The objective of this paper is to make recommendations for the perioperative management of antirheumatic treatment based on the best available evidence. A systematic review was performed including studies in which patients with rheumatic diseases treated with biological and non-biological disease-modifying antirheumatic drugs (DMARDs) had undergone surgery. A total of 5285 studies were recorded, of which 27 were finally included. These contained information on 5268 patients and 7933 surgeries. The majority were women (mean age 55years) diagnosed with rheumatoid arthritis, and the most studied drug was methotrexate (MTX). The final recommendations include: maintaining treatment with MTX or leflunomide in the perioperative period in the absence of other risk factors for postoperative complications (Level of Evidence 1c, Grade D recommendation). Treatment with DMARDs should be temporarily suspended, or the surgery scheduled as far as possible from the last dose, and if there were other risk factors a space at least two doses (Level of Evidence 2c; Grade D recommendation).
Con el objetivo de proponer recomendaciones para el manejo perioperatorio de los fármacos modificadores de la enfermedad (FAME) en pacientes con enfermedades reumáticas que van a ser sometidos a cirugía, se ha realizado una revisión sistemática de la literatura. Se realizó una búsqueda de todos los estudios publicados y de los resúmenes de congresos, recopilando 5.285 documentos, de los que finalmente se incluyeron 27 estudios que proporcionan información de 5.268 pacientes y 7.933 cirugías. La mayoría eran mujeres (edad media: 55 años), estaban diagnosticados de artritis reumatoide y el fármaco más estudiado fue el metotrexate (MTX). Las recomendaciones finales son las siguientes: mantener el tratamiento con MTX o leflunomida en el período perioperatorio en ausencia de otros factores de riesgo de complicaciones posquirúrgicas (Nivel de evidencia 1c; Grado de recomendación D) y con respecto a los FAME biológicos, suspenderlos momentáneamente o programar la cirugía lo más alejada posible a la última dosis, espaciando al menos 2 dosis si existieran otros factores de riesgo (Nivel de evidencia 2c; Grado de recomendación D).
A high number of patients with inflammatory rheumatic disease are submitted to surgical operations, specifically orthopaedic surgeries, throughout the course of their illness. In Spain, just for rheumatoid arthritis (RA), it is estimated that 26% of patients will be subject to some orthopaedic procedure.1 Surgical complications can vary, the rate of major complications in orthopaedic surgery for RA being 3.4 in every 100 patients per year.2 The development of postoperative infections is of particular concern; they occur in around 2% or more2 of interventions,3,4 according to the series.
Discontinuing both biological and synthetic disease-modifying anti-rheumatic drugs (DMARDs) is habitual practice before surgical operations on patients with inflammatory rheumatic disease. The objective of this procedure lies in the immunosuppressive characteristic of these drugs, which theoretically increase the probability of postoperative infection. It is also based on the unverified belief that these drugs can affect surgical wound healing. On the other hand, discontinuing the primary medication for an inflammatory disease can lead to its reactivation, a situation associated with all kinds of complications, including an increased risk of infection. Consequently, other rheumatologists are reluctant toward said discontinuation. Data that support 1 practice or another are scarce, leaving many clinical questions unanswered, including whether discontinuation is really necessary, how long before the surgery treatment should be discontinued and how long after DMARD treatment should start again, etc.
The objective of this document was to develop recommendations for perioperative management of both biological and synthetic DMARDs that are used in treating rheumatic diseases. In addition, these recommendations were to be based on the best evidence available.
Materials and methodsA systematic literature review was performed, following the Cochrane5 methodology, of all studies where patients diagnosed with any rheumatic disease treated with biological or synthetic DMARDs were to have surgical interventions.
Search strategyThe search for references was done by 2 reviewers (BH and LC) using the following electronic databases: Medline (from 1950 to 14 June 2010), the Cochrane Library (from 1972 to 14 June 2010) and EMBASE (from January 1961 to 14 June 2010). The initial search was broadened with a manual search of summaries from the last 5 European League Against Rheumatism (EULAR) conferences (2007–2011) and American College of Rheumatology (ACR) conferences (2006–2010). In addition, all the bibliographical citations from the studies included were actively searched. The search results were processed by a reference manager in order to eliminate duplicates and select those that complied with selection criteria, based on titles and summaries. Articles with titles related to the subject, but without a summary, were included for closer reading.
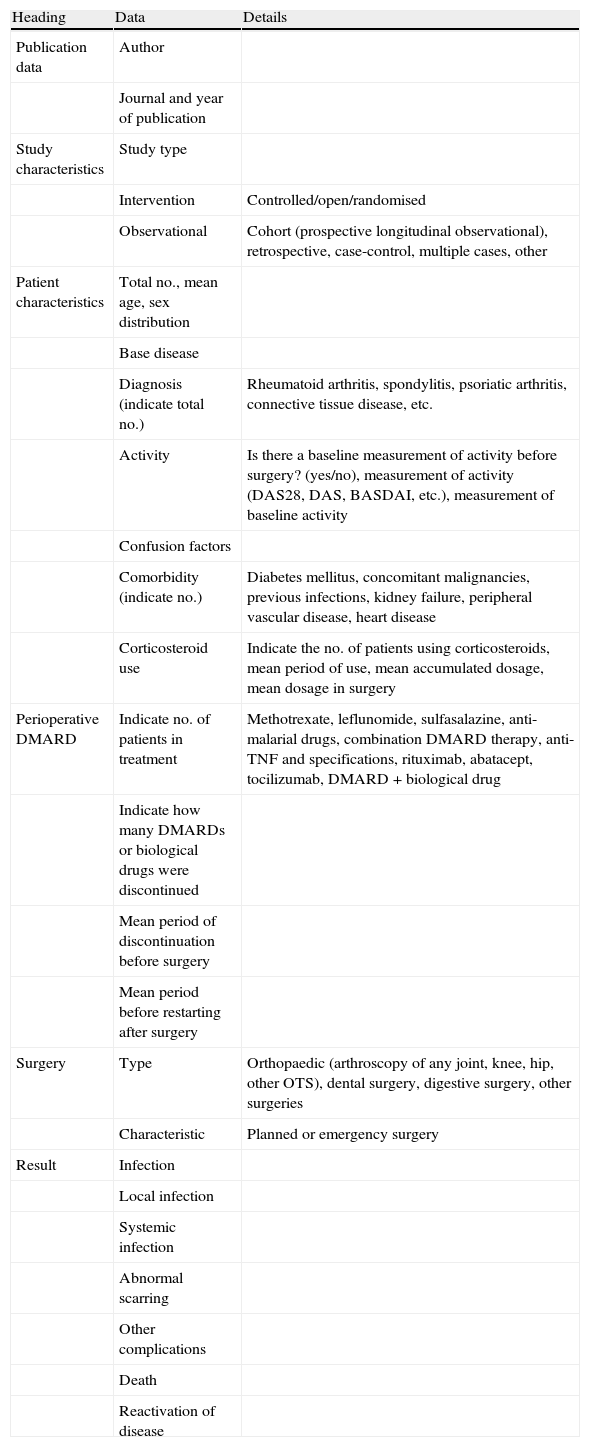
Selection criteria, data collection and analysisThe selected studies included inflammatory rheumatic disease patients being treated with classic and biological DMARDs whose objectives were: (1) to compare perioperative strategies of DMARD treatment (discontinue vs. continue treatment), (2) to measure risk of using DMARDs in relation to surgical complications, and/or (3) to measure the frequency of complications. Studies concerning isolated clinical cases were excluded. Two independent reviewers (AB and LO) selected the articles, according to title and summary, and a third reviewer (LC) compared the selected articles. Four reviewers (AB, LO, MG and BH) performed a detailed analysis of the selected articles, gathering data (Table 1) independently on paper, and a fifth (DT) included these data in an Excel® file. Quality was evaluated using the New Castle-Ottawa6 scale for risk of bias in observational studies and the Jadad7 scale for clinical trials. A meta-analysis would be performed (LC) if homogeneity existed in at least 3 studies (in study type, population and result measurement). It would also be performed in observational studies, as the few clinical trials existing were of low quality. The final level of evidence in supporting the recommendations was established based on the levels of evidence from the Oxford Centre for Evidence-Based Medicine.8
Data gathered from the articles.
