To evaluate the effectiveness and safety of a single intravenous dose of tranexamic acid in order to reduce blood loss in total knee replacement.
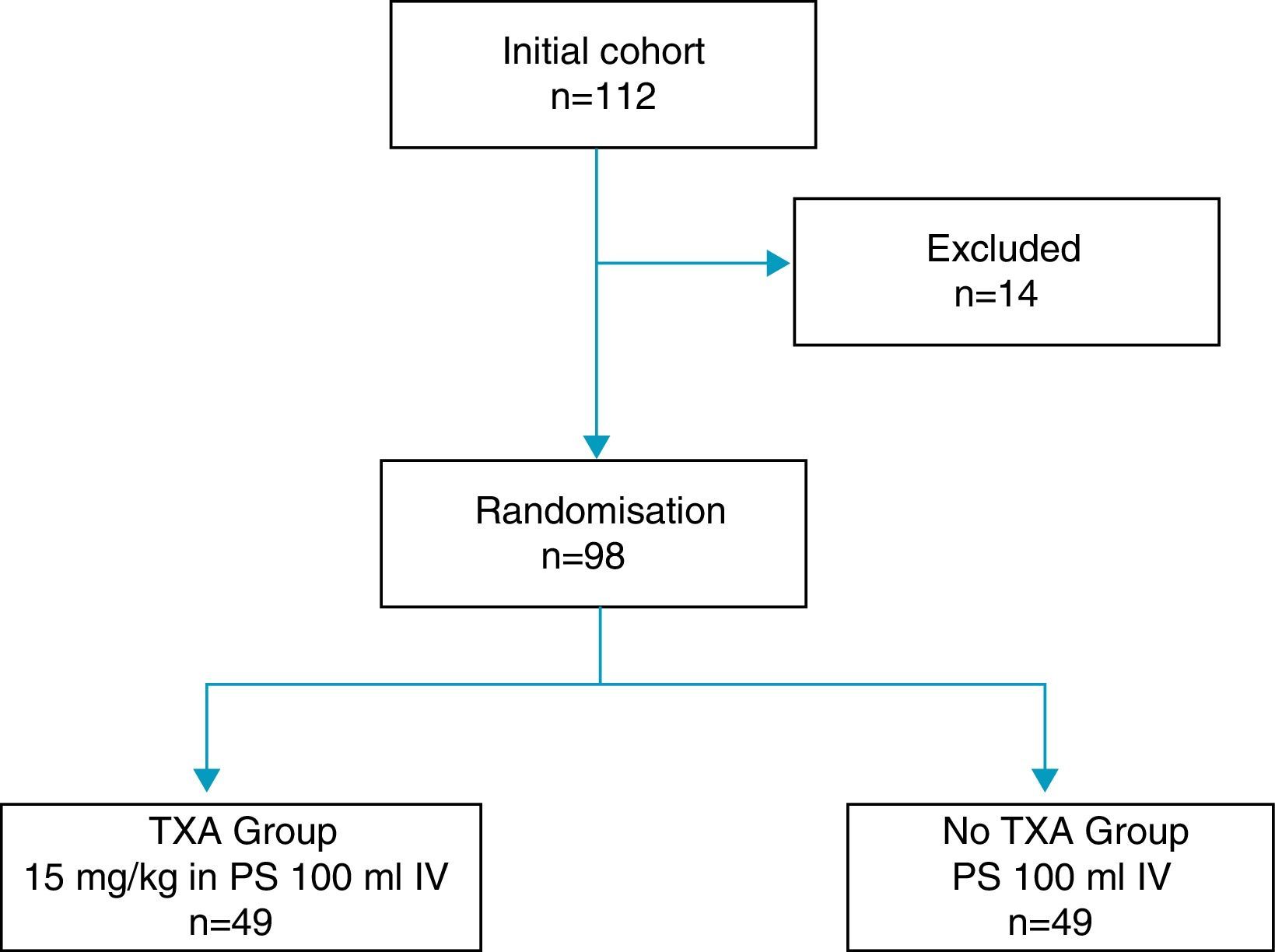
Materials and methodsProspective observational study of the administration of tranexamic acid in patients undergoing primary total knee arthroplasty from November 2013 to February 2015, in which an autologous blood recovery system was used. The study included 98 patients, distributed into two groups of 49 patients according to whether or not they received intravenous tranexamic acid. The primary endpoint was the number of patients requiring autologous transfusion from the recovery system autologous blood recovery system.
