Periacetabular osteotomy (PAO) is an accepted and worldwide technique recognised for residual dysplasia treatment and even in unstable hips with limited acetabular coverage. The aim of this study is to analyse the functional, radiological and complication results in patients treated with mini-invasive PAO.
Material and methodsWe performed a retrospective study in which we analysed 131 cases undergoing mini-invasive PAO at our centre. The degree of joint degeneration was evaluated with Tönnis scale, Wiberg angle, acetabular index (AI), anterior coverage angle (AC), joint space, complications and functional outcome with the Non-Arthritic Hip Score (NAHS) were analysed preoperatively and at the end of follow-up.
ResultsThe average age was 32.3±9.5 (SD) years, 102 (77.9%) were female and 29 (22.1%) were male. 7.7±2.8 (SD) years follow up. The radiological parameters improved between the pre-surgical phase and the end of follow-up, Wiberg angle +18.5° (18.3° versus 36.8°, 95% CI 17.3–19.7), AC angle +13.5° (26.2° versus 39.7°, 95% CI 11.6–15.4) and the AI −11.1° (19.5° versus 8.4°; 95% CI −12.1 to −10.1). In addition, the functional results, with the NAHS scale, improved +31.3 points (60.7 pre-surgical versus 92 at the end of follow-up, 95% CI 28.7–33.8). The most common complication was transient lateral femoral cutaneous nerve hypoaesthesia in 10 cases (7%).
ConclusionThe mini-invasive PAO approach is a reproducible technique, it allows restoration of acetabular coverage and provides an improvement in functional scales as confirmed by our series.
La osteotomía periacetabular (OPA) es una técnica utilizada para el tratamiento de la displasia residual, incluso en caderas inestables con cobertura acetabular limitada. El objetivo de este estudio es analizar los resultados funcionales, radiológicos y las complicaciones en pacientes tratados mediante OPA mini-invasiva.
Materiales y métodosEstudio retrospectivo que analiza 131 casos intervenidos con OPA en nuestro centro. Se determinó de forma prequirúrgica y al final del seguimiento el grado de degeneración articular con la escala de Tönnis, el ángulo de Wiberg, el índice acetabular, el ángulo de cobertura anterior, el espacio articular, las posibles complicaciones y el resultado funcional mediante la escala Non-Arthritic Hip Score.
ResultadosLa edad media de 32,3±9,5 (DE) años, 102 (77,9%) fueron mujeres y 29 (22,1%) fueron hombres. El seguimiento fue de 7,7±2,8 (DE) años. Se obtuvo una mejora en los parámetros radiológicos entre el momento prequirúrgico y al final del seguimiento, ángulo de Wiberg de+18,5° (18,3° versus 36,8°, IC 95%: 17,3 a 19,7), ángulo de cobertura anterior de+13,5° (26,2° versus 39,7°, IC 95%: 11,6 a 15,4) y el índice acetabular de –11,1° (19,5° versus 8,4°; IC 95%: –12,1 a –10,1). Además, los resultados funcionales con la escala Non-Arthritic Hip Score mejoraron en+31,3 puntos (60,7 prequirúrgico versus 92 último seguimiento posquirúrgico; IC 95%: 28,7 a 33,8). La complicación más frecuente fue la disestesia transitoria del nervio fémoro-cutáneo lateral en 10 casos (7%).
ConclusiónLa osteotomía periacetabular mediante el abordaje mini-invasivo es una técnica reproducible, permite restaurar la cobertura acetabular y proporciona una mejora en las escalas funcionales según confirma nuestra serie.
Residual and developmental dysplasia of the hip, known in the English-speaking literature as developmental dysplasia of the hip (DDH), consists of a series of anatomical abnormalities, the most notable of which is insufficient acetabular coverage, resulting in abnormal load distribution. Consequently, there is an increase in contact pressure at the level of the articular cartilage and joint instability, predisposing to damage to the chondrolabral complex, periarticular structures and possible coxarthrosis.1–3
The aim of hip preservation surgery is to fundamentally correct the anatomical abnormalities and chondrolabral damage that lead to early joint degeneration, with the intention of preventing or delaying the onset of secondary osteoarthritis.4 Therefore, in the absence of articular cartilage degeneration, young patients (15–40 years of age), who are active and with these symptomatic anatomical changes are candidates for surgical treatment.
Periacetabular osteotomy (PAO), the Ganz osteotomy, also known as the Bernese osteotomy, has been gradually gaining in acceptance in recent decades, and is now the gold-standard in skeletally mature patients.4,6,7
PAO consists of an osteotomy around the acetabulum, with a polygonal cut, which enables its reorientation, allowing the following:
- -
Balanced distribution of the loads on the femoral head.
- -
Better acetabular coverage in all planes.
- -
Maintenance of contact of the acetabular hyaline cartilage with the femoral head.
Ganz et al.6 described this technique in 1988 through a modified Smith-Petersen approach with disinsertion/reinsertion of the anterosuperior iliac spine and anterior rectus muscles, thus correctly exposing the acetabulum.
In 2008 the Aarhus school (Prof. Søballe; Troelsen et al.7) described a mini-invasive modification of the classical POA technique, based primarily on a change in the surgical approach. This approach consists of a trans-sartorial inguinal approach, and has several benefits over the classic technique,6 including a clear reduction in surgical time, directly in relation to the learning curve, but with less blood loss and reduced transfusion requirements, less postoperative pain, rapid functional recovery and aesthetic benefits, since the incision is made in the same direction as the Langer's lines.
In addition to benefits inherent to the different types of approach,8,9 the relevance of the clinical-functional results of these patients is also of note. Short-medium term clinical follow-up shows symptom relief and functional improvement in 40%–97% of patients.10,11 In addition, up to 71% have been described a return to sports activities, similar or even more intense after the PAO.12–14 Clinical improvement in relation to pain is of note in this group of patients, and resumption of sports activities at preoperative levels is not compromised.12
The study objectives are:
- 1.
