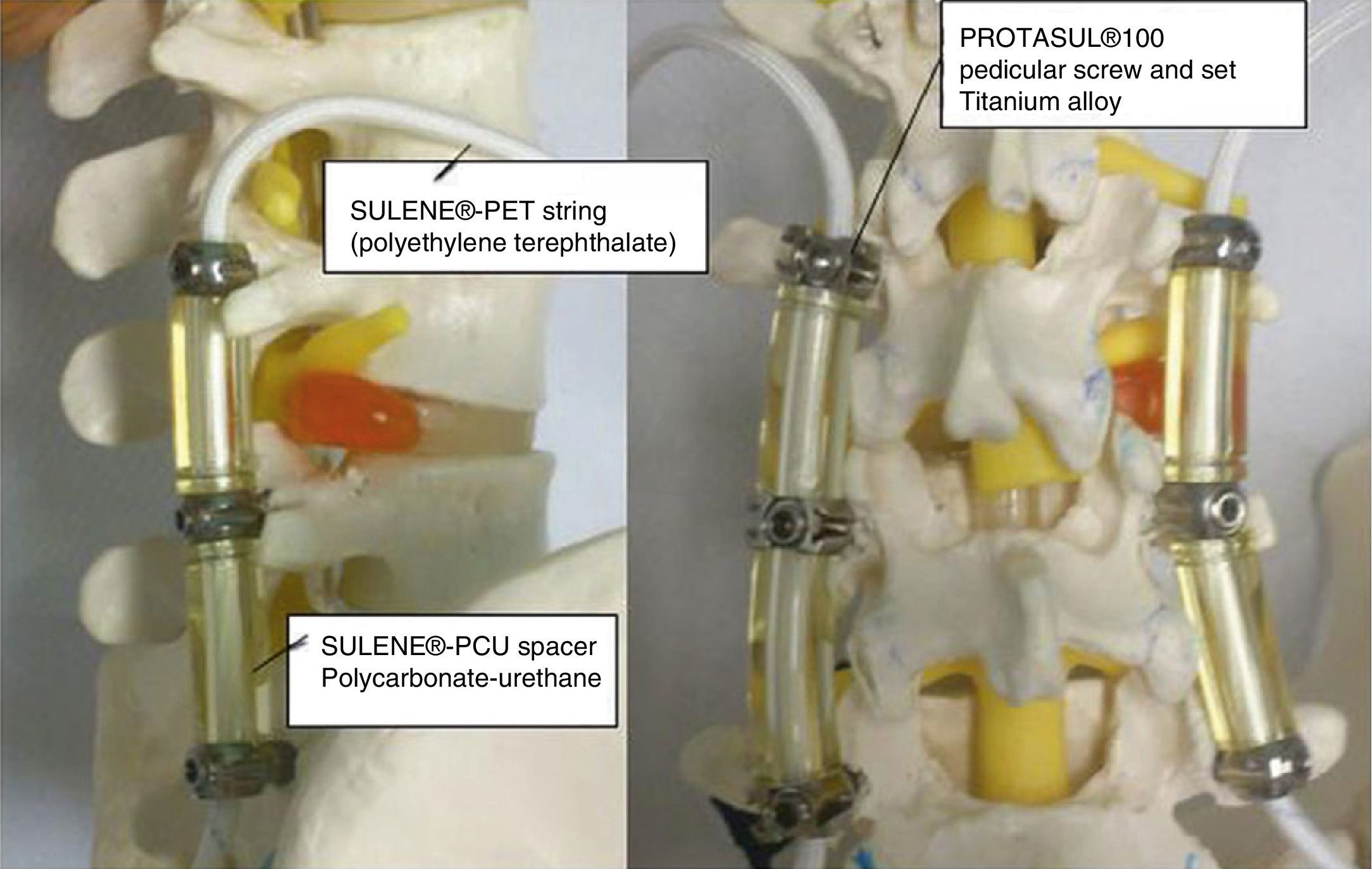
Degenerative spine diseases have a great impact on functional capacity and pain. Among them is degenerative disc disease, associated or not with spinal canal stenosis. Traditionally, the accepted surgical treatment for these diseases has been decompression associated to fusion.1 Such treatment has been associated to certain complications, such as loosening of screws, breakage of stems, hypermobility of the adjacent segment and nonunion of the instrumented segment, which in many cases lead to revision surgery.2 Several dynamic stabilization systems, like the Dynesys® system (Zimmer, Inc., Warsaw, IN, USA), have been developed in order to avoid these undesirable effects. The Dynesys® dynamic stabilization system was presented by Dr. Gilles Dubois and was first used in France in 1994.3,4 It was introduced into clinical practice in Europe in the year 2000 and was approved in the USA in 2009 to provide spinal stabilization for patients with degenerative spinal diseases causing lumbar and radicular pain.5,6 The system replaces rigid fusion stems by pedicular screws manufactured with Ti-Al-Nb and joined together by polyethylene terephthalate (Sulene-PET®) strings running through the center of a polycarbonate-urethane (Sulene-PCU®) cylindrical spacer, thus allowing a certain degree of flexibility of the instrumented segments7 (Fig. 1). There is scarce literature assessing the functional outcomes and complications of long-term implantation in patients with degenerative lumbar disc disease. Only 1 study has been published in our country.8 The aim of our study is to evaluate the functional and lumbar pain results in these patients, and to evaluate the complications related to the material by measuring the prevalence of breakage and loosening of screws after a follow-up period of 4 years.