ResultsNo drop-outs were recorded during follow-up. There were no significant differences between groups as regard the preoperative and hospital variables. The mean preoperative haemoglobin and haematocrit at 24 and 48h postoperatively were similar in both groups. The average volume of bleeding in the autologous blood recovery system and estimated average blood loss was lower in patients who had been administered tranexamic acid, with significant differences. No patients in the group that was administered tranexamic acid required blood autotransfusion. The transfusion rate was zero in the two groups. No adverse events related to the administration of tranexamic acid were recorded.
ConclusionsIntravenous administration of tranexamic acid, according to the described protocol, has presented a non-autotransfusion or allo-transfusion rate of 100%, with no increased incidence of thrombotic events. Thus, its use in this group of patients is recommended. The indication should be individualised, its use justified in the patient medical records, and informed consent is mandatory.
Evaluar la eficacia y seguridad de la administración de una dosis única intravenosa de ácido tranexámico como medida de ahorro transfusional en prótesis total primaria de rodilla.
Material y métodosEstudio observacional prospectivo de la administración de ácido tranexámico en pacientes intervenidos de prótesis total primaria de rodilla desde noviembre de 2013 a febrero de 2015, en los que se utilizó un sistema de recuperación de sangre autóloga. Se incluyeron en el estudio 98 pacientes distribuidos en dos grupos de 49 pacientes según la exposición a la administración de ácido tranexámico. La variable principal del estudio fue el número de pacientes que precisaron autotransfusión del sistema de recuperación de sangre autológa.
ResultadosNo se registraron pérdidas durante el seguimiento. No hubo diferencias significativas entre ambos grupos con respecto a las variables preoperatorias y hospitalarias. Los valores medios de hemoglobina y hematocrito preoperatorios, a las 24 y 48 h postoperatorias eran similares en ambos grupos. El volumen medio de sangrado en el sistema de recuperación de sangre autóloga y la pérdida media estimada de sangre fue menor en los pacientes a los que se había administrado ácido tranexámico, siendo las diferencias significativas. Ningún paciente del grupo en el que se administró ácido tranexámico precisó autotransfusión sanguínea. No se precisó alotransfusión sanguínea en los pacientes de la cohorte. No se registraron eventos adversos relacionados con la administración del ácido tranexámico.
ConclusionesEl uso de una dosis única 15 mg/kg de ATX intravenoso en PTR primaria ha presentado una tasa de no autotransfusión ni alotranfusión sanguínea del 100%, sin aumento en la incidencia de eventos trombóticos. Por ello recomendamos su utilización en este grupo de pacientes, con una indicación que debe ser individualizada, justificar su uso en la historia clínica y precisar del consentimiento informado del paciente.
Nivel de evidencia III.
Knee replacement surgery (KRS) is a procedure for which between 39% and 67% of patients require an allogenic blood transfusión.1 The release of the ischaemia at the end of the intervention causes bleeding due to the increase in the fibrinolytic activity which sometimes necessitates an allogenic blood transfusion in patients, that is not without complications and risks. Moreover, these transfusions may also have a negative affect on the outcome of surgery and increase the risk of perioperative infection, hospital stay and health care costs.2,3 As a consequence, it is recommended that transfusional policies be applied to reduce blood loss in perioperative stages.4
The use of perioperative autologous blood recovery (ABR) systems reduces the relative risk of allogenic transfusion by 42% according to Cochrane's study in KRS, with several blood quality conditions an absolute guarantee.5 For this, blood flow above 400ml needs to be in the system during the first 4h, which must be transfused before 6h have passed. Large volumes of blood for transfusion are not recommended, due to the risks and complications this may entail.6
Tranexamic acid (TXA) is a synthetic derivative of lysine with a pure antifibrinolytic activity. Its mechanism of action is based on it binding to the lysine bond of plasminogen. This prevents the fibrin from binding to the complex formed by the plasminogen-plasmin tissue activator complex and degrading fibrin.7 Another possible effect is the protection of platelets based on its antiplasmin effect and the inhibition of the platelet activation in addition to the reduction of the loss of intracapilliary albumin maintaining intravascular volume.8 The administration of TXA as a means of cost saving in transfusion in KRS is strongly evident in literature due to its efficacy and safety, in randomised and meta-analysis studies, with very low perioperative allogenic blood transfusion rates.9–11
The working hypothesis was that the administration of a single 15mg/kg dose of intravenous TXA would prevent allotransfusion and autotransfusion in primary KRS.