| Heading | Data | Details |
| Publication data | Author | |
| Journal and year of publication | ||
| Study characteristics | Study type | |
| Intervention | Controlled/open/randomised | |
| Observational | Cohort (prospective longitudinal observational), retrospective, case-control, multiple cases, other | |
| Patient characteristics | Total no., mean age, sex distribution | |
| Base disease | ||
| Diagnosis (indicate total no.) | Rheumatoid arthritis, spondylitis, psoriatic arthritis, connective tissue disease, etc. | |
| Activity | Is there a baseline measurement of activity before surgery? (yes/no), measurement of activity (DAS28, DAS, BASDAI, etc.), measurement of baseline activity | |
| Confusion factors | ||
| Comorbidity (indicate no.) | Diabetes mellitus, concomitant malignancies, previous infections, kidney failure, peripheral vascular disease, heart disease | |
| Corticosteroid use | Indicate the no. of patients using corticosteroids, mean period of use, mean accumulated dosage, mean dosage in surgery | |
| Perioperative DMARD | Indicate no. of patients in treatment | Methotrexate, leflunomide, sulfasalazine, anti-malarial drugs, combination DMARD therapy, anti-TNF and specifications, rituximab, abatacept, tocilizumab, DMARD+biological drug |
| Indicate how many DMARDs or biological drugs were discontinued | ||
| Mean period of discontinuation before surgery | ||
| Mean period before restarting after surgery | ||
| Surgery | Type | Orthopaedic (arthroscopy of any joint, knee, hip, other OTS), dental surgery, digestive surgery, other surgeries |
| Characteristic | Planned or emergency surgery | |
| Result | Infection | |
| Local infection | ||
| Systemic infection | ||
| Abnormal scarring | ||
| Other complications | ||
| Death | ||
| Reactivation of disease |
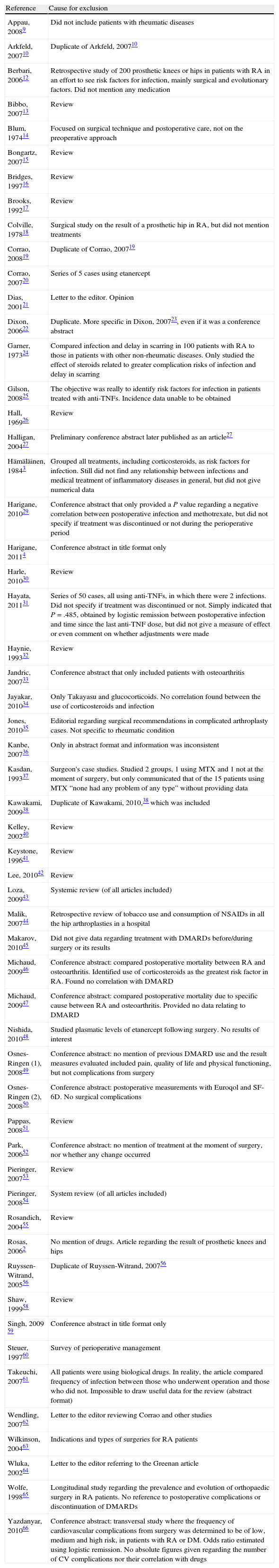
A total of 5285 documents were collected, of which, after eliminating duplicates and performing the first title and summary screening, 82 were selected and evaluated in detail. Of these, 56 were excluded due to causes indicated in Annex 1. Ultimately, 27 studies (Annex 2) published between 1991 and 2011 and the abstracts of 5 conference communications were included,11,23,79,82,84 in addition to 5 clinical trials,67,70,75,84,87 which were of questionable quality (all with a Jadad7 score of 2 or lower). The rest consisted of retrospective longitudinal studies, case series and 2 case-controls. Even though 2 cases were called case-controls, in reality they were retrospective comparative studies with different types of patients, not divided by result but by drug.39,77 The authors were from the United States (n=10), Japan (n=7), the United Kingdom (n=4) and France (n=3).
In total, these documents provided information about 5226 patients on whom 6327 surgeries were performed. In this sample, 4128 (79%) patients were female and the mean age was 56.8years (minimum: 17; maximum: 94). All patients were diagnosed with RA in accordance with ACR criteria,89 with the exception of 3 studies: 20% of those in the Ruyssen-Witrand57 study were diagnosed with spondylitis, and small percentages of psoriatic arthritis (4%) or juvenile idiopathic arthritis (JIA) (2%) were found in other studies.73,85 The postoperative follow-up duration was obtained in 19 studies and had a mean of 6months (0.5–24). Surgery type was difficult to specify, as this information was incomplete. From the data analysed, surgeries were typified as follows: 957 (15%) knee arthroplasties, 774 (12%) hip arthroplasties, 412 (6%) ankle and foot surgeries, 135 (2%) hand and wrist surgeries, 124 (2%) elbow surgeries, 114 (2%) shoulder surgeries, 64 (1%) arthroscopies and 1935 (30%) orthopaedic surgeries of another kind. In 1587 (25%) cases, the type of trauma surgery was not specified. Furthermore, 614 (9%) surgical procedures were included that were not trauma surgeries, but predominantly digestive surgery. Emergency surgery occurred in only 10 cases.
Comorbidity data and possible risk factors for postoperative infection were limited. Comorbidity was reported in 7% of the cases: ischaemic cardiopathy in 210 (4%) patients, DM in 120 (2%) patients and less than 1% for the group of high blood pressure (n=21), kidney failure (n=12) bronchiectasis (n=9) and malignancies (n=4). Previous report of infection was not found in any of the cases. In general, the description of the patients and the comparability of the study groups were not very satisfactory, except for noted exceptions (Table 2). Dixon23 did not provide data on comorbidity or predisposing factors for developing infection, but he adjusted for them in multivariate reference models. In the Giles74 study, comorbidity was analysed, but no numerical data were given. Furthermore, there were only references to results that had no significant influence. In the Fuerst73 study, the author referred to the collection of comorbidity data, but did not provide them nor adjust for them in reference models of different drugs. The Alarcón67 study did not specify characteristics of the population studied, despite being a clinical trial. The author simply referred to the comparison groups as homogenous.
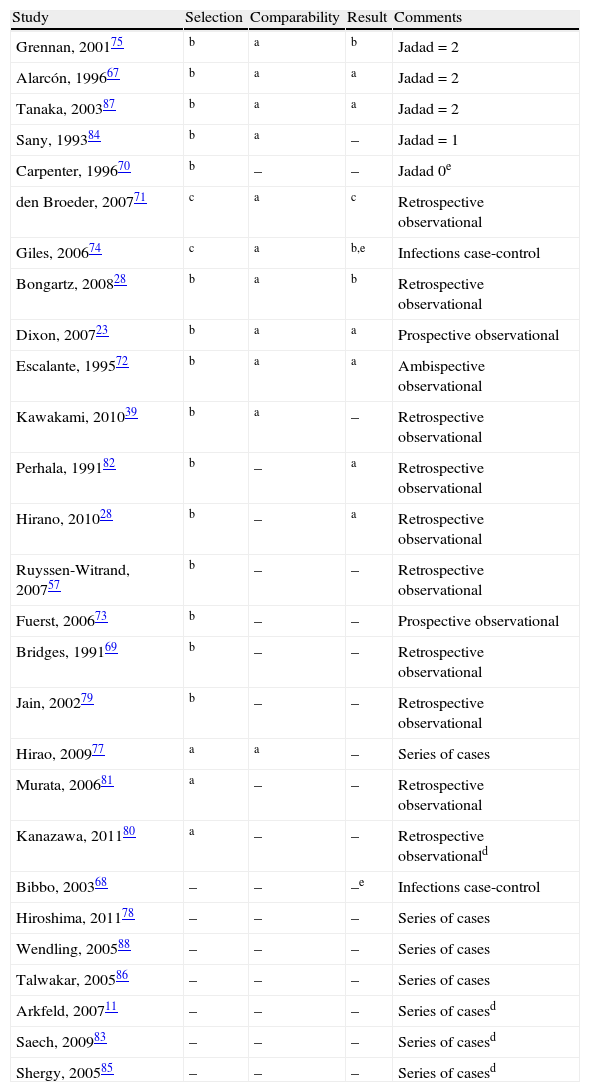
Quality (absence of bias) of the studies included, in descending order.
| Study | Selection | Comparability | Result | Comments |
| Grennan, 200175 | b | a | b | Jadad=2 |
| Alarcón, 199667 | b | a | a | Jadad=2 |
| Tanaka, 200387 | b | a | a | Jadad=2 |
| Sany, 199384 | b | a | – | Jadad=1 |
| Carpenter, 199670 | b | – | – | Jadad 0e |
| den Broeder, 200771 | c | a | c | Retrospective observational |
| Giles, 200674 | c | a | b,e | Infections case-control |
| Bongartz, 200828 | b | a | b | Retrospective observational |
| Dixon, 200723 | b | a | a | Prospective observational |
| Escalante, 199572 | b | a | a | Ambispective observational |
| Kawakami, 201039 | b | a | – | Retrospective observational |
| Perhala, 199182 | b | – | a | Retrospective observational |
| Hirano, 201028 | b | – | a | Retrospective observational |
| Ruyssen-Witrand, 200757 | b | – | – | Retrospective observational |
| Fuerst, 200673 | b | – | – | Prospective observational |
| Bridges, 199169 | b | – | – | Retrospective observational |
| Jain, 200279 | b | – | – | Retrospective observational |
| Hirao, 200977 | a | a | – | Series of cases |
| Murata, 200681 | a | – | – | Retrospective observational |
| Kanazawa, 201180 | a | – | – | Retrospective observationald |
| Bibbo, 200368 | – | – | –e | Infections case-control |
| Hiroshima, 201178 | – | – | – | Series of cases |
| Wendling, 200588 | – | – | – | Series of cases |
| Talwakar, 200586 | – | – | – | Series of cases |
| Arkfeld, 200711 | – | – | – | Series of casesd |
| Saech, 200983 | – | – | – | Series of casesd |
| Shergy, 200585 | – | – | – | Series of casesd |
a,b,cAccording to the New Castle-Ottawa bias scale for cohorts (adapted in EC) or case-controls studies.
dOnly abstract available.
eThis is a case-control study. This table refers to risk exposure, and not to the outcome, which would be the criteria for cases selection.