MethodsType of studyThis was a retrospective case series study based on registry data conducted at Hospital General Universitario of Elche with patients undergoing interventions with the Dynesys® technique between January and December 2008 and with postoperative follow-up during the 4 years after surgery.
Inclusion criteriaPatients with lumbar and/or radicular pain resistant to conservative treatment with non-opioid analgesics (NSAIDs, paracetamol, metamizole), codeine, tramadol and/or buprenorphine, oxycodone and/or physical therapy for at least 6 months.
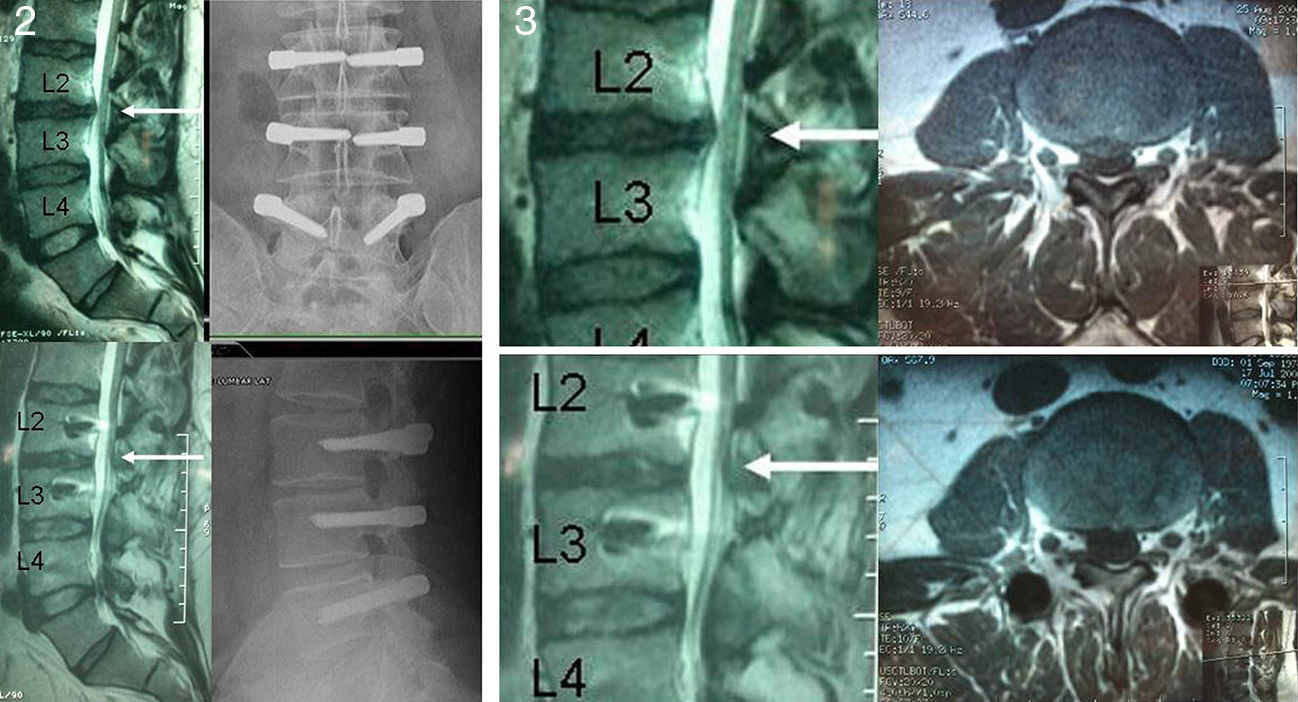
Positive magnetic resonance imaging (MRI) scan for disc degeneration in stages 2, 3 and 4 of the Pfirrmann classification9 (Figs. 2 and 3).