Description of our initial series of patients treated for DDH by mini-invasive PAO.
- 2.
Description of our patients’ functional results.
- 3.
To enumerate the technical advice of the procedure, based on our experience.
- 4.
Description of the complications, with the learning curve completed, related with this procedure.
We conducted a retrospective study of patients operated at our centre using a mini-invasive approach, described by Troelsen et al.,7 over a period of 9 years from 2007 to December 2016.
Patients were included in the study if they had persistent mechanical hip pain, hip dysplasia, congruent joint interline, joint space greater than 3mm, hip flexion greater than 110 and internal hip rotation less than 15°.
Of a total of 145 PAO with a minimally invasive approach performed in the period indicated, 3 were excluded because the radiological parameters were not included in our digital radiological system, 4 due to a lack of both digital and functional radiological parameters, and 7 because they were not fully monitored. Consequently, 131 cases were included in 118 patients (13 bilateral).
The demographic parameters (age, sex), side of intervention, radiological parameters (Wiberg's angle or lateral centre edge angle,15 angle of lateral coverage,16 acetabular index or Tönnis angle17 and joint interline of the coxo-femoral joint) were determined as variables, preoperatively and at the end of follow-up. In addition, complications (transient dysaesthesia of the lateral femoral cutaneous nerve, sciatic nerve paresis, conversion to total hip prosthesis, femoroacetabular impingement) were measured during follow-up and functional results using the Non-Arthritic Hip Score18 were measured preoperatively and at the end of the PAO follow-up.
Surgical techniqueThe patient is placed in a supine position on a radiolucent table. Antibiotic prophylaxis (cefazolin 2g) and tranexamic acid (1g) are administered prior to surgery. The anaesthetic, general, should be given with low doses of muscle relaxant (Table 1).
Tips and tricks for performing mini-invasive peri-acetabular osteotomy.
| Pre-operatively |
| Pre-operative planning of the peri-acetabular cuts and the expected acetabular orientation |
| Radiological |
| X-ray equipment must be able to move from an AP view to a Lequesne's false profile. Otherwise, we will not be precise in carrying out each of the cuts that make up the PAO |
| Lequesne's false profile requires a 30°–40° slope, allowing for better exposure of the posterior spine |
| Modifying the height of the surgical table allows increased frontal view with the X-ray equipment, to allow centring of the pubic symphysis, in an attempt to make it as similar as possible to the orthostatic pelvic X-ray |
| Neurological |
| Collaboration from a neurophysiologist to monitor the motor and sensory responses of the femoral, sciatic and obturator nerve |
| Vascular |
| Collaboration from a vascular surgeon due to risk of injury to the corona mortis which is usually located at 1cm medial to the pubic cut |
| Use a system of continuous autotransfusion to minimise blood loss during the procedure |
| Surgical |
| Position the patient in supine position on a radiolucent table |
| General anaesthesia with a low dose of muscle relaxant so as not to interfere with the neurophysiological measurements. Epidural anaesthesia is not recommended as it could mask a vascular and neurological complication |
| Osteotomes must always be sufficiently sharp |
| Follow the strict order of the osteotomies |
| Do not over pull the iliopsoas muscle, which can damage the lateral femoral cutaneous nerve and the femoral nerve |
| During the 2nd, 3rd and 5th cut the hip should rest with a slight external rotation of 20°–30° and 90° knee flexion to move laterally and keep the sciatic nerve and gluteal arteries relaxed. |
| In the 4th cut it could be useful to demarcate the osteotomy by inserting a Kirschner wire next to the cortex of the lateral ilium, and to use a blunt retroverted radiolucent retractor to protect the upper gluteal vessels |
| Do not place the blunt retroverted retractor too high so as not to damage the lumbar plexus |
| Acetabular reorientation should be performed with the hip in flexion. If performed in extension, the capsule and iliofemoral ligaments are tightened and this could lead to retroversion of the fragment |
| For acetabular reorientation the Hernández-Ros clamp or an 8mm threaded bicortical Schanz screw used in the form of a joystick can be helpful. It should be placed in the most super-medial part of the fragment so as not to force it; otherwise there is a risk of unexpected rupture |
| Postoperative pain |
| Use a local intralesional catheter for the first 24–48h to inject a bolus of 20cc containing 10cc of ropivacaine .7%, 9cc of saline and 1cc of ketorolac (30mg) every 8h. |
In our centre this intervention is always performed with X-ray equipment (OEC Fluorostar 7900 series, GE OEC Medical Systems Inc, Wendelstein, Germany), intraoperative neurophysiological monitoring, a vascular surgeon and a continuous autotransfusion system (Continuous AutoTransfusion System C.A.T.S® plus, Fresenius Kabi AG, Bad Homburg, Germany) (Table 1). The X-ray equipment is placed under the operating table, and before placing the patient, we must ensure anteroposterior (AP) and Lequesne's false profile views (Table 1). Intraoperative neurophysiological monitoring is performed by a neurophysiologist who records the motor and sensory activity of the abductor muscle groups, rectus abdominis, vastus medialis, hamstrings, gastrocnemius muscle, long fibula, posterior tibial and first toe adductor. This allows us to monitor the motor and sensory signals of the femoral, sciatic and obturator nerve, while we perform osteotomies (Table 1). The presence of a vascular surgeon is due to the risk of injury to the corona mortis, which is a retropubic anastomosis between the external iliac artery (or deep epigastric vessels) and the obturator artery. This artery is usually located 1cm medial to the pubic cut (Table 1).