Data regarding previous rheumatic disease activity and treatment were not reported systematically. A report was found on some measure of activity in 21 articles. However, in only 1 case was this measurement an activity index (DAS28). In the rest, acute phase reactants or non-validated semiqualitative measures were used, such as the doctor's opinion, or 20% deterioration in the inflamed joint count. Regarding concomitant treatments, 1287 of the 2230 patients (58%) reported consuming corticosteroids with a mean dosage of 7.5mg (5–10). Furthermore, in some studies, the use of corticosteroids was the only RA treatment before surgery. Synthetic DMARDs that some patients used before surgery were methotrexate (MTX) (11%) and leflunomide (LEF) (2%). The drug most studied was MTX, found in 9 studies, including clinical trials. Leflunomide was analysed in 2 studies, 1 being a clinical trial.87 In 1399 (22%) cases, consumption of synthetic DMARDs was noted without type specification, thus making it impossible to obtain clear information regarding combinations. Biological DMARDs were used with 2033 (32%) patients, of which anti-TNFs were the most used. Tocilizumab and rituximab treatments were reported in isolated cases. Biological DMARDs were included in 8 observational studies but no clinical trials. The evidence found for drug treatment is provided in Annex 2. In several studies, no specific DMARD was studied, but rather all were studied as risk factors.
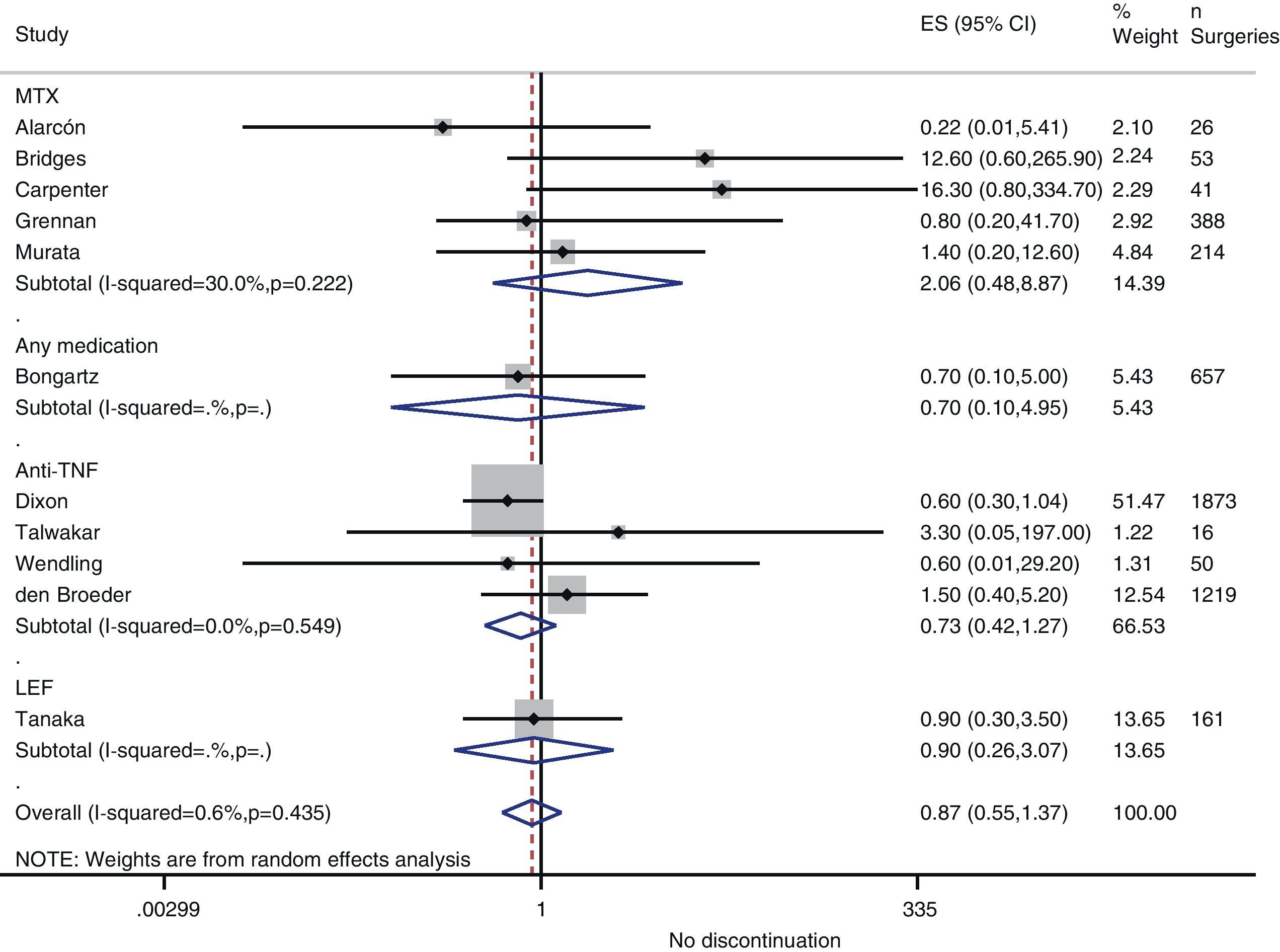
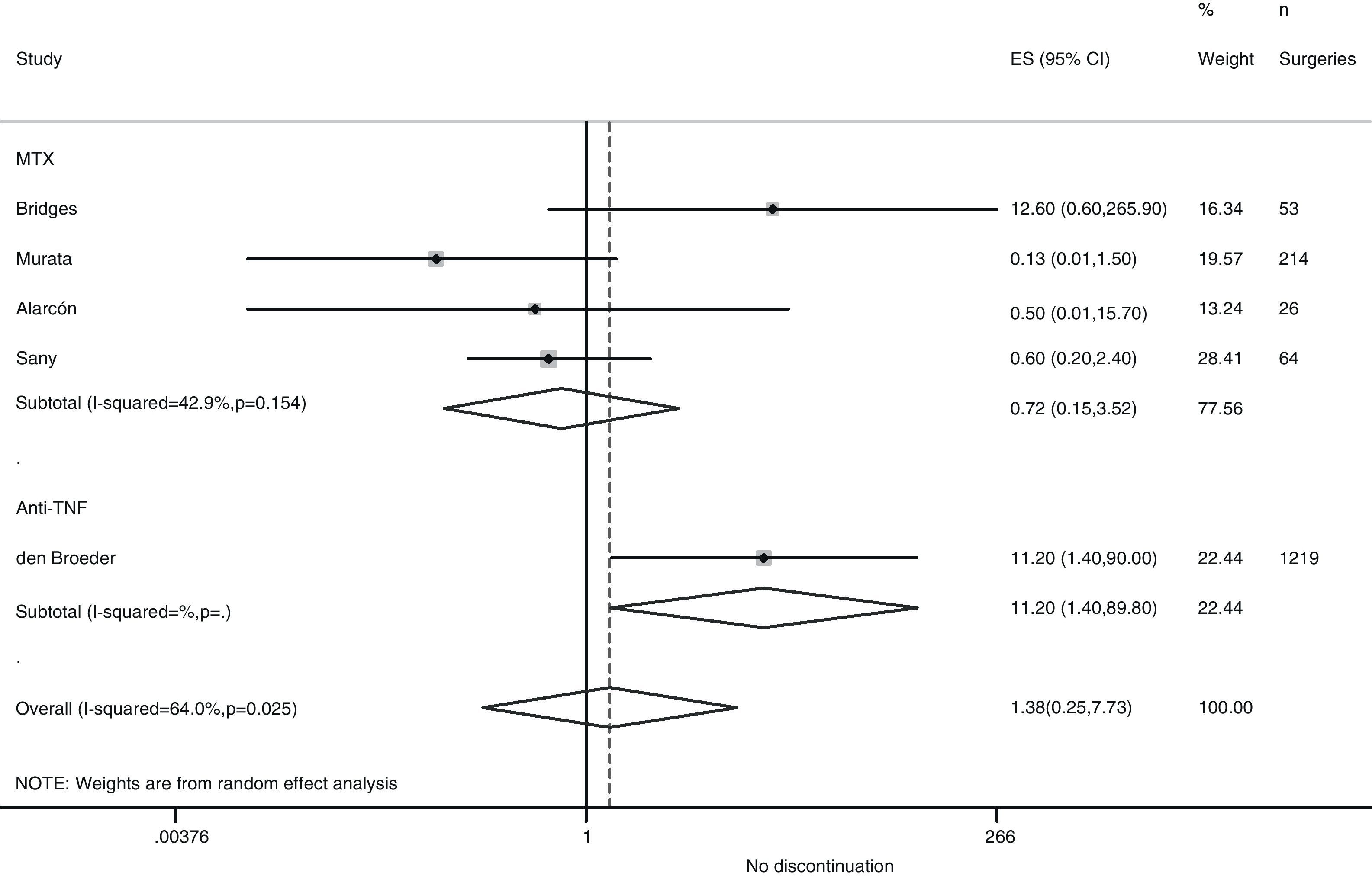
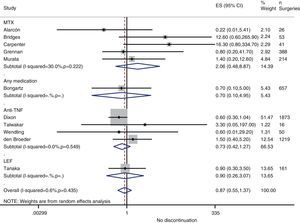
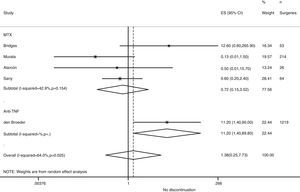
Risk of complications between strategies: discontinuation vs. continuationTable 3 shows data regarding risk of complications in studies that directly compared strategies. We performed a meta-analysis of the surgical infections and abnormal scarring: the pooled odds ratio (OR) for presenting infection complications with any DMARD whose use was not discontinued in the perioperative period was 0.8 (95% confidence interval [CI], 0.6–1.4). There were no apparent variations between DMARD types (Fig. 1). The meta-analysis of abnormal scarring did not show a defined grouped estimator for any strategy; OR 1.4, 95% CI 0.2–7.7 (Fig. 2).