Changes in disc morphology consisting in disc prolapse, disc hernia and/or lumbar canal stenosis.
Exclusion criteriaPatients with degenerative disc changes of Pfirrmann grade 5.
Disc extrusion or disc sequestration.
Spondylolisthesis.
Lumbar scoliosis greater than 10 degrees.
Presence of malignant tumors.
Body mass index over 30kg/m2.
History of alcohol or drug abuse.
All patients gave their informed consent to be assessed and the study was approved by the Bioethics Committee of our hospital.
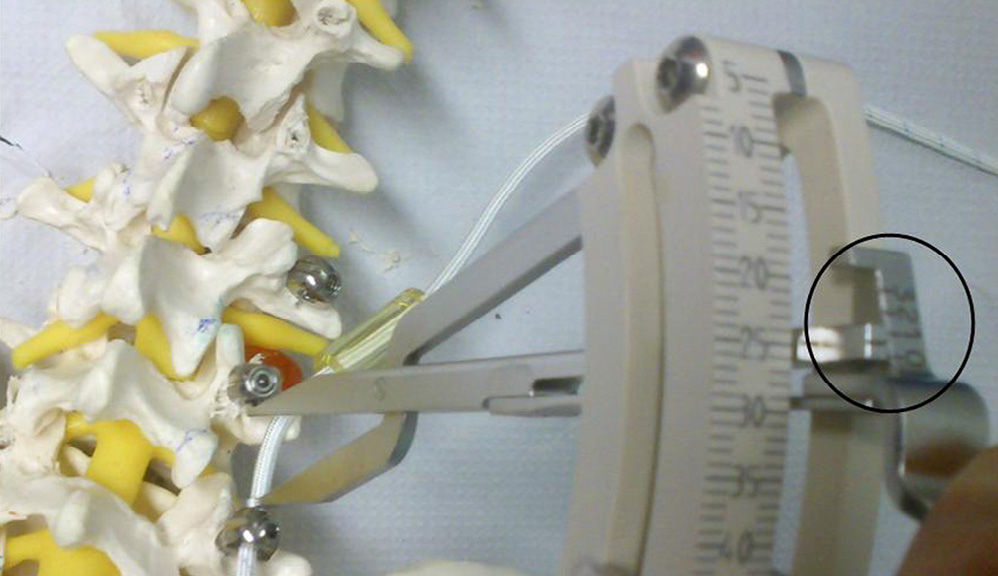
Surgical procedureAll surgical procedures were performed by the same surgeon (D.F-S) through a Wiltse paramedian approach.10 The titanium screws were anchored in the pedicle and vertebral body preserving the facet joints, under intraoperative radiographic control. After the pedicular screws were placed, the interpedicular distance was measured with the dynamometric compass included in the instrumentation, and distraction was performed up to mark number 1 of the 3 preset marks in the instrument. Next, the Sulene-PCU® cylinder was cut to the resulting length and introduced using the Sulene-PET® string (Fig. 4. In those cases requiring associated nerve root decompression, this was performed through an incision in the midline and unilateral or bilateral laminotomy, preserving the spinous apophysis and supra- and interspinous ligament.
Sitting and walking were allowed on the day after surgery, without a lumbar orthosis or any limitation of activities of daily living.
Clinical and radiographic variables were collected from the medical records of patients and a personal interview conducted during the last outpatient visit.
Clinical assessmentFunctional outcomes were measured using the Oswestry disability index (ODI) in its Spanish version, whilst back and leg pain was assessed by means of a visual analog scale (VAS).11 The ODI is represented on a scale of 0–100%, where higher values describe greater functional limitations. Between 0% and 20%: minimal functional limitations; 20–40%: moderate; 40–60%: severe; 60–80%: disability, and above 80%: maximum functional limitation.