Material and methodA prospective observational study was designed for the administration of TXA in patients undergoing primary KRS where an ABR system was used. This began in November 2013 and terminated in February 2015. Previous studies referring to an autotransfusion rate of 34%12 of the ABR system in KRS were reviewed in order to calculate the sample size. In keeping with perioperative blood reduction results recorded in literature,13,14 49 patients were required for each group so as to obtain a confidence level of 95% and a statistical power of 90%, and with a maximum auto transfusion rate for the ABR system in patients to whom TXA was administered at 10%. Randomisation of patients was made using the balanced random assignment method previously made statistically. Sealed opaque envelopes, one per sample subject, were opened in the operating theatre by the anaesthetist, to determine whether the physiological saline was to be administered alone or with TXA.
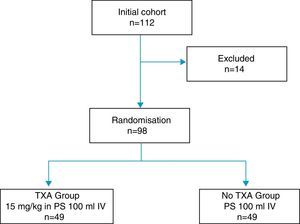
During said period 112 patients who had been diagnosed with osteoarthritis of the knee and referred for KRS were included in the study. Exclusion criteria of the study were established as: allergy to TXA, severe ischaemic cardiomyopathy, severe kidney failure, severe lung failure, liver failure, haematological disease, retinopathy, allogenic blood administration rejection, INR>1.4 and arterial or venous thromboembolic disease. The trial was approved by the hospital's Ethics Committee and obtained the informed consent from all patients.
14 of the total patients were excluded: 6 due to severe comorbidities, 4 due to consumption of dicoumarinics, and 4 due to a history of thromboembolic disease, with the remaining 98 patients constituting the study cohort, distributed into two groups 49 patients each, according to the exposure to intravenous administration of TXA (Fig. 1). One group of patients received an intravenous dose of 15mg/kg of TXA (Anchafibrin®, Rottapharm) in 100ml physiological saline between 15 and 20min prior to the release of ischaemia. The Bellovac® ABR system (ABT system of the Wellspect Group) was used in all patients, for replacing blood loss during surgery. The time for blood collection and re-infusion was between 4 and 6h after surgery, and quantities over 400ml and under 1000ml were re-used.
All operations were performed by three orthopaedic surgeons with experience in KRS using the internal parapatellar approach with saddle block anaesthesia. The KRS model was identical in all patients (Genesis®, Smith and Nephew) and established afterwards with the cemented components. The cement used was Copal® G+C (Heraeus). The use of ischaemia was protocoled from the beginning to the end of the intervention in all patients and released after applying the compression bandage and once the ABR draining system had been established.
Femoral artery blockage was performed on all patients as a measure of perioperative analgesic control. Conventional analgesic, intravenous therapy was also used for the first 48h (paracetamol, metamizol and tramadol). Cefazolin or vancomycin were used preoperatively and continued for 24h postoperatively as antibiotic prophylaxis followed by antithrombotic treatment with enoxaparine for 30 days after hospital discharge. No posterior doses of TXA were administered on the hospital ward. 24h later the patients were up in a chair and began active physiotherapy according to the instructions given by the rehabilitating doctor. After 48h the wound was covered, the ABR system was removed and the patient was allowed to use a walking frame.
A blood transfusion was indicated if haemoglobin levels were lower than 8g/dl in asymptomatic patients with no history of heart disease and lower than 9g/dl in symptomatic patients or those with associated heart disease.
The main variable of the study was the number of patients who required an autotransfusion from the ABR system. Secondary variables were recorded which included: epidemiologic variables, pre and post operative haemoglobin and haematocrit levels at 24 and 48h, bleeding in the ABR system, number of patients who required allogenic blood transfusions, hospital stay, re-admittance and adverse events related to the use of TXA for the three months after the operations. The TXA usage rate was also determined in the saving of heath care costs. Patient follow-up was for 3 months after surgery.
Two methods were used to calculate blood loss in surgery. The first was to determine the blood in the ABR system. The second consisted in applying the Mercuriali formula based on preoperative haematocrit levels and haematocrit levels after 48h.15
The SPSS 17.0 for Windows was used for data collection and data processing. To determine normal distribution the Kolmogorov–Smirnov test was used. The Fisher test was applied for contrasting qualitative variables and the student t test for comparing the means of separate samples from numerical variables with normal distribution. A p value equal to or lower than 0.05 was considered significant.