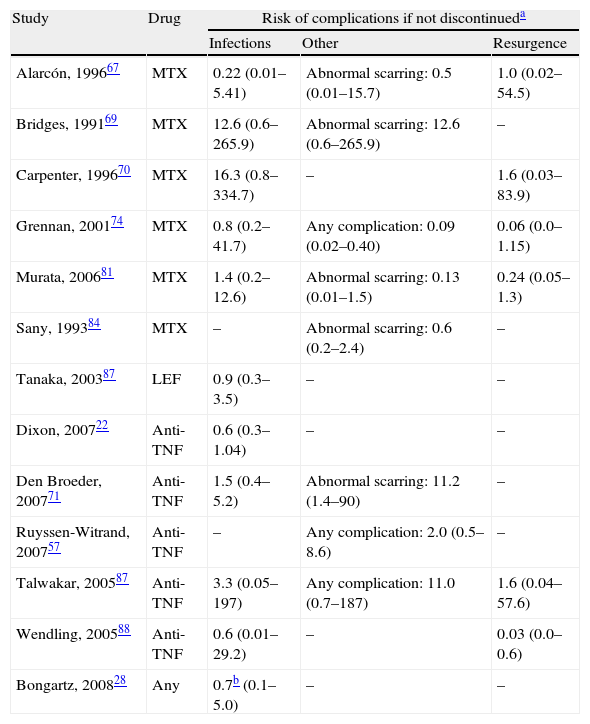
Compared risk of complications between discontinuing and continuing treatment in the perioperative period.
| Study | Drug | Risk of complications if not discontinueda | ||
| Infections | Other | Resurgence | ||
| Alarcón, 199667 | MTX | 0.22 (0.01–5.41) | Abnormal scarring: 0.5 (0.01–15.7) | 1.0 (0.02–54.5) |
| Bridges, 199169 | MTX | 12.6 (0.6–265.9) | Abnormal scarring: 12.6 (0.6–265.9) | – |
| Carpenter, 199670 | MTX | 16.3 (0.8–334.7) | – | 1.6 (0.03–83.9) |
| Grennan, 200174 | MTX | 0.8 (0.2–41.7) | Any complication: 0.09 (0.02–0.40) | 0.06 (0.0–1.15) |
| Murata, 200681 | MTX | 1.4 (0.2–12.6) | Abnormal scarring: 0.13 (0.01–1.5) | 0.24 (0.05–1.3) |
| Sany, 199384 | MTX | – | Abnormal scarring: 0.6 (0.2–2.4) | – |
| Tanaka, 200387 | LEF | 0.9 (0.3–3.5) | – | – |
| Dixon, 200722 | Anti-TNF | 0.6 (0.3–1.04) | – | – |
| Den Broeder, 200771 | Anti-TNF | 1.5 (0.4–5.2) | Abnormal scarring: 11.2 (1.4–90) | – |
| Ruyssen-Witrand, 200757 | Anti-TNF | – | Any complication: 2.0 (0.5–8.6) | – |
| Talwakar, 200587 | Anti-TNF | 3.3 (0.05–197) | Any complication: 11.0 (0.7–187) | 1.6 (0.04–57.6) |
| Wendling, 200588 | Anti-TNF | 0.6 (0.01–29.2) | – | 0.03 (0.0–0.6) |
| Bongartz, 200828 | Any | 0.7b (0.1–5.0) | – | – |
In the Grennan75 randomised clinical trial (RCT), it was evident that the incidence rate for complications was lower in the group that continued with MTX (2%) than in the group that discontinued its use (15%). The OR for complications after continuing treatment is 0.09 (95% CI: 0.02–0.40). Six months after surgery, no patient who had continued using MTX showed disease reactivation, compared to 6 (8%) patients who had discontinued. There were no variations of activity over the long term.
In the Alarcón67 study, a RCT of strategies was not performed. The sample size was not large enough due to doctors abandoning the study because they did not consider it very ethical to continue or discontinue treatment. Patients (26) were randomised to receive MTX or a placebo before and after surgery (13 in each group) with an observation period of 12weeks after surgery. There were 5 (38%) complications with MTX and 2 (15%) with the placebo. In each group, 9 (70%) knee surgeries were performed on only the patients who showed complications. There was no resurgence of the disease in any of the groups, and physical functioning after 12weeks was comparable. However, the sample size prevented a reliable conclusion from being reached, as shown in Table 3 by the wide confidence intervals calculated. An observational study with the same group69 analysed data for 38 patients undergoing MTX treatment, who underwent planned surgery. There were 8 complications among the 19 patients who continued with MTX until at least 2weeks before surgery, compared to no complications in the 34 patients who discontinued MTX treatment 4weeks or more before surgery. There were groups with other similar risk factors. The percentage of knee replacements was greater among patients who continued, as well as the percentage of diabetic patients. The evaluation was not performed independently and the confidence intervals were exceedingly wide.
Carpenter et al.70 performed an open clinical trial, in which the surgeons decided, according to their preferences, whether to discontinue MTX 2weeks before surgery or not. This was done without a blind assessment and thus had many biases. Even though the sample size was small and the confidence intervals were vague, a greater tendency toward infection was detected in the group that continued MTX treatment, as found in previous studies.
Sany84 carried out a similar study, although with randomised assignment. Of the 32 patients who did not discontinue MTX use, 13% presented some kind of complication, compared to 19% who discontinued treatment at least 1week beforehand. No group reported infection.
Murata81 performed a retrospective study of complications resulting from surgical procedures in RA in patients who had continued using MTX, compared to procedures whose patients had discontinued treatment at least 2weeks before surgery. The groups were quite comparable, except that all patients in each group were referred by a different source. There were 4 complications in the group that continued with MTX and 3 in the group that discontinued its use. There were 3 cases of the disease reactivating in each group (5% of those who continued and 14% of those who discontinued).
In the Loza43 review, the Sany and Grennan studies were meta-analysed, and no variations regarding morbidity related to surgical scarring were found between those who discontinued MTX treatment and those who did not (OR, 0.69; 95% CI, 0.23–2.02).
Discontinuing leflunomideThe Tanaka87 RCT analysed the effect of discontinuing leflunomide over 4weeks (2 before and 2 after surgery) on the rate of postoperative infections. Patients could also be treated with other DMARDs, but all treatments were discontinued before intervention in the 2 groups. The groups were quite comparable, both with more than 80% of patients using corticosteroids, although at low doses. The rate of infection was practically the same in both groups. The study did not provide data regarding resurgence of RA activity.
Discontinuing anti-TNFIn the British registry of biologists, Dixon23 examined the risk of severe postoperative infection (30days) associated with discontinuing or continuing anti-TNF treatment (28 safety days). Adjusting for age, sex, activity, diabetes and steroids, the OR for severe postoperative infection when anti-TNF use was discontinued was 0.56 (95% CI, 0.30–1.04). This corresponded to an infection rate of 7.3% continuing treatment and of 4.8% discontinuing (n=1694). Van den Broeder,71 in a retrospective study, examined the combined risk of early infection (less than 30days) and delayed infection in patients being treated with anti-TNFs, those who discontinued its use and those who did not, depending on whether or not the period up until the surgery represented 4 half-lives. The rate of surgical infection was 4% in patients not exposed, 5.8% in those exposed who discontinued use and 8.7% in those exposed who continued use. Perioperative use of anti-TNF drugs was not significantly associated with an increase in infection (OR, 1.5; 95% CI, 0.4–5.2), but was significantly associated with abnormal scarring (OR, 11.2; 95% CI, 1.4–90).
In the Ruyssen-Wytrand57 study, the rate of complications among patients who discontinued anti-TNF use more than 5 half-lives before the surgery (36 surgeries) was 19.4% compared to 18.4% among those who discontinued use later or did not discontinue at all (P=.48). If use was discontinued more than 2 half-lives before surgery, the rate of complications was 17.6% compared to 30% among those who discontinued use later or not at all (P=.24).
In the 16 surgeries exposed to anti-TNF drugs in the Talwakar86 study, no infection was found in the group that discontinued or the group that maintained anti-TNF use. One patient in the discontinuation group experienced resurgence (using etanercept). In the Wendling88 study, no serious complications occurred, and no infection was found in any of the groups. There were 6 cases (12%) of moderate reactivation with each anti-TNF, and orthopaedic surgery was significantly correlated with discontinuation.