The surgical fields are then placed, the trans-sartorial inguinal approach described by Söballe (Troelsen et al.7) is carried out, with special emphasis on the identification and subfascial release of the lateral femoral cutaneous nerve, in order to provide maximum displacement, and the osteotomies are performed in the following order:
- 1.
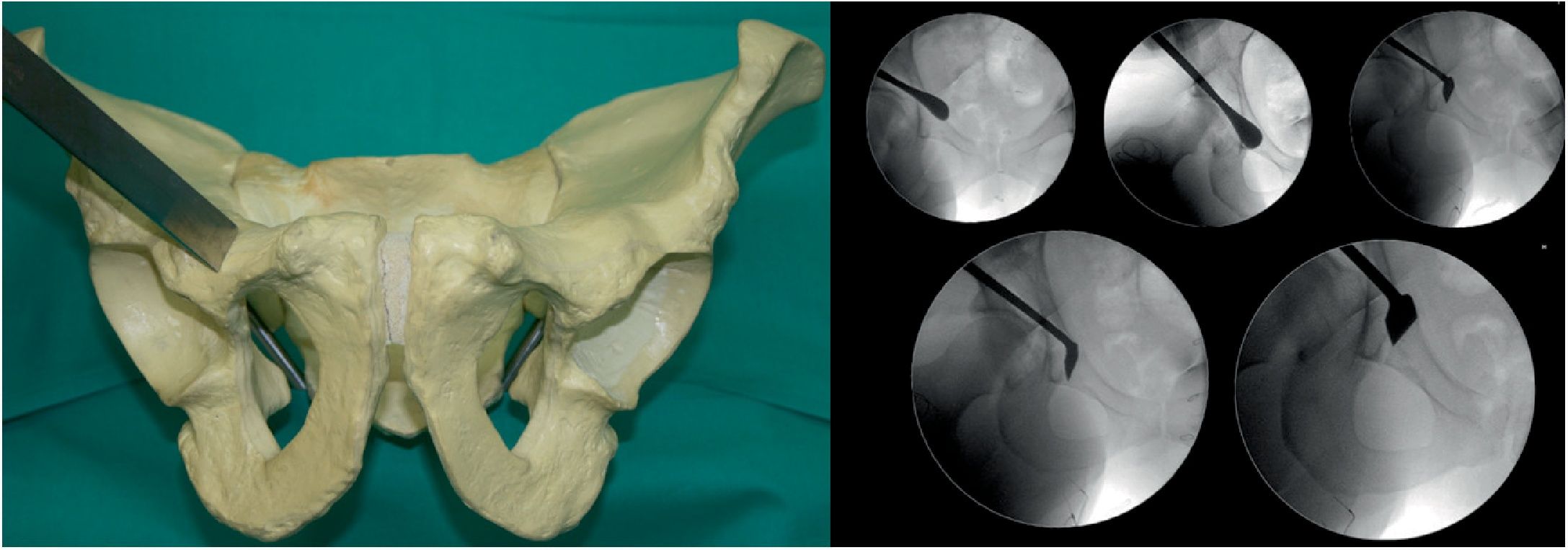
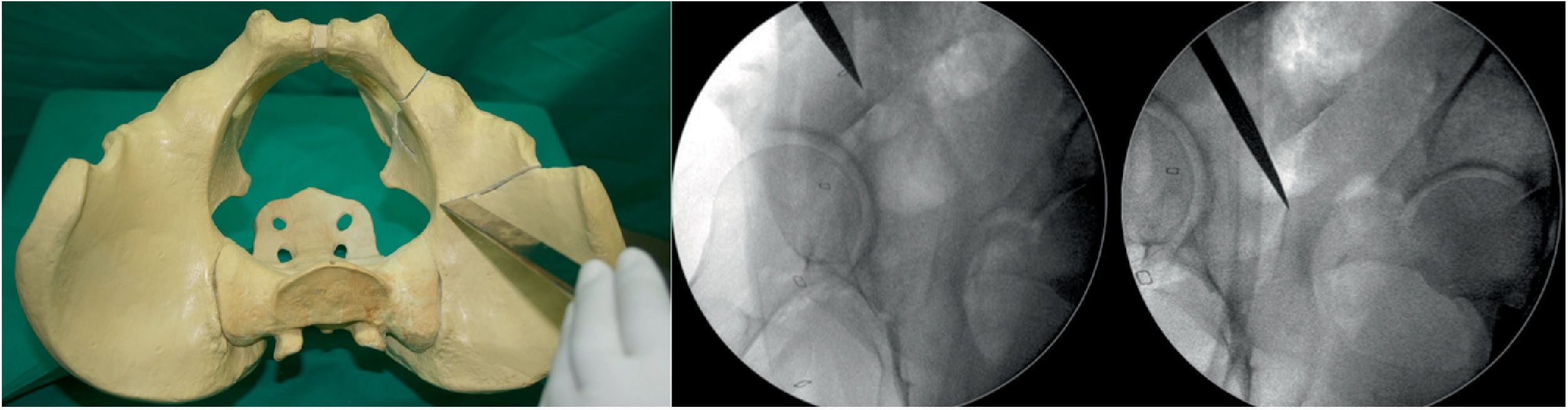
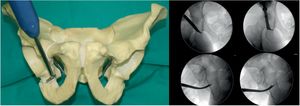
Osteotomy, with chisel, from the iliopubic branch to 1cm medial to the acetabulum. It is performed by flexing the hip from 80° to 90° and a slight internal rotation to relax the iliacus psoas and the femoral nerve. The osteotomy must be precise, no more than 1cm of the superolateral angle of the obturator foramen and is monitored by X-ray with AP and neutral and oblique inlet and outlet views (Fig. 1).
- 2.