ResultsNo losses were recorded during follow-up.
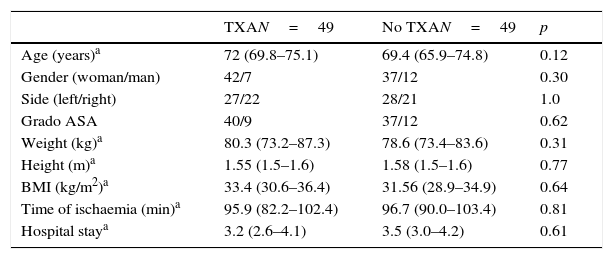
There were no significant differences between both groups with respect to age, sex, side, ASA grades, weight, size, size, BMI, mean ischaemia time during surgery and hospital stay (Table 1).
General data of the series.
| TXAN=49 | No TXAN=49 | p | |
|---|---|---|---|
| Age (years)a | 72 (69.8–75.1) | 69.4 (65.9–74.8) | 0.12 |
| Gender (woman/man) | 42/7 | 37/12 | 0.30 |
| Side (left/right) | 27/22 | 28/21 | 1.0 |
| Grado ASA | 40/9 | 37/12 | 0.62 |
| Weight (kg)a | 80.3 (73.2–87.3) | 78.6 (73.4–83.6) | 0.31 |
| Height (m)a | 1.55 (1.5–1.6) | 1.58 (1.5–1.6) | 0.77 |
| BMI (kg/m2)a | 33.4 (30.6–36.4) | 31.56 (28.9–34.9) | 0.64 |
| Time of ischaemia (min)a | 95.9 (82.2–102.4) | 96.7 (90.0–103.4) | 0.81 |
| Hospital staya | 3.2 (2.6–4.1) | 3.5 (3.0–4.2) | 0.61 |