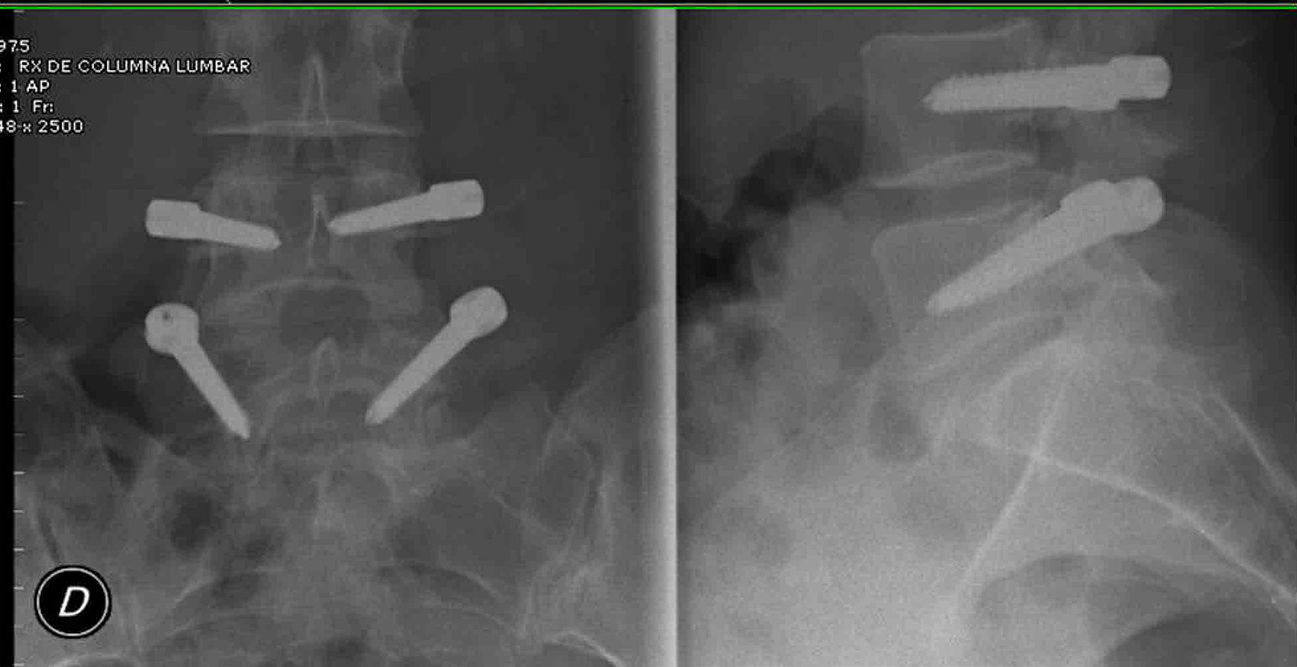
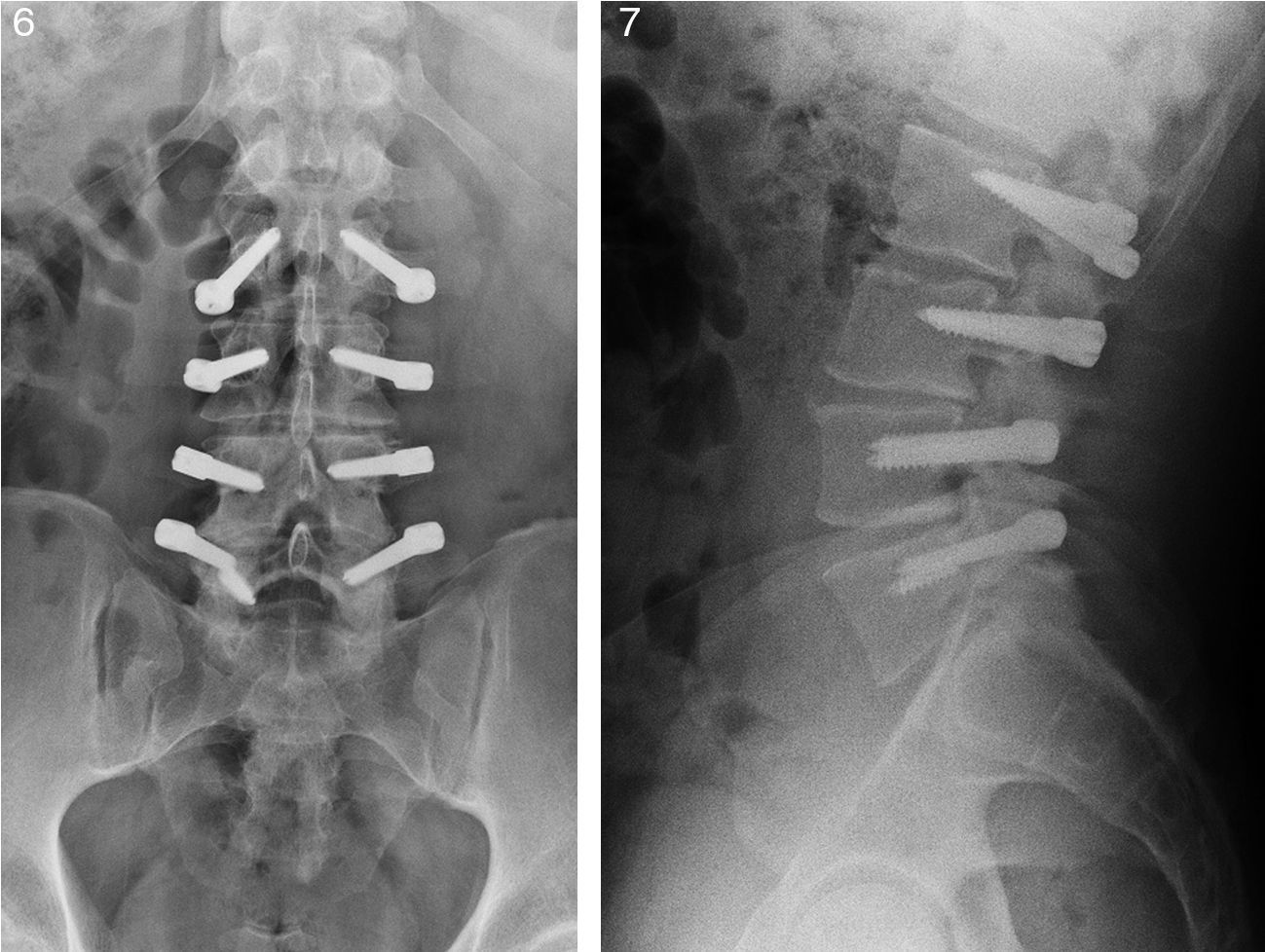
Radiographic assessmentLumbar radiographs in anteroposterior and lateral projections were obtained using the Risolution® (MediAlfa Corp., Miami, FL, USA) digitized system. Each image was magnified and contrast was adjusted to obtain the best possible visualization. An orthopedic surgeon (M.S-T), reviewed the presence of breakage and loosening of the screws, considering as positive the presence, in both projections, of a radiolucent circumference around the screw of 1mm or more, surrounded by a radiopaque bone line with greater density; “Double halo sign”12 (Fig. 5).
Statistical analysisWe used the mean and standard deviation for quantitative variables with normal distribution and the frequency for categorical variables. We performed the Wilcoxon test for the analysis of repeated functional outcomes over time and the Mann–Whitney test was used to detect differences between different groups. The