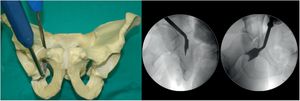
Second osteotomy (Fig. 2). Osteotomy, with chisel, of the infra-acetabular fossa under fluoroscopic control with AP and Lequesne's views. First a blunt dissection is performed with long Metzenbaum scissors to create a hole through which the Ganz or Matta osteotome can be introduced on the outer edge of the obturator hole. The osteotomy must not go beyond the posterior cortex of the ischium. It is important to keep approximately 1cm in front of the posterior cortex of the ischium so as not to create a pelvic discontinuity.
- 3.
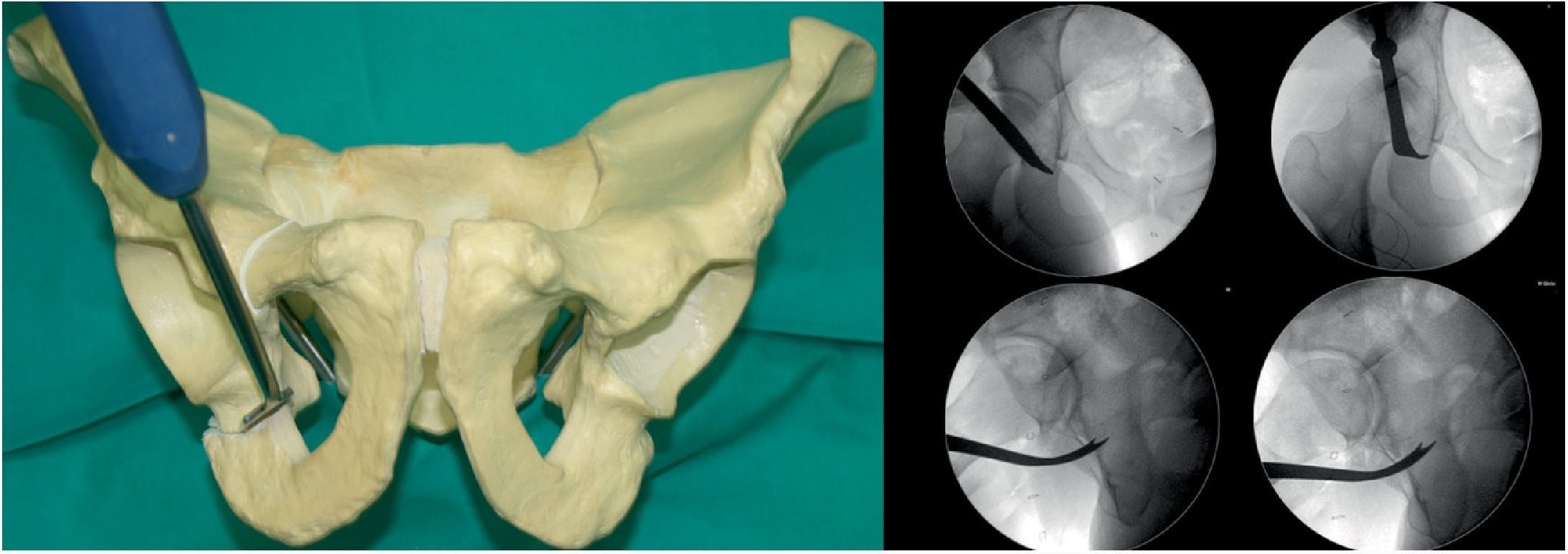
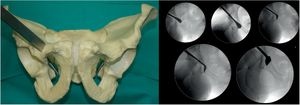
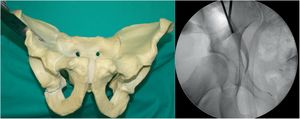
Third osteotomy (Fig. 3). A cut is made using the double-ended Ganz angled chisel, 2.5cm, which connects a point halfway between the acetabular joint line and the sciatic spine. This cut is made in Lequesne's alar view (it will give us the length) and anteroposterior (it will give us the width) and with the hip in flexion of 80°–90° with discrete external rotation to relax the sciatic nerve, due to its proximity.
- 4.
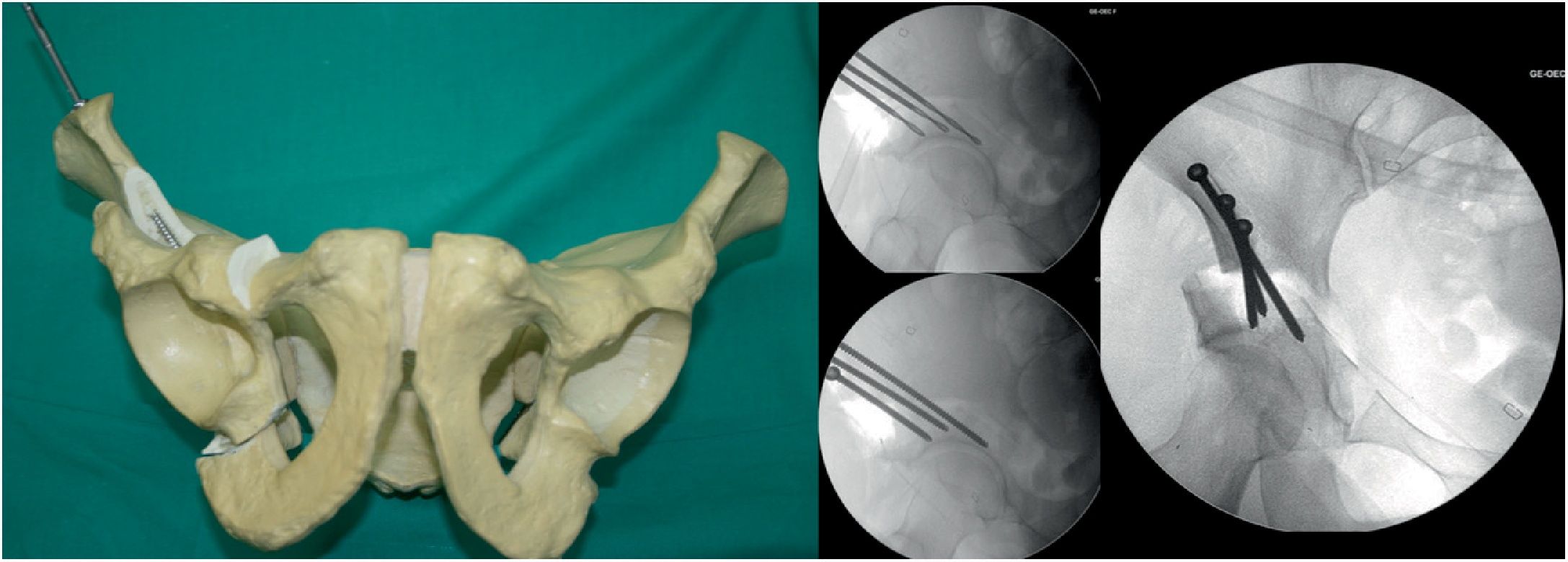
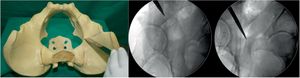
Fourth osteotomy (Fig. 4). The only cut that is performed with an oscillating saw and direct visualisation. The osteotomy runs from the iliac wing, below the anterosuperior iliac spine (3.5cm supracetabular), to 1cm of the pelvic edge (Table 1). It is controlled by fluoroscopy in AP pelvic view.