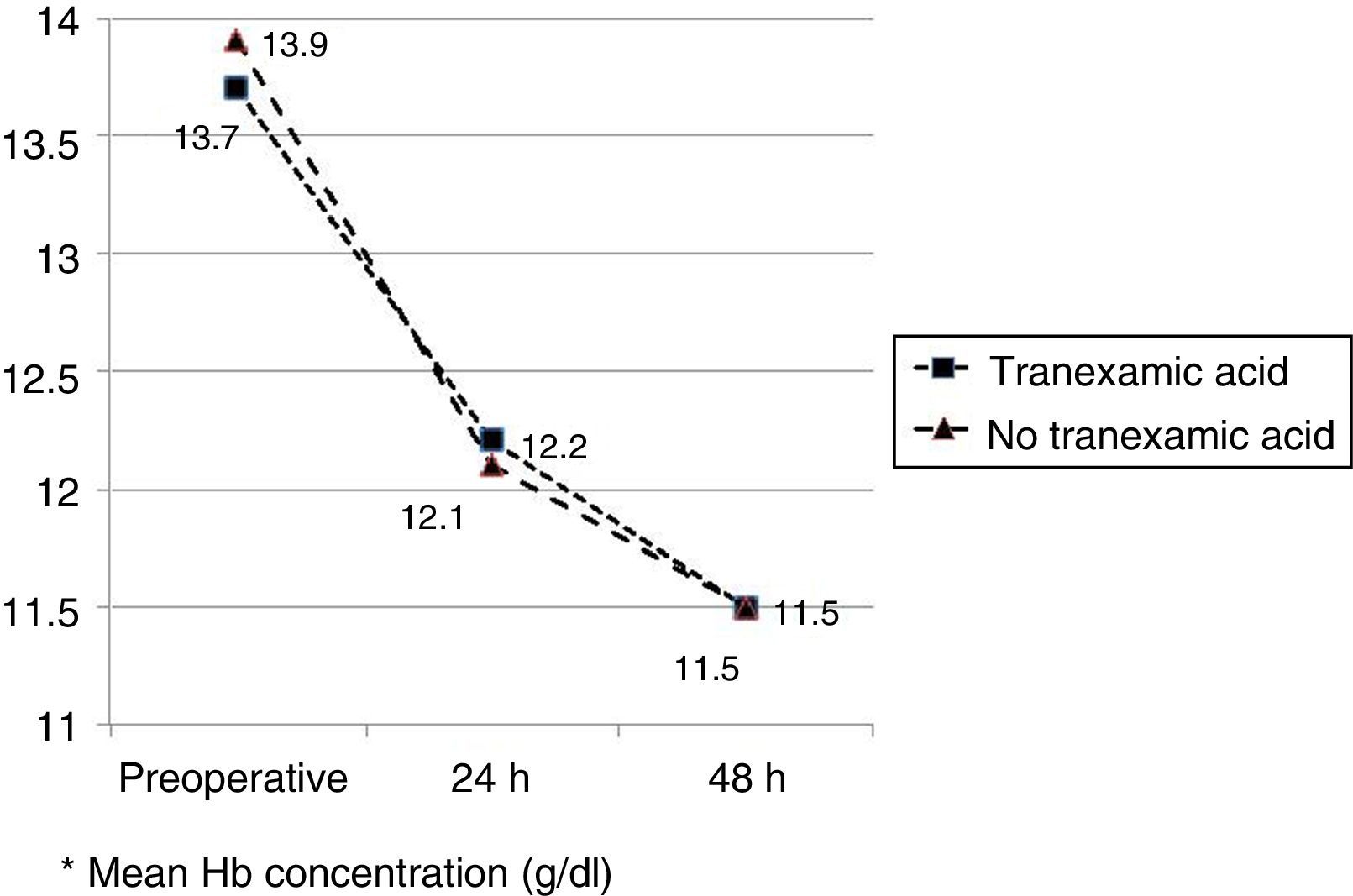
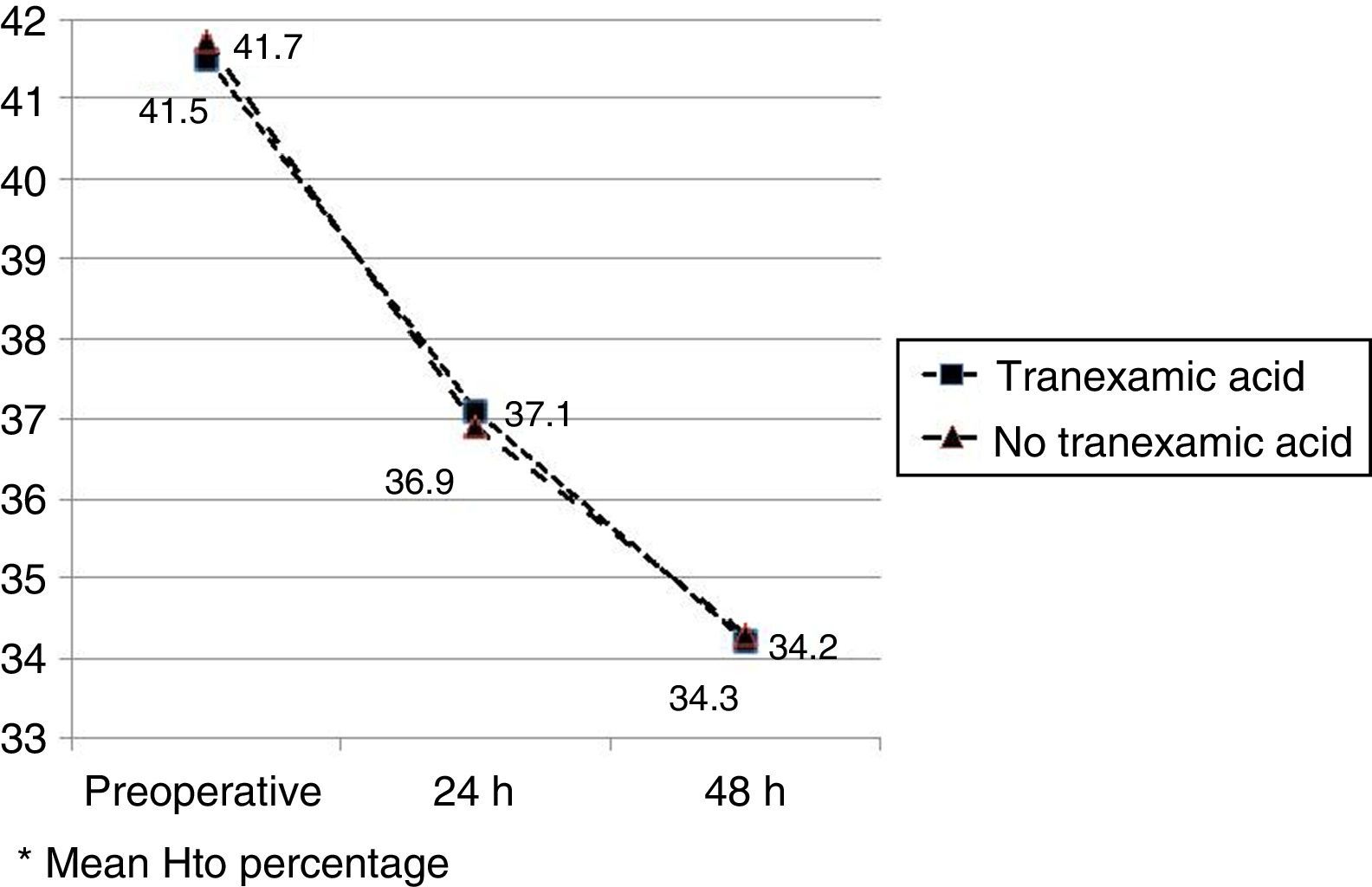
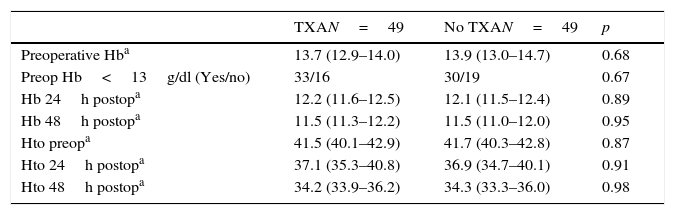
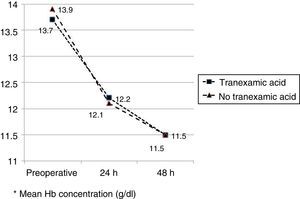
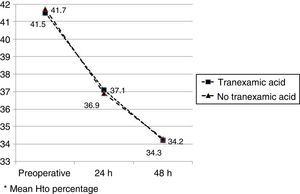
The mean preoperative and postoperative haemoglobin and haematocrit levels were similar in both groups. In 33 (67.3%) of the patients who had been administered TXA and in 30 (61.2%) of the patients who had not been administered TXA, the preoperative haemoglobin level was lower than 13g/dl, with no significant differences. The mean decrease in the postoperative haemoglobin and haematocrit levels was similar in both groups. After 24h it was 1.6 and 4.4 points in the group of patients treated with TXA; and 1.7 and 4.8 points in the group who had not been administered with the drug; and after 48h levels were 2.2 and 2.9; and 2.4 and 2.6, respectively (Table 2; Figs. 2 and 3).