Discontinuing any disease-modifying anti-rheumatic drugIn the Bongartz15 study, discontinuing any DMARD at the moment of surgery was associated with decreased risk. However, its relationship with prosthetic infection was not statistically significant (OR, 0.65; 95% CI, 0.09–4.95).28
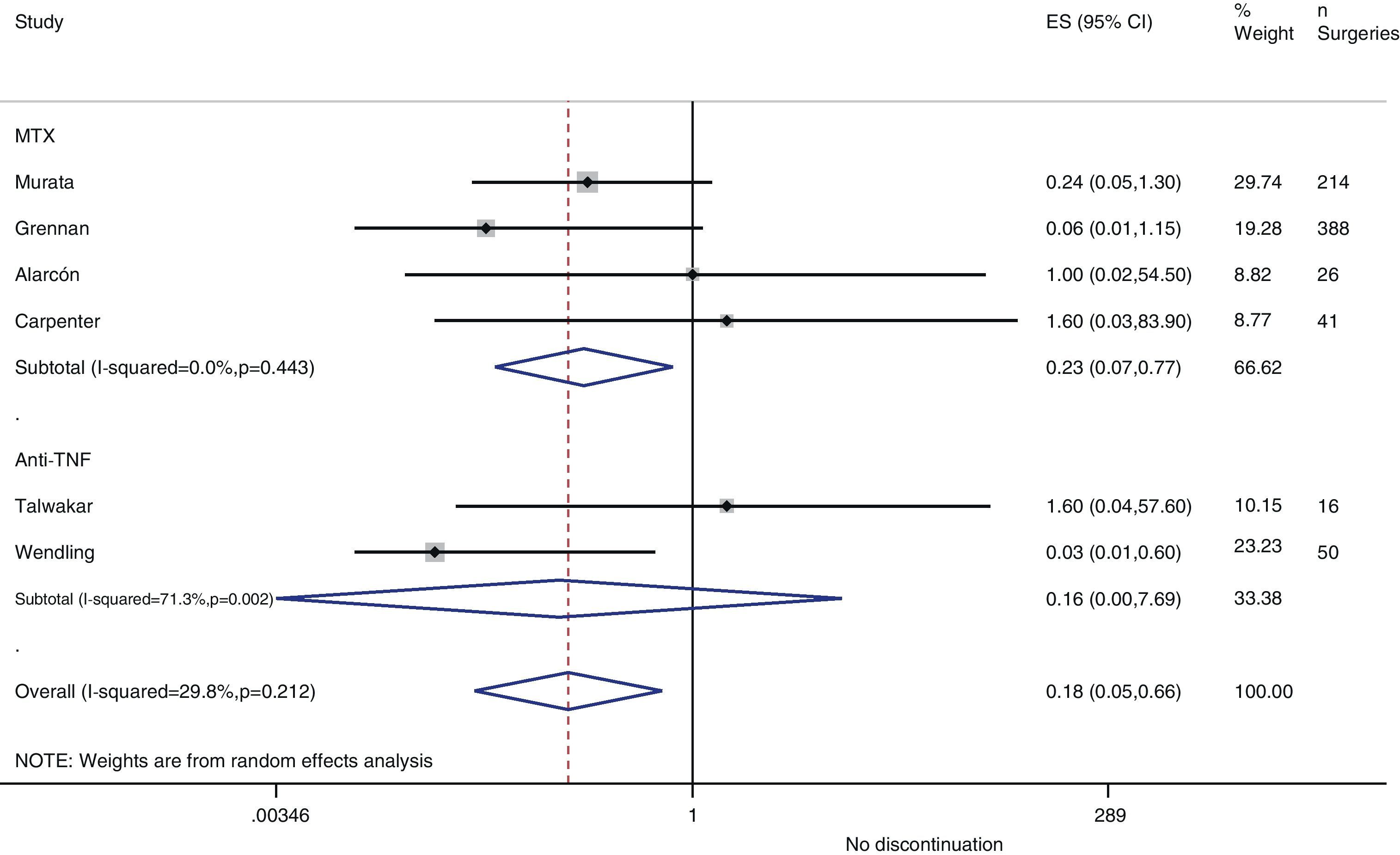
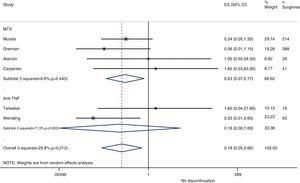
Resurgence of activityReference to baseline activity of the disease before surgery existed in only 8 studies and only 2 provided numeric data.75,77 In the postoperative period, an increase in phase reactants is typically produced and many habitual measures of disease activity thus remain altered. In the case of reactivation, if treatment was discontinued in the perioperative period, the pooled OR was 0.2 (95% CI, 0.05–0.7), in favour of not discontinuing. However, this meta-analysis had high heterogeneity (I2=29.8%), mainly in the results of the anti-TNF studies (Fig. 3). In the Kawakami39 study, which compared biological drugs to non-biological, the presence of arthralgia was used as a criterion for disease recurrence. Eleven cases of recurrence were found among those who discontinued anti-TNF use. Percentages of discontinuation were not given for any group, thus we cannot know what associations existed with discontinuation itself.
In the Alarcón67 and Carpenter et al.70 studies, activity resurgence was not found in any group (neither among those who discontinued nor those who continued MTX treatment). However, they did not explain how they defined resurgence, nor did they provide baseline disease activity. In the Sany84 study, all patients who discontinued MTX more than 4weeks showed a resurgence, but the authors did not clarify how many discontinued for that length of time nor how resurgence was defined.
Kanazawa80 demonstrated that, in 68 operations, activity resurgence (without definition) occurred in 3 patients who discontinued etanercept treatment more than 12days. In addition, activity resurgence appeared in all patients who discontinued more than 21days. The study concluded that the preoperative use of biological drugs did not constitute an independent risk factor for infection.
Other result measurementsTwo Japanese studies were centred on the appearance of fever or increase in C-reactive protein (CRP) in patients with RA who underwent surgery. In the Hirao77 study, body temperature and CRP were analysed in 22 surgeries exposed to tocilizumab and 22 exposed to a non-biological DMARD. At first, no complications were observed in either group. However, patients using tocilizumab did not show fever or elevated CRP. Hiroshima78 analysed 8 surgeries in 5 patients using tocilizumab, comparing them to 16 using anti-TNFs and 16 using classic DMARDs. All patients using tocilizumab discontinued its use 4weeks before surgery and restarted treatment 4weeks after it. Temperature and CRP levels increased in both the anti-TNF and the DMARD groups, but not in the tocilizumab group. There was no comment as to whether there were complications.
Kawakami39 found a greater difference (significant) between the pre- and postoperative CRP in those exposed and those not exposed to biological drugs. In addition, an association between the use of biological drugs and deep vein thrombosis (DVT) was found (OR, 3.0; 95% CI, 1.1–7.8), while MTX was not associated with DVT (OR, 1.2; 95% CI, 0.4–3.4).
Hirano76 examined the time up until total recovery of the surgical wound and did not find variations between those exposed to anti-TNFs and those not. Nor were there differences in postoperative fever or anaemia between the 2 groups.
Comparison between drugsSeveral studies did not provide comparative data between strategies, but they provided data on the risk of complications associated with drugs.
Regarding MTX, in the Grennan75 article, a group of patients not using MTX was included. In comparing this group to those exposed to MTX, whether they had discontinued use or not in the operative period, the number of complications did not generally differ between the groups (OR, 0.75; 95% CI, 0.37–1.53). Furthermore, no group showed more reactivation (OR, 0.95; 95% CI, 0.33–2.72). Likewise, a group not using MTX was included in the Murata81 study. In comparing the rates of infections, no variations were found (OR, 1.05; 95% CI, 0.26–4.33), nor were they found in reactivation (OR, 0.89; 95% CI, 0.29–2.76). Perhala82 retrospectively compared the proportion of complications among RA patients exposed to MTX to those among patients not exposed, the figures being 9% and 6%, respectively (OR for infections, 1.5; 95% CI, 0.4–5.9). Jain79 compared several postoperative results between 4 groups, divided according to the drugs being used at the moment of surgery. The perioperative guidelines were not modified in any group: 48 used only MTX, 30 used only prednisolone, 30 used both and 21 did not use any drug. There was a 5% rate of infection in the surgical wound among those using MTX and 4% among those who not using it (P>.05, but without adjustment for other risk factors).
In the case of leflunomide, Fuerst73 used logistic regression to see the effect of continued treatment during the perioperative period with MTX, leflunomide, etanercept, infliximab or corticosteroids on postoperative infections. No association was found for any of the drugs, not even corticosteroids, except for leflunomide (OR, 3.5; 95% CI, 1.3–9.2). It was not clear whether the data were adjusted for risk factors of infection.
Regarding biological DMARDs, Arkfeld10,11 compared the number of infections after elbow surgery in 11 patients exposed to anti-TNFs and 11 patients not exposed. Four (36%) of the exposed elbows were infected compared to 1 (9%) of those not exposed (calculated OR for anti-TNF, 5.7; 95% CI, 0.5–62.7).
Giles74 performed a case-control to see the effect that anti-TNF treatment would have on postoperative infections. Anti-TNF therapy was significantly correlated with the development of postoperative infections in the bivariate analysis (OR, 4.4; 95% CI, 1.1–18.4) and after adjustment for age, sex, use of corticosteroids, diabetes and rheumatoid factor (OR, 5.3; 95% CI, 1.1–24.9).
Dixon23 examined the risk of severe postoperative infection (30days) associated with exposure to anti-TNFs (discontinued or not) compared to non-biological DMARDs during the surgical period. Adjusting for age, sex, activity, diabetes and steroids, the OR for severe postoperative infection using DMARDs compared to anti-TNFs was 0.75 (95% CI, 0.44–1.28). In the DMARD group, the rate of infection was 5.9% compared to 7.1% in the anti-TNF group.