- 5.
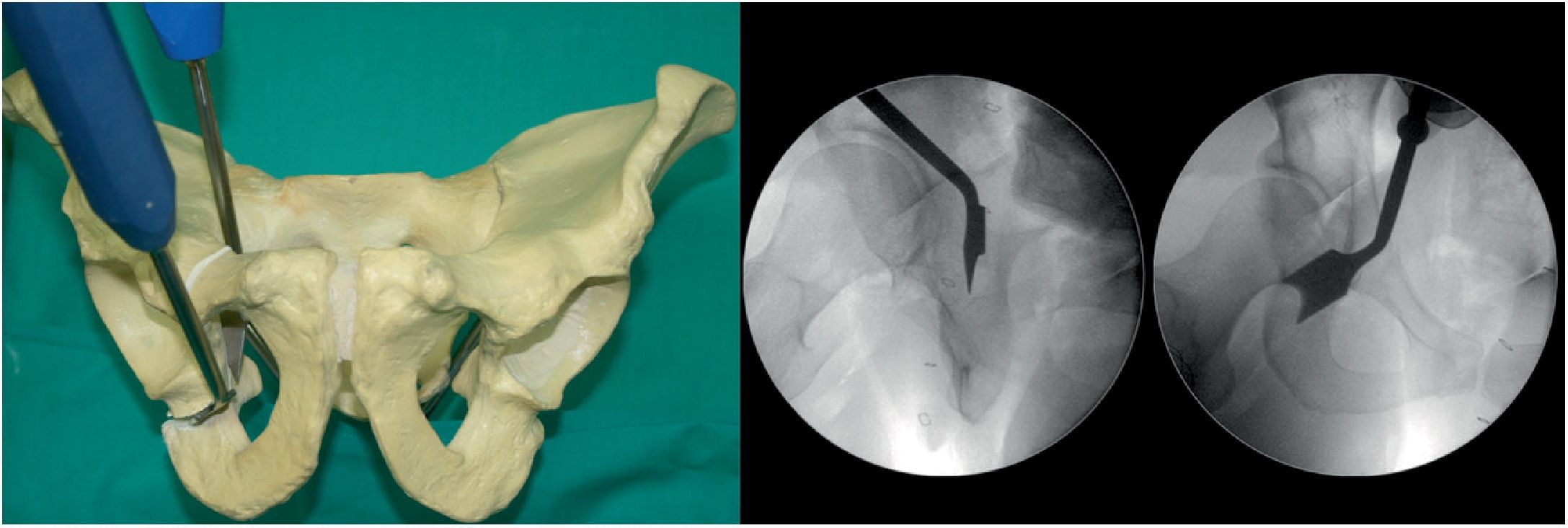
Fifth osteotomy (Fig. 5). Called “junction osteotomy”. It goes from 1cm from the pelvic wing to joining with the highest part of the third osteotomy. This osteotomy, in contrast to all the authors, is performed with 2 Lambotte osteotomes, and under fluoroscopic control with Lequesne's and AP view. At this point the acetabulum is separated from the rest of the pelvis.
- 6.
Acetabular reorientation. This is the most complex step (Table 1). Sometimes, the anaesthetist must induce additional muscle relaxation (especially in very muscular patients) in order to reorientate the acetabulum. The goal is to obtain a slight medialisation of the acetabular fragment and lateral coverage, and physiological anteversion as planned. To this end:
- -
The centre of the femoral head should be located 4 or 5mm from the posterior acetabular rim.
- -
The anterior acetabular rim should not cross the posterior rim and should be projected approximately at 1/3 from the posterior wall.
- -
The sciatic spine should not be visible in the strict AP view (which would mean retroversion) and the second osteotomy (infra-acetabular-ischium) should have seen angulation corresponding to that achieved in the femoral head coverage.
- -
- 7.