level of statistical significance was set at P<.05. Data were assessed using the software package SPSS® version 15.0 (SPSS Inc., Chicago, IL, USA).
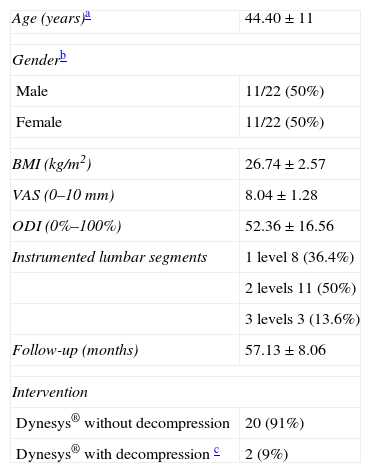
ResultsThe total sample consisted of 23 patients, of whom 22 were included in the study (1 patient could not be examined due to a change of residence). The gender distribution was 11 females and 11 males, and the mean age was 44.40±11 years. The mean follow-up period was 53.45 months (range: 48–60 months). A total of 20 patients (91%) were implanted with the Dynesys® system through a Wiltse paramedian approach without any associated decompression maneuvers, whilst 2 patients (9%) were implanted after a unilateral laminectomy and discectomy due to a herniated disc. The mean body mass index (BMI) was 26.74±2.57kg/cm2. A monosegmental stabilization was performed in 8 patients (36.4%), whilst 2 segments were instrumented in 11 (36.4%) cases, and 3 segments in 3 cases (13.6%) (Table 1).