Den Broeder71 compared those exposed and not exposed to anti-TNFs. The OR for surgical infections in those exposed compared to those not exposed was 0.8 (95% CI, 0.3–2.0). Furthermore, sulfasalazine was identified as a protective factor against infection, with an OR of 0.21.
Kawakami39 compared the rate of infections among patients exposed and not exposed to anti-TNFs. These patients were grouped by age, sex and type of surgery. The rate of infection was clearly greater among those exposed (adjusted OR, 21.8; 95% CI, 1.2–386.1). In the Hirano76 study, the total number of complications in the group using anti-TNFs (5%) was not different from that in the unexposed group (7%), with an OR of 0.7 (95% CI, 0.1–4.0).
Ruyssen-Witrand57 provided the rate of postoperative complications with anti-TNF treatment at approximately 19% (24/127), including infections (9%), thrombosis (<1%) and scarring complications (5%). In the Shergy85 study, the rate of infection with infliximab was 3% and the rate of complications in general was 9%.
In the Saech83 study, 13 patients using rituximab who underwent orthopaedic surgery experienced a soft tissue infection and another experienced a urinary tract infection, although neither was severe. There were also 3 cases of abnormal scarring.
For other DMARDs, Bibbo68 did not find any association between postoperative infection in RA patients who underwent foot or ankle surgery and those who were exposed to a non-biological DMARD. Escalante72 studied risk factors for complications and did not find any association with DMARDs, just with azathioprine (RR=2.13; 95% CI, 1.04–4.4). The risk of complications was the same among surgical procedures in patients exposed to and not exposed to DMARDs. Furthermore, there was no difference for prednisone (RR=1.3; 95% CI, 0.9–1.8).
Other factors associated with postoperative risk of complicationsIn addition to DMARDs, other factors (for both the patient and the surgery) important when selecting patients at greater risk were studied. Among the patient factors, the use of steroids4,23, diabetes79 and hypertension80 stand out. Bongartz28 demonstrated that RA is a risk factor for surgical complications. However, age was not identified as an important risk factor. Regarding the disease, no study found any association with duration, functional class or CRP levels before the operation.28,81
Regarding factors of the surgery itself, Ruyssen-Witrand57 found a rate of complications of 12% for orthopaedic procedures, and of 6% for infections, while 50% of abdominal procedures had complications, all of them infections. Furthermore, complications in emergency orthopaedic procedures had a rate of 20%. Den Broeder71 found a greater risk of complications in elbow, foot and hand surgeries. Kanazawa80 found greater risk in knee surgeries. Bongartz28 identified the presence of infections from previous operations as a clear risk factor.
DiscussionUpon initiating this review, we decided to include studies of any quality, since a previous search alerted us to the lack of clinical trials. Conclusions and recommendations should be prudent and based, if possible, on high quality studies.
Performing clinical trials in the perioperative context is complicated, as Alarcón67 revealed in a clinical trial on perioperative strategies, which ultimately did not achieve the planned sample size. The main obstacles in the Alarcón study—besides budget cuts and little cooperation from surgeons in recruitment—were the preconceived ideas, from both rheumatologists and traumatologists, regarding how immunosuppression should be managed during this period. Strangely enough, the proportion of physicians who did not consider discontinuation to be very ethical was similar to that of those who thought the same of continuation. In both cases, these physicians abandoned the study. This polarisation in opinion also became evident in the Steuer60 study, a survey of rheumatologists in which 35% of rheumatologists and 46% of traumatologists considered MTX to be clearly correlated with postoperative complications. Even in the same centre, it was difficult to predict which patients’ treatment would be discontinued, as the decision was not often based on the patient's age, the severity of their underlying disease or their comorbidities.28 It was not even homogenous within the same centre.27
Regarding the nuances that should be considered when accepting study conclusions as valid, we were able to show that the definition of discontinuation varied from 1 study to the next. In some studies, the definition was very sophisticated, with timetables, etc. Furthermore, it especially concerned us that the definition of a half-life should vary so much between the studies. To say a patient had discontinued medication before an operation was especially complicated in observational studies, since they were based on collecting the dates of the last dose before surgery and of the surgery itself. Consequently, there was a lack of supporting evidence, not for discontinuing or continuing medication, but for how long it should be discontinued.
On the other hand, the definition of complications was fairly constant, mainly regarding postoperative infections and abnormal scarring. That allowed us to perform a meta-analysis. Even though the definition for disease reactivation was unclear and not homogenous, our meta-analysis resulted in favour of continuing medication.
At the moment of deciding a perioperative strategy, it is important to consider other factors, primarily those that increase risk of infection, such as age, diabetes, kidney failure or the use of corticosteroids in medium-high doses.3,22,23 There does not seem to be a firm relationship between clinical factors related to disease expression and complications. In a case-control concerning risk factors for developing infections—not included as it did not provide data regarding drugs—Hämäläinen3 did not find any association, not even with the previous disease duration, nor with the Steinbrocker criteria, ESR or the rheumatoid factor. However, factors related to surgery or admission did seem associated: hospitalisation period,3 day of hospitalisation (greater risk on Monday),3 ischaemia period3 and type of operation (greater risk in prosthetic knee and hand synovectomy2,4). In addition, Hämäläinen3 highlighted another variable (collected in only the Bongartz28 study) plausibly and clearly correlated with greater risk: the presence of infection in previous operations. In general, as use of corticosteroids was a constant risk factor among the studies,23,25 it seems reasonable to not discontinue immunosuppressive treatment if discontinuation makes an increase in corticosteroid dose obligatory.
It is important to indicate that in this review we found studies primarily about RA and in planned orthopaedic surgery above all. While this could be the most frequent situation, it is not possible to generalise from this. Thus, we concluded that the data was in favour of continuing treatment in order to avoid reactivation, and also that no data supported discontinuation to avoid complications. However, perhaps it is more important to consider other factors besides drugs when deciding whether or not to discontinue medication during the perioperative period.
From this review it may be inevitably concluded that, in addition to the need to perform quality studies, comparisons should be made between discontinuation and continuation strategies of immunosuppression. Studies should include control of confusion factors and objective measures of results, including primary disease activity and complications.
In conclusion, it is recommended that when a patient with an inflammatory rheumatic disease undergoes surgery, risk of infection should be considered, in accordance with perioperative risk factors and DMARD type. The following are risk factors to be considered: age, diabetes, kidney failure or use of corticosteroids in medium-high doses, hospitalisation period, ischaemia period, type of operation (greater risk in prosthetic knee and hand synovectomy) and the presence of infections in previous operations.
For the patient treated with synthetic DMARDs who does not present other risk factors of postoperative complications—such as old age, diabetes, corticosteroid treatment, kidney failure or certain surgeries—maintaining treatment with MTX or leflunomide is recommended during the perioperative period (Level of evidence 1c; Grade of recommendation D). The evidence analysis did not support a specific strategy of discontinuing or continuing the use of immunosuppressive drugs, but there were data that identified diabetes, corticosteroids and some kinds of surgery as greater risks of complications; consequently, we thought it simpler to not make any modifications in treatment regarding the surgery. Furthermore, simple strategies are easier to achieve and expose patients to fewer safety problems. Maintaining treatment is thus the desirable option in most cases.
For the patient treated with biological DMARDs without other associated risk factors for postoperative complications, discontinuing treatment momentarily, or planning the surgery as far ahead as possible from the last dose, is recommended. In the presence of other risk factors for postoperative complications, such as diabetes or corticosteroid treatment, surgery should be put off for the period of at least 2 dosages (Level of evidence 2c; Grade of recommendation D).
Level of evidenceLevel of evidence 3.
Ethical responsibilitiesHuman and animal protectionThe authors declare that no experiments were performed with humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.