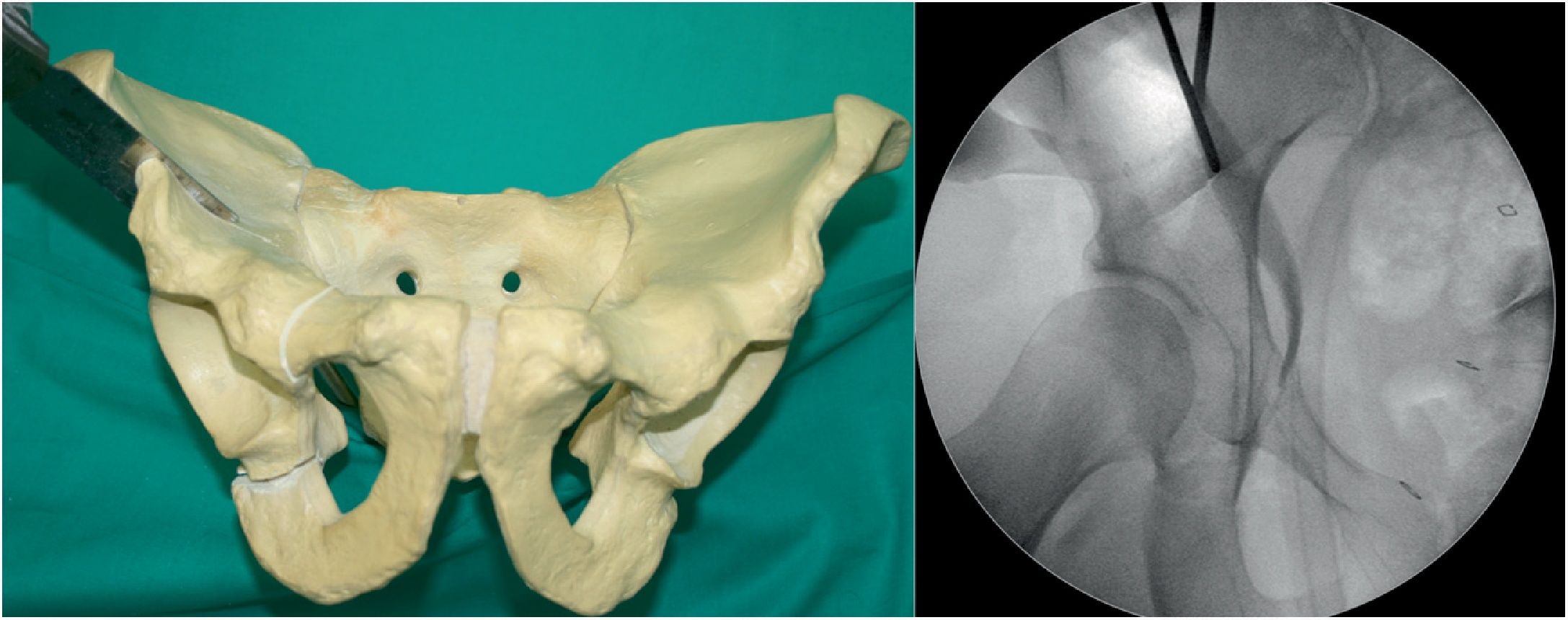
Acetabular fixation (Fig. 6). We use 2 or 3 4.5mm bicortical screws (Matta Pelvic System, Stryker Trauma AG, Selzach, Switzerland) varying from 60 to 140mm in length, starting from lateral to medial in an inverted V. During fixation we perform AP and Lequesne's views to ensure that we do not invade the coxo-femoral joint
Closure is in planes (taking care not to trap the lateral femoral cutaneous nerve), we do not use drains, and for the skin we use continuous intradermal absorbable suture.
Postoperative follow-up and rehabilitation protocolFor postoperative pain we use a local intralesional catheter for the first 24–48h to inject 20cc bolus containing 10cc of 0.7% Ropivacaine, 9cc of saline and 1cc of ketorolac (30mg) every 8h (Table 1). Additional rescue analgesia may be used, if required, or even a patient-controlled analgesia pump system may be applied.
The length of hospital stay is usually 6–8 days. With the patient haemodynamically stable (no haemoglobin loss below 3g/l is expected) rehabilitation is usually started on the first postoperative day. In the first 6 weeks passive pendulum movements and gradual, gentle kinesitherapy are performed without exceeding 90° flexion, 40° abduction, 60° external rotation, 20° adduction and 20° internal rotation (an excess of internal rotation would greatly force acetabular fixation). In addition, isometric quadriceps, gluteus maximus and gluteus medius exercises are performed. Walking starts with partial weight-bearing from 48h with the help of 2 English canes. According to the radiological progress, in the seventh week the external support is removed from the operated side and in the ninth week walking without external support and strengthening of the pelvitrochanteric musculature begins. From the eleventh week, proprioception exercises are performed.
Check-ups are carried out at 3, 6 and 12 weeks post-operatively, where a physical examination and radiological control is performed through antero-posterior and false Lequesne profile views.
Statistical analysisStata version 12.0 for Macintosh (Data Analysis and Statistical Software, Texas, USA) was used for the statistical analysis. A descriptive study of the variables was performed, and they were expressed in means and standard deviation (SD). In addition, the Shapiro–Wilk test was used to confirm the normal distribution of variables. When the normal distribution could not be confirmed or the requirements to perform the Student's t-test for paired data were not met, a non-parametric test, the Wilcoxon test, was used. A probability level of .05 was accepted as the criterion for statistical significance for all statistical tests and the confidence intervals were calculated, where possible, at a 95% confidence level.
ResultsThe mean age of the patients was 32.3±9.5 (SD) years, 102 (77.9%) were women and 29 (22.1%) were men. Fifty-three point four percent (70) of the PAOs were performed on the right side and 46.5% (61) on the left. The mean follow-up was 7.7±2.8 (SD) years.