Baseline characteristics of the patients in the sample.
| Age (years)a | 44.40±11 |
| Genderb | |
| Male | 11/22 (50%) |
| Female | 11/22 (50%) |
| BMI (kg/m2) | 26.74±2.57 |
| VAS (0–10mm) | 8.04±1.28 |
| ODI (0%–100%) | 52.36±16.56 |
| Instrumented lumbar segments | 1 level 8 (36.4%) |
| 2 levels 11 (50%) | |
| 3 levels 3 (13.6%) | |
| Follow-up (months) | 57.13±8.06 |
| Intervention | |
| Dynesys® without decompression | 20 (91%) |
| Dynesys® with decompression c | 2 (9%) |
BMI: body mass index; ODI: Oswestry disability index; VAS: analog visual scale.
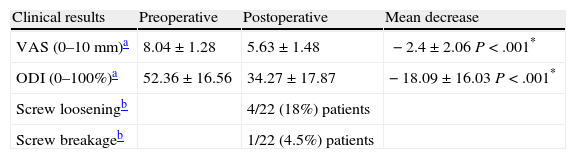
The assessment of back and leg pain by a VAS (0–10mm) improved from a preoperative value of 8.04±1.28 to 5.63±1.48, with a mean decrease of 2.4±2.06mm (P=.0001) (Table 2).
Clinical results and complications of the implant after 4 years follow-up.
| Clinical results | Preoperative | Postoperative | Mean decrease |
| VAS (0–10mm)a | 8.04±1.28 | 5.63±1.48 | −2.4±2.06 P<.001* |
| ODI (0–100%)a | 52.36±16.56 | 34.27±17.87 | −18.09±16.03 P<.001* |
| Screw looseningb | 4/22 (18%) patients | ||
| Screw breakageb | 1/22 (4.5%) patients |
ODI: Oswestry disability index; VAS: analog visual scale.
Significant difference using the Wilcoxon test, P<.05.
The preoperative value of the ODI on the absolute scale from 0% to 100% was 52.36±16.56% (severe functional limitation). After surgery, this value was 34.27±17.87% (moderate functional limitation), with a mean decrease of 18.09±16.03% (P=.001). The results were slightly better in the 2 patients in whom laminectomy was associated, with a decrease in the ODI of 30% in one case and 20% in the other.
When comparing the clinical outcomes by gender, males presented better results on the ODI scale, without this difference being statistically significant (P=.075). No correlation between functional outcome and BMI or age was found.
A total of 19 patients (86.4%) reported that their symptoms improved after surgery, while 3 patients (13.6%) reported a worse outcome after surgery. Of these 3 patients, 2 worsened their ODI scores by 10 points, while the third patient worsened by 30 points. The 3 patients were female and did not differ in age and BMI compared to patients who reported improvement.
Radiographic monitoringA total of 4 (18%) patients presented signs of loosening of screws. The improvement in ODI among these patients was lower than in patients without these signs, specifically 9.75 points compared to 19.94 points (on the absolute scale of 0–100%). This difference was not statistically significant (P=.10).
One patient (4.5%) suffered breakage of 1 screw. The patient did not suffer a worsening of clinical status relative to the preoperative condition (Figs. 6 and 7).
DiscussionThis retrospective observational study presents the experience of the authors with the Dynesys® system. According to the study published by Ostelo et al. (establishing the relevant minimum difference as 10 points in the ODI and 1.5 points in the VAS scale) patients included in our study achieved a clinically relevant reduction of pain and disability after surgery.13 When we compare our results with those reported by other published series of cases, we can see that the margin of improvement in our patients was not as extensive as those reported by other studies. In this regard, Lee et al.14 published the largest decrease in the ODI recorded to date, with a variation of 57.43%, from 79.58% to 22.17% (P<.001), in 19 patients with spondylolisthesis and lumbar stenosis. Sapkas et al.15 also obtained better results than those in our study, with a variation in the ODI of 35% in 114 patients with degenerative disc disease and spinal canal stenosis. Recently, Fay et al.16 have reported a decrease of 4.1 points on the VAS scale (P<.001) and 23.3% in the ODI (P<.001) in patients with similar characteristics to the previous set. These differences between the aforementioned studies and our own, assuming that the patients were comparable, could be due to the fact that the former associated decompression in 100% of patients, whereas in our study only 2 (9%) patients underwent decompression along with the Dynesys® device. It seems clear that, in cases in which a decompressive maneuver is associated, this could act as a confounding factor, making it impossible to isolate the effect of Dynesys® per se. In this sense, our study, in which 91% of patients did not undergo decompression, provides an approximate reference to the functional improvement that may be obtained by using the Dynesys® device alone.