| Reference | Cause for exclusion |
| Appau, 20089 | Did not include patients with rheumatic diseases |
| Arkfeld, 200710 | Duplicate of Arkfeld, 200710 |
| Berbari, 200612 | Retrospective study of 200 prosthetic knees or hips in patients with RA in an effort to see risk factors for infection, mainly surgical and evolutionary factors. Did not mention any medication |
| Bibbo, 200713 | Review |
| Blum, 197414 | Focused on surgical technique and postoperative care, not on the preoperative approach |
| Bongartz, 200715 | Review |
| Bridges, 199716 | Review |
| Brooks, 199217 | Review |
| Colville, 197818 | Surgical study on the result of a prosthetic hip in RA, but did not mention treatments |
| Corrao, 200819 | Duplicate of Corrao, 200719 |
| Corrao, 200720 | Series of 5 cases using etanercept |
| Dias, 200121 | Letter to the editor. Opinion |
| Dixon, 200622 | Duplicate. More specific in Dixon, 200723, even if it was a conference abstract |
| Garner, 197324 | Compared infection and delay in scarring in 100 patients with RA to those in patients with other non-rheumatic diseases. Only studied the effect of steroids related to greater complication risks of infection and delay in scarring |
| Gilson, 200825 | The objective was really to identify risk factors for infection in patients treated with anti-TNFs. Incidence data unable to be obtained |
| Hall, 196926 | Review |
| Halligan, 200427 | Preliminary conference abstract later published as an article27 |
| Hämäläinen, 19843 | Grouped all treatments, including corticosteroids, as risk factors for infection. Still did not find any relationship between infections and medical treatment of inflammatory diseases in general, but did not give numerical data |
| Harigane, 201029 | Conference abstract that only provided a P value regarding a negative correlation between postoperative infection and methotrexate, but did not specify if treatment was discontinued or not during the perioperative period |
| Harigane, 20114 | Conference abstract in title format only |
| Harle, 201030 | Review |
| Hayata, 201131 | Series of 50 cases, all using anti-TNFs, in which there were 2 infections. Did not specify if treatment was discontinued or not. Simply indicated that P=.485, obtained by logistic remission between postoperative infection and time since the last anti-TNF dose, but did not give a measure of effect or even comment on whether adjustments were made |
| Haynie, 199332 | Review |
| Jandric, 200733 | Conference abstract that only included patients with osteoarthritis |
| Jayakar, 201034 | Only Takayasu and glucocorticoids. No correlation found between the use of corticosteroids and infection |
| Jones, 201035 | Editorial regarding surgical recommendations in complicated arthroplasty cases. Not specific to rheumatic condition |
| Kanbe, 200736 | Only in abstract format and information was inconsistent |
| Kasdan, 199337 | Surgeon's case studies. Studied 2 groups, 1 using MTX and 1 not at the moment of surgery, but only communicated that of the 15 patients using MTX “none had any problem of any type” without providing data |
| Kawakami, 200938 | Duplicate of Kawakami, 2010,38 which was included |
| Kelley, 200240 | Review |
| Keystone, 199641 | Review |
| Lee, 201042 | Review |
| Loza, 200943 | Systemic review (of all articles included) |
| Malik, 200744 | Retrospective review of tobacco use and consumption of NSAIDs in all the hip arthroplasties in a hospital |
| Makarov, 201045 | Did not give data regarding treatment with DMARDs before/during surgery or its results |
| Michaud, 200946 | Conference abstract: compared postoperative mortality between RA and osteoarthritis. Identified use of corticosteroids as the greatest risk factor in RA. Found no correlation with DMARD |
| Michaud, 200947 | Conference abstract: compared postoperative mortality due to specific cause between RA and osteoarthritis. Provided no data relating to DMARD |
| Nishida, 201048 | Studied plasmatic levels of etanercept following surgery. No results of interest |
| Osnes-Ringen (1), 200849 | Conference abstract: no mention of previous DMARD use and the result measures evaluated included pain, quality of life and physical functioning, but not complications from surgery |
| Osnes-Ringen (2), 200850 | Conference abstract: postoperative measurements with Euroqol and SF-6D. No surgical complications |
| Pappas, 200851 | Review |
| Park, 200652 | Conference abstract: no mention of treatment at the moment of surgery, nor whether any change occurred |
| Pieringer, 200753 | Review |
| Pieringer, 200854 | System review (of all articles included) |
| Rosandich, 200455 | Review |
| Rosas, 20062 | No mention of drugs. Article regarding the result of prosthetic knees and hips |
| Ruyssen-Witrand, 200556 | Duplicate of Ruyssen-Witrand, 200756 |
| Shaw, 199958 | Review |
| Singh, 2009 59 | Conference abstract in title format only |
| Steuer, 199760 | Survey of perioperative management |
| Takeuchi, 200761 | All patients were using biological drugs. In reality, the article compared frequency of infection between those who underwent operation and those who did not. Impossible to draw useful data for the review (abstract format) |
| Wendling, 200762 | Letter to the editor reviewing Corrao and other studies |
| Wilkinson, 200463 | Indications and types of surgeries for RA patients |
| Wluka, 200264 | Letter to the editor referring to the Greenan article |
| Wolfe, 199865 | Longitudinal study regarding the prevalence and evolution of orthopaedic surgery in RA patients. No reference to postoperative complications or discontinuation of DMARDs |
| Yazdanyar, 201066 | Conference abstract: transversal study where the frequency of cardiovascular complications from surgery was determined to be of low, medium and high risk, in patients with RA or DM. Odds ratio estimated using logistic remission. No absolute figures given regarding the number of CV complications nor their correlation with drugs |
| Study | Patients | Treatments evaluated | Sx | Measurements |
| Studies that compared strategies | ||||
| Alarcón, 199667U.S. 12-weekControlled, multicentre RCT | n=26 RA (26 Sx)No reference to baseline activityNo comorbidity data | 13 (50%) discontinued MTX 2weeks before until 2weeks after 13 (50%) did not discontinue MTX | 18 knee arthroplasties8 hip arthroplasties | % postoperative infections% local infections% systemic infections% abnormal scarring% disease resurgence |
| Grennan, 200175United Kingdom 1-yearControlled RCT in a centre | n=388 RA (388 Sx); Mean age: 61 (range: 17–95); 82% femaleMeasure of baseline activity=joint count DM 4%, heart disease 5%, HBP 5%, bronchiectasis 2%, diverticulitis 0.8%, asthma 7%, osteoporosis 12 (3.1)Used corticosteroids 155 (40%) | 88 using MTX since 6weeks before and did not discontinue use 72 discontinued MTX 2weeks before until 2weeks after Sx 228 did not receive MTX | 77 planned knee Sx44 planned hip Sx275 other planned orthopaedic Sx | % postoperative infections% systemic infections% abnormal scarring% disease reactivation% other complications |
| Carpenter, 199670U.S. 1-yearOpen, controlled non-randomised CT | n=32 RA (41 Sx); Mean age: 60 (range: 35–78); 78% femalesNo reference to baseline activityNo comorbidity data69% using corticosteroids with a mean dosage of 6mg | 19 (59%) (25 Sx) discontinued MTX 2weeks before Sx 13 (41%) (16 Sx) continued with the same dosage | 10 planned knee Sx12 planned hip Sx6 planned wrist Sx | % postoperative infections% disease resurgence |
| Sany, 199384France 8-monthOpen, controlled RCT | n=64 RA (64 Sx); Mean age: 50 (range: 26–70); 91% femaleNo reference to baseline activityNo comorbidity data28% using corticosteroids with a mean dosage of 10mg | 32 continued MTX during the week of Sx 32 discontinued MTX at least 1week before until 1month after Sx | 89 planned orthopaedic Sx | % postoperative infections% abnormal scarring% disease reactivation |
| Tanaka, 200387Japan 1-yearOpen, controlled RTC | n=82 RA (161 Sx); Mean age: 57 (range: 28–77); 82% femaleNo reference to baseline activityNo comorbidity data80% using corticosteroids with a mean dosage of 5mg | 41 (82 Sx)a continued LEF 41 (79 Sx)a discontinued LEF 2weeks before until 2weeks after Sx | 99 planned knee Sx33 planned hip Sx29 other planned orthopaedic Sx | % postoperative infections |
| Murata, 200681JapanRetrospective longitudinal observational | n=122 RA (214 Sx); Mean age: 60 (range: 45–80); 82% femaleNo reference to baseline activity11% DM, 10% kidney failure, 13% hyperthyroidism | 48 (77 Sx) using MTX more than 6weeks before surgery and continued 12 (21 Sx) using MTX discontinued at least 2weeks before Sx 56 (103 Sx) not using MTX | 28 planned knee Sx82 planned hip Sx99 other planned orthopaedic Sx | % postoperative infections% abnormal scarring% disease reactivation |
| Ruyssen-Witrand, 200757France 1-yearRetrospective longitudinal observational | n=92 (127 Sx); Mean age: 54 77.2% RA, 20% SpA; no reference to baseline activityNo comorbidity data | 92 patients using anti-TNF (127 Sx) discontinued: 10<2 half-lives before Sx 55 between 2 and 5 half-lives before Sx 36>5 half-lives before Sx (6 had septic arthritis and 20 were excluded as the date of the last dose was unknown) | 13 (10%) knee Sx16 (13%) hip Sx28 (24%) arthrodesis 28 (32%) other orthopaedic Sx6 (5%) digestive Sx2 (2%) gynaecological Sx12 (9%) other Sx10 (8%) emergency Sx | % postoperative infections% abnormal scarring |