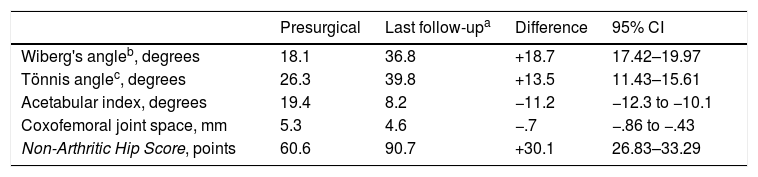
With regard to the radiological results (Table 2), the Wiberg's angle went from 18.3±7.6 (SD) to 36.3±7.83 (SD). The change was statistically significant with a gain in coverage of +18.5 (95% CI 17.26–19.74; Student's t-test for paired samples, p=.000). The anterior coverage angle increased from 26.2±9.71 (SD) to 39.7±9 (SD) at the end of follow-up. Thus, a gain was obtained of+13.5 (95% CI 11.6–15.42; Student's t-test for paired samples, p=.000). The acetabular index improved from 19.6±6.4 (SD) to 8.6±3.6 (SD) at the end of the follow-up. This reduction was also statistically significant (−11.1, 95% CI −12.13 to −10.12; Student's t-test for paired samples, p=.000).
Radiological results.
| Presurgical | Last follow-upa | Difference | 95% CI | |
|---|---|---|---|---|
| Wiberg's angleb, degrees | 18.1 | 36.8 | +18.7 | 17.42–19.97 |
| Tönnis anglec, degrees | 26.3 | 39.8 | +13.5 | 11.43–15.61 |
| Acetabular index, degrees | 19.4 | 8.2 | −11.2 | −12.3 to −10.1 |
| Coxofemoral joint space, mm | 5.3 | 4.6 | −.7 | −.86 to −.43 |
| Non-Arthritic Hip Score, points | 60.6 | 90.7 | +30.1 | 26.83–33.29 |
| Postoperative Tönnis scale17 | ||||
|---|---|---|---|---|
| Preoperative Tönnis scale17 | 0 | 1 | 2 | 3 |
| 0 | 37 | 31 | 3 | 0 |
| 1 | 0 | 33 | 4 | 0 |
| 2 | 0 | 0 | 2 | 3 |
| 3 | 0 | 0 | 0 | 0 |
CI: confidence interval.
With regard to the functional results the NAHS improved from 60.7±10.42 (SD) points to 92±11.3 (SD), the increase being +31.3 points (95% CI: 28.7–33.8, p=.000). Table 2 shows the cervical-diaphyseal femoral angle, the joint space and the degree of joint degeneration according to the Tönnis scale.17
We had complications in 20 cases (15.26%) of the 131 included in the study, which we differentiated into minor (not requiring second surgery) and major (requiring second surgery). Minor complications (12.21%): the most frequent complication was transient lateral femoral cutaneous nerve dysaesthesia (LFN), which occurred in 10 cases (58% overall of all complications). In addition, we had one case with sympathetic algodystrophy, one case of coxa saltans, one case of asymptomatic pseudoarthrosis of the iliopubic branch, one case of superficial skin infection that resolved with antibiotic therapy, one case of transient paresis of the external branch of the sciatic nerve that resolved with medical and rehabilitative treatment in less than one year, one case of delayed consolidation of the posterior spine, and one case of gluteal midline tendinitis. Major complications (2.29%): none of them neurovascular. In one case required conversion to short-stem total hip arthroplasty with ceramic-on-ceramic pair, and 2 cases with an alpha-femoral angle of more than 55° required conversion to mini open femoral-acetabular osteoplasty. These latter complications are now directly avoided by combining PAO with arthroscopic femoral osteoplasty.
In terms of the degree of progression according to the Tönnis scale, we observed that 37.6% of the patients with Tönnis 0 progressed to Tönnis 1, and 9.8% of the patients with Tönnis 1 progressed to Tönnis 2 (Table 2).
DiscussionThis retrospective study evaluates functional results and acetabular orientation capacity by means of mini-invasive PAO and found that patients treated with this technique achieve correct acetabular coverage with few complications and with a significant improvement in functional results.
There are few national studies that assess results following a PAO,19,20 and of these only the study by Díaz et al.19 performs PAO according to the technique described by Ganz et al.,6 using the modified Smith-Petersen approach and not the mini-invasive approach described by Troelsen et al.7 In 2007 we learned from Professor Søballe about the mini-invasive approach,7 and because of its advantages (same acetabular orientation capacity, reduced surgical time, very moderate blood loss [in our case no more than 500cc, which was retransfused once filtered]), less postoperative pain, less soft tissue manipulation and lower proportion of complications: we have been using this type of approach ever since.
Our results, with the mini-invasive approach, are similar and comparable to those reported in the literature,7,21,22 and to those with an established experience curve that use the modified Smith-Petersen approach.4,7,10,22,23 We found, at the end of the follow-up, a Wiberg's angle of 36.8 and an acetabular index of 8.4 (Table 2). Troelsen et al.23 conducted a study that analysed 263 PAO (165 using the mini-invasive approach and 98 using the ilioinguinal approach) and observed similar correction with both approaches, but there were more advantages with the mini-invasive approach than with the ilioinguinal approach.24 We cannot compare our results with the results of PAOs using the ilioinguinal approach,4,11,19 because it is not the same approach, and they also use different functional scales to those we have used.
Nevertheless, considering that our work is not comparative, based on the literature we were able to assess the advantages of the mini-invasive approach in relation to other approaches, which comprise, according to Troelsen et al.,7 less muscle damage with selective involvement of the sartorius muscle, less surgical time due to less time for the approach and closure. When the trans-sartorius mini-invasive approach is performed, the sartorius and iliopsoas muscles protect the femoral vessels and nerves from indirect injury, which is why the reported prevalence of moderate and severe neurovascular injuries is zero (0%), compared to the iliofemoral approach, where the prevalence of moderate and severe neurovascular injuries is 2%–3%.7 In relation to blood loss, the literature reports a loss of approximately 0.7–2l,6,23 using the modified Smith-Petersen approach, according to the results reported by Trousdale and Cabanela,25 mean blood loss was 350ml, and using the trans-sartorial mini-invasive approach the mean loss was approximately 2l6. Regarding the transfusion requirement, Troelsen et al.,7 using the mini-invasive approach, describe that it was necessary in approximately 3% of the procedures, while Bryan et al.,26 using the modified Smith-Petersen approach, describe a transfusion rate of approximately 21%.