The ODI results were slightly better in the 2 cases (9%) in which decompression was associated. This is consistent with the results published by Grob et al. and Bothmann et al.,17,18 who found better results when dynamic stabilization was combined with decompression of the spinal canal or nerve root. All this evidence suggests that, as is the case with fusion, the main maneuver is decompression, with dynamic stabilization providing scarce additional benefit in those cases requiring decompression of the spinal canal or foramen due to a stenosing lesion.19
Dynesys® versus fusionThe Dyensys® system has not demonstrated superior functional results compared to fusion. In this regard, Yu20 found no significant differences in the ODI when comparing a group of patients treated with the Dynesys® system versus another group treated with posterior interbody fusion (P=.254). Moreover, during the clinical trial conducted to obtain approval of the Dynesys® system by the FDA in the USA in 2009 the Dynesys® system was compared against rigid posterolateral fusion and the difference in the functional results was not statistically significant at 2 years follow-up (P=.34). However, it should be mentioned that some authors argue that, with all results being equal, the Dynesys® system provides other benefits, as it does not require an extraction of iliac crest graft and it also shortens the surgical time.21
In the Spanish context, 1 study has been published which reported positive results regarding the improvement in disability at 2 years follow-up. However, this study did not specify whether these differences were statistically significant nor the percentage of patients undergoing decompression together with the Dynesys® system.10 At an international level, only 4 studies have published results with the Dynesys® system with more than 4 years follow-up.15,21–23 In this sense, the long-term results are still uncertain, with the most extensive records to date being those reported by Hoppe,22 with 7.1 years follow-up.
In the analysis of screw loosening we obtained similar results to those published by other authors with this technique. In this sense, Wu et al.24 found 19.8% of cases with radiographic signs of loosening among 126 patients treated with this technique. Some authors argue that the percentage of screw loosening with the dynamic system may be lower than with rigid systems because the flexible stems would allow some degree of mobility, reducing the load on the pedicular screws.23 However, the studies by Yu et al.20,25 compared these 2 systems and obtained a similar percentage of screw loosening with the Dynesys® system compared to the rigid fusion system; 14.3% versus 20% of patients, respectively (P=.728).
LimitationsThere are several stages within degenerative disc disease.9 We present our experience in cases suffering mild disc degeneration, Pfirrmann stages 2, 3 and 4, and with healthy joints facets, where most cases (91%) were treated with the Dynesys® device alone. In our clinical practice we would opt for fusion surgery and/or decompression in those cases presenting a more advanced stage of disc degeneration and/or facet arthrosis. This means that we must be cautious when comparing our results to other populations with different stages of disc disease and undergoing interventions which combine dynamic stabilization after decompression.
ConclusionSurgery with the Dynesys® device presents favorable long-term clinical outcomes in patients with degenerative lumbar disc disease. However, the range of improvement in our series was lower than those reported by other studies. This could be due to the difference in the percentage of decompressions associated with Dynesys® in the results reported by other authors. Comparative studies between the Dynesys® device and decompression should be performed in order to isolate the benefit offered by dynamic stabilization from that offered by decompression. Complications associated with loosening of the material are not uncommon.
Level of evidenceLevel of evidence IV.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare having obtained written informed consent from patients and/or subjects referred to in the work. This document is held by the corresponding author.
Conflict of interestsThe authors declare that they do not have any financial or personal relationship with any institution related to the content of this publication which could entail a conflict of interests.
Please cite this article as: Segura-Trepichio M, Ferrández-Sempere D, López-Prats F, Segura-Ibáñez J, Maciá-Soler L. Sistema pedicular de estabilización dinámica. Resultados funcionales y complicaciones del implante en pacientes con enfermedad degenerativa discal lumbar, tras un seguimiento mínimo de 4 años. Rev Esp Cir Ortop Traumatol. 2014;58:85–91.