| Wendling, 200588France 1-yearSeries of cases | n=30 RA (50 Sx); Mean age: 54; 83% femaleJoint count as measurement of baseline activity. Postoperative resurgence was defined as increase in joint count and overall CAP>20%No comorbidity data 82% using corticosteroids with a mean dosage of 8.2mg | 32 continued anti-TNF18 discontinued anti-TNF | 2 planned knee Sx4 planned hip Sx29 other planned orthopaedic Sx6 planned digestive Sx5 other planned Sx | % postoperative infections% abnormal scarring% disease reactivation |
| Talwalkar, 200586United KingdomCase series | n=11 (16 Sx); Mean age: 57; 55% female 91% RA; no reference to baseline activityNo comorbidity data | 12 continued anti-TNF4 discontinued anti-TNF | 16 major orthopaedic Sx (arthroplasties and arthrodesis) and minor orthopaedic Sx (outpatient) | % complications |
| Bridges, 199169U.S. Case series | n=38 RA (53 Sx); Mean age: 59;70% femaleNo reference to baseline activity 10% DM 63% using corticosteroids with a mean dose of 8mg | 19 (50%) received MTX in the 4weeks previous to Sx 25 (66%; 6 also in the other group) discontinued MTX more than 4weeks before without using DMARD | 24 planned knee Sx20 planned hip Sx9 other planned orthopaedic Sx | % postoperative infections% local infections% abnormal scarring |
| Studies that compared pharmaceuticals | ||||
| Dixon, 200723bUnited Kingdom 30-dayCohort | n=1503 RA (1873 Sx)No reference to baseline activity or comorbidity (an abstract) | 1348 (1694 Sx) exposed to anti-TNF 1421 Sx among those who continued use 273 Sx among those who discontinued use ≥4weeks before 155 (179 Sx) not exposed to anti-TNF | 1873 total Sx1399 (75%) planned orthopaedic Sx | % postoperative infections |
| den Broeder, 200771Holland 1-yearRetrospective longitudinal observational | n=768 RA (1219 Sx); Mean age: 60; 77.3% femaleNo reference to baseline activity DM 76 (6%); Heart failure 178 (15%)Used corticosteroids 388 (32%) | Cohort 1: 1023 Sx anti-TNF-naïve Cohort 2: anti-TNF 104 Sx discontinuing anti-TNF beforehand (4 times the half-life period) 92 Sx continuing anti-TNF | 195 (16%) knee Sx172 (15%) hip Sx280 (23%) ankle and foot Sx114 (9%) shoulder Sx102 (8%) elbow Sx29 (3%) other Sx | % postoperative infections% abnormal scarring% other complications |
| Perhala, 199182U.S. 6-monthRetrospective longitudinal observational | n=121 RA (202 Sx); Mean age: 54 (range: 26–87); 82% femaleNo reference to baseline activityNo comorbidity dataUse of corticosteroids with a mean dosage of 4.78mg | 66 (92 Sx) using MTX61 (110 Sx) not using MTX | 92 total knee and hip arthroplasties | % postoperative infections% local infections% systemic infections% abnormal scarring |
| Jain, 200279 UnitedKingdom 1-yearRetrospective longitudinal observational | n=80 RA (129 Sx); Mean age: 53 (range: 28–81); 77.5% femaleNo reference to baseline activity DM 6 (5%); Cancer 4 (3%); Heart failure 11 (8.5%) 45% using corticosteroids with a mean dosage of 7.6mg | 28 (48 Sx) MTX only 18 (30 Sx) steroids only 18 (30 Sx) MTX+prednisolone 16 (21 Sx) none | 129 planned orthopaedic hand Sx | % postoperative infections% local infections% systemic infections% abnormal scarring% disease resurgence |
| Fuerst, 200673Germany 2-monthProspective longitudinal observational | n=201 (201 Sx); Mean age: 62 (range: 28–82); 85% female 94% RA; 4% PAs; 2% JIA; no reference to baseline activityNo comorbidity data 49% using corticosteroids with a mean dosage of 5.9mg | 124 MTX (65+corticosteroids) 32LEF (28+corticosteroids)25 MTX+LEF (20+corticosteroids)5 ETN (4+corticosteroids)11 MTX+ETN (9+corticosteroids)3 MTX+IFX (2+corticosteroids)1 LEF+IFX+corticosteroids | 6 planned arthroscopies of any location 44 planned knee Sx38 planned hip Sx119 other planned orthopaedic Sx | % postoperative infections |
| Kawakami, 201039JapanRetrospective longitudinal observationald | n=112 RA (128 Sx); Mean age: 57 (range: 47–61); 75% femaleArthralgia as measure of baseline activity DM 5 (5%) | 49 (64 Sx) exposed to biological drugs (IFX 21, ETN 19, TCZ 2)63 (64 Sx) not exposed to biological drugs | 33 knee Sx8 hip Sx23 other orthopaedic Sx | % postoperative infections% local infections% abnormal scarring% deep vein thrombosis% disease reactivation |
| Bongartz, 200828U.S. 1-yearRetrospective longitudinal observational | n=462 RA (657 Sx); Mean age: 64; 67% femaleNo reference to baseline activityNo comorbidity data 52% using corticosteroids with a mean dosage of 10mg | 429 Sx with DMARD (biological or non)222 (34% Sx) discontinuedb,e228 Sx without DMARD | 238 knee Sx164 hip Sx | % postoperative infections |
| Escalante, 199572U.S. 2-monthRetrospective longitudinal observational | n=204 RA (367 Sx); Mean age: 52, 90% female Steinbrocker IV 18% 10% diabetes, 40% using corticosteroids | 228 Sx using any DMARDc139 Sx without exposure to DMARD | 119 knee Sx106 hip Sx128 other orthopaedic Sx | % postoperative infections% local infections% systemic infections% prosthetic infection with discontinuation% abnormal scarring |
| Giles, 200674U.S. 30-dayCase-control | n=91 RA (91 Sx); Mean age: 59; 85% femaleNo reference to baseline activityNo comorbidity data 43% using corticosteroids | 35 (35 Sx) using anti-TNF56 (56 Sx) not using anti-TNF | 35 planned arthroscopies of any location 66 other planned orthopaedic Sx | % postoperative infections% local infections |
| Bibbo, 200368U.S.Unspecified periodCase-control | n=104 RA (725 Sx); Mean age: 56 (range: 23–83); 84% femaleNo reference to baseline activityExcluding cases of DM and peripheral vascular disease and peripheral neuropathyUsed corticosteroids 48 (46%) | 40 MTX 16 hydroxychloroquine9 goldenseal 68 combined therapy | 100% multiple planned ankle and foot Sx | % postoperative complications% abnormal scarring |
| Arkfeld, 200710,11U.S. 8-monthCase series | n=15 RA (22 Sx) No reference to baseline activityNo comorbidity data | 11 with elbows exposed to anti-TNF11 with elbows not exposed | 22 elbow plasties | % postoperative infections% local infections |
| Hirano, 201076 Japan 1-monthRetrospective longitudinal observational | n=113 RA (113 Sx); Mean age: 61 (range: 30–77); 86% female ESR, CRP and Steinbrocker functional degree as measurements of baseline activityNo comorbidity dataUse of corticosteroids with a mean dosage of 3.5mg | 39 (39 Sx) using anti-TNF (always discontinued)64 (64 Sx) using another DMARD (no discontinuation data) | 65 (58%) planned knee Sx30 (27%) planned hip Sx18 (16%) other planned Sx | % local infections% abnormal scarringDays until stitches taken outMean haemoglobinPostoperative fever |
| Hirao, 200977Japan 2-weekCase series | n=44 RA (44 Sx); DAS28-PCR as measurement of baseline activityNo comorbidity dataUse of corticosteroids with a mean dosage of 7mg | 22 Sx using tocilizumab without discontinuation22 Sx using non-biological DMARD (6 MTX, 10 sulfasalazine, 3 bucilamine, 1 D-penicillamine, 17 prednisolone (coupled by age and Sx) | 15 (68%) planned arthroscopies of any location 9 (41%) planned knee Sx1 (5%) planned hip Sx7 (32%) other planned orthopaedic Sx | Fever and abnormal acute reactant phases |
| Hiroshima, 201178Japan2weeksCase series | n=5 RA (8 Sx); Mean age: 57 (range: 47–69); 98% femaleNo reference to baseline activityNo comorbidity data | 5 (8 Sx) using tocilizumab (discontinued 4weeks before) and coupled by age and Sx type: 16 Sx using anti-TNF 16 Sx using non-biological DMARD | 1 (13%) planned knee Sx3 (38%) planned hip Sx4 (50%) other planned orthopaedic Sx | Fever and abnormal acute reactant phases |
| Kanazawa, 201180JapanUnspecified periodRetrospective longitudinal observational | n=442 RA (887 Sx); Mean age: 61; 90% femaleNo reference to baseline activityNo comorbidity dataNo corticosteroid data | 347 patients not using biological drugs33 using ETN62 using other biological drugs | 887 planned orthopaedic Sx | % infections |
| Saech, 200983GermanyCase series | n=13 RA (18 Sx); Mean age: 61; 53% femaleNo reference to baseline activityNo comorbidity dataWith the exception of a depletion in CD19+B lymphocytesAll using corticosteroids with a mean dosage of 7.5mg | 13 using rituximab | 14 orthopaedic Sx4 other Sx | % postoperative infections% abnormal scarring |
| Shergy, 200585U.S.Case series | n=73 RA (76 Sx)No reference to baseline activityNo comorbidity data | 73 using infliximab | 76 unspecified Sx | % postoperative infections |
DM: diabetes mellitus; ETN: etanercept; IFX: infliximab; LEF: leflunomide; MTX: methotrexate; RCT: randomised clinical trial; Sx: surgery.
Could have also been treated with D-penicillamine, goldenseal, sulfasalazine or MTX, but all were discontinued before the Sx.
In this study, strategies were also compared in the group exposed to anti-TNF, but we included it under this heading only to avoid duplicating it in the table.
Compared patients with complications to patients without complications, but also specified that DMARD treatment was not discontinued in the institution, thus leading us to assume that most patients continued.
Described by the authors as a case-control, because patients not using anti-TNFs were selected, coupled by age, sex and surgery type.
In accordance with a table of days for each drug: MTX 8, leflunomide 85 or 14 using cholestyramine, oral goldenseal 8, intramuscular 29, sulfasalazine goldenseal 8, hydroxychloroquine 85, azathioprine 8, cyclosporine 8, cyclophosphamide 8, D-penicillamine 15, etanercept 8, adalimumab 15, infliximab 57 and anakinra 8.
Please cite this article as: Del Olmo L, et al. Manejo perioperatorio de los fármacos modificadores de la enfer-medad en Reumatología: recomendaciones basadas en un metaanálisis. Rev Esp Cir Ortop Traumatol. 2012;56:393–412.