However, we observe that, regardless of the approach used, PAO improves the patient's functional status. Alcobía Díaz et al.19 obtained 14.3 points on the Merle-D’Aubigne-Postel scale out of a possible 18, corresponding to a good outcome. Steppacher et al.4 observed that after 20 years of follow-up, the patients achieved a score of 15.8±2.1 points out of a possible 18 on the Merle-D’Aubigne-Postel scale. Using the Harris Hip Score, Peters et al.27 obtained an improvement from 54 (range, 20–81) points preoperatively to 87 (range, 49–100) points at the end of follow-up. We, with the NAHS scale, obtained an increase in the score from 60.7 points preoperatively to 92 points at the end of follow-up (Table 2).
Progression of coxarthrosis can occur in from 5% to 33%.5,28 In our results we observed that 37.6% of patients with Tönnis 0 progressed to Tönnis 1 and 9.8% of patients with Tönnis 1 progressed to Tönnis 2 (Table 2). Alcobía Díaz et al.19 observed that an increase of at least one degree on the Tönnis scale occurred in 20% of patients at 5 years of follow-up, and 54% at 10 years. Matta et al.29 demonstrated a progression of coxarthrosis by 21% in patients with Tönnis 1, 35% with Tönnis 2 and 83% for Tönnis 3.
The complication rate is related to the learning curve of the orthopaedic surgeon.30 In general, PAO carries a high risk of developing some type of complication. Complications and their severity, such as neurological or vascular lesions, occur at very low rates in the hands of expert surgeons according to Zaltz et al. and the ANCHOR group30; there is a 5.9% chance of complications that would involve further surgery.30
The ANCHOR group classifies complications into 5 grades: grade I are those that do not require treatment and do not impair the postoperative course, grade II are those that impair the normal postoperative course, requiring pharmacological treatment or more frequent controls, grade III are those that require surgical intervention and unplanned re-admission, grade IV are those that are life-threatening if not treated or that have the potential for permanent disability, and grade V are complications that cause death.30
Wells et al.31 conducted a study involving 154 PAOs using the method described by Ganz6 with a minimum follow-up of 4 years. They observed that 66 of the 154 PAOs (42.8%) developed some complication, the most frequent were those that did not require postoperative treatment (48.31%), such as the presence of asymptomatic heterotopic ossification, asymptomatic non-union or dysaesthesia of the LFC nerve. Troelsen et al.,23 when comparing the mini-invasive approach with the ilioinguinal approach observed that in the ilioinguinal group there were 3/98 cases of arterial thrombosis, while in the mini-invasive group there were no neurovascular complications or moderate/severe complications deriving from the technique.
We had complications in 20/131 cases (15%). The most frequent complication (7.63%) was temporary dysaesthesia of the LFC nerve, a grade I complication according to ANCHOR,30 which resolved satisfactorily in all patients during follow-up.
Five percent of complications were grade II. One case with sympathetic algodystrophy, one case of coxa saltans, one case of asymptomatic pseudoarthrosis of the pubic ramus, one case of superficial skin infection, one case of transient sciatic nerve paresis, one case of delayed consolidation and one case of gluteal midline tendinitis.
Only 3/131 cases (2.29%) had grade III complications according to the ANCHOR criteria, requiring postoperative surgical treatment (one conversion to total hip prosthesis and 2 osteoplasties due to femoroacetabular impingement using the mini-invasive technique). As an adjuvant treatment, when femoroacetabular impingement occurs after a PAO, surgical treatment offers satisfactory results.32–34 We had no complications that could be classified as grade IV or V.
Long-term cohort survival has been reported. Steppacher et al. describe 60% survival in their series at 20 years of follow-up.4Similarly, as reported by Ziran et al.,35 60% of patients undergoing PAO maintain their native hip after 20 years of follow-up. Although conversion from a PAO to a total hip arthroplasty (THA) could be considered a therapeutic failure, it should not be seen as such, but rather as an adjunct to THA. Baqué et al.36 concluded that PAOs prior to THA optimise patient recovery and provide greater stability to the hip.
We acknowledge that our study has certain limitations. First, it is a retrospective study and therefore has the inherent limitations of this type of study. Second, we did not conduct a comparative study with other PAO techniques, we focussed only on the technique used at our centre. Third, we did not analyse whether age influenced the progression of degenerative changes according to the Tönnis scale.17
In summary, based on our results, we have described our series of patients treated for DDH using mini-invasive PAO, describing their functional results, the technical steps of the procedure based on our experience, and the complications related to the procedure.
ConclusionPeri-acetabular osteotomy using the mini-invasive approach is a reproducible technique, allowing restoration of acetabular coverage and providing improvement on the functional scales, as confirmed in our series.
With a low complication rate that we understand, and this is also reflected in the literature, this is a safe procedure in the hands of expert surgeons. Our data suggest that most of these complications are not associated with the need for further surgical procedures.
We strongly believe in the need to extend our expertise to a greater number of hip surgeons interested in the mini-invasive treatment of residual dysplasia in children and young adults.
Level of evidenceLevel of evidence III.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ramírez-Núñez L, Payo-Ollero J, Comas M, Cárdenas C, Bellotti V, Astarita E, et al. Osteotomía periacetabular en el tratamiento de displasia de cadera mediante técnica mini-invasiva. Nuestros resultados a medio plazo en 131 casos. Rev Esp Cir Ortop Traumatol. 2020;64:151–159.