Haemoglobin and haematocrit levels.
| TXAN=49 | No TXAN=49 | p | |
|---|---|---|---|
| Preoperative Hba | 13.7 (12.9–14.0) | 13.9 (13.0–14.7) | 0.68 |
| Preop Hb<13g/dl (Yes/no) | 33/16 | 30/19 | 0.67 |
| Hb 24h postopa | 12.2 (11.6–12.5) | 12.1 (11.5–12.4) | 0.89 |
| Hb 48h postopa | 11.5 (11.3–12.2) | 11.5 (11.0–12.0) | 0.95 |
| Hto preopa | 41.5 (40.1–42.9) | 41.7 (40.3–42.8) | 0.87 |
| Hto 24h postopa | 37.1 (35.3–40.8) | 36.9 (34.7–40.1) | 0.91 |
| Hto 48h postopa | 34.2 (33.9–36.2) | 34.3 (33.3–36.0) | 0.98 |
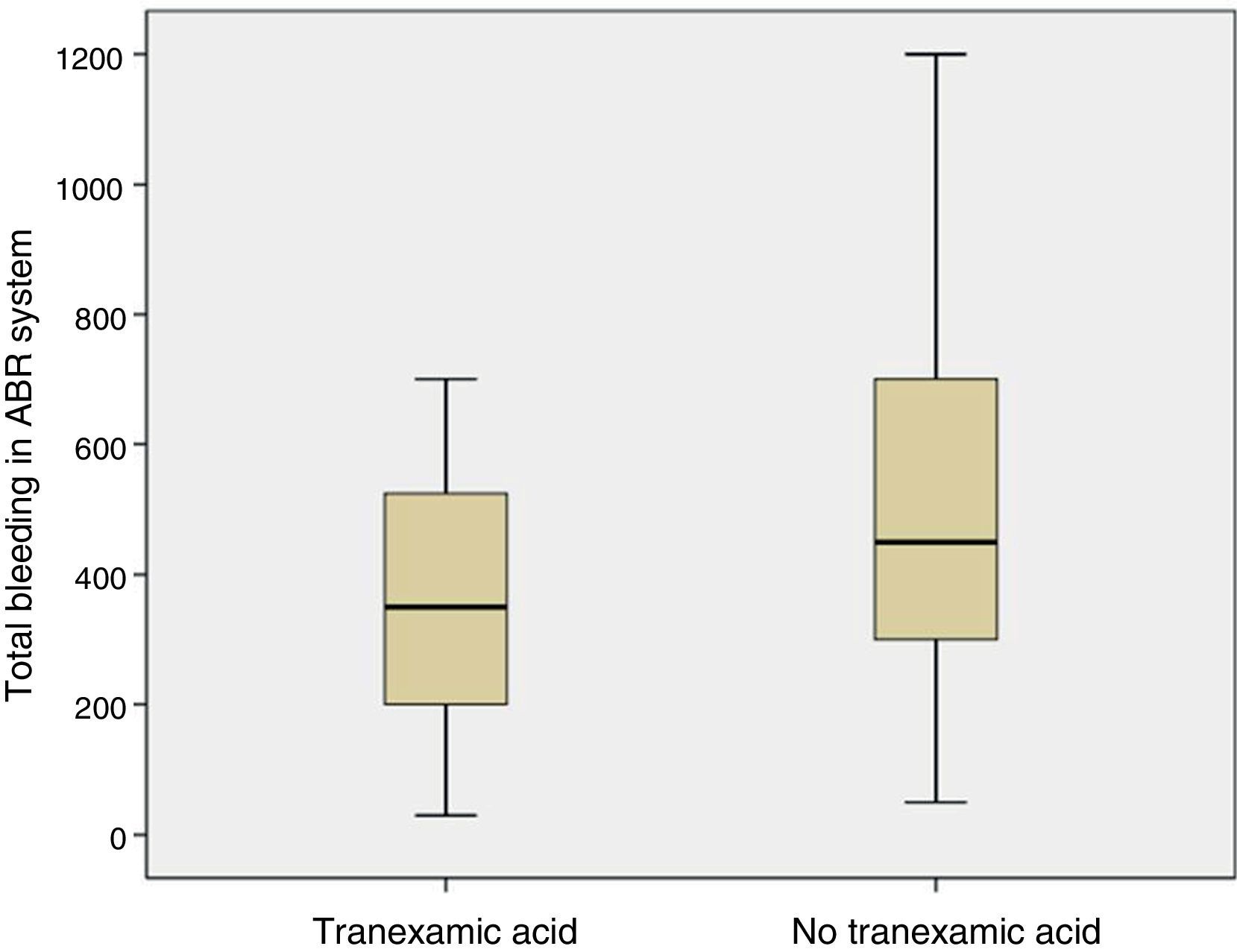
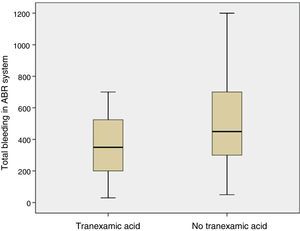
The mean volume of blood in the ABR system 4h after surgery was lower in patients who had been administered TXA than in patients who had not been administered the drug (p=0.05). The mean bleeding in the ABR system between 4 and 48h after surgery was also lower in the group of patients who had been administered TXA than in patients who had not been (p=0.05). The estimated blood loss was lower in the group of patients who had been administered TXA compared with those who had not been administered the drug (p=0.03) (Table 3; Fig. 4).
No patient group which had been administered with TXA need an autotransfusion with blood volume above 400ml in the first 4h after surgery, compared with 39 (69.3%) patients in the group who had not been administered it (p<0.001). No allogenic blood transfusion was required in the cohort patient group.
Two patients (4.0%) presented with arterial hypotension during autologous blood re-infusion, with no subsequent effects. No clinical symptoms or signs of thromboembolic complications were recorded during hospital stay nor during the first three months of follow-up. None of the patients were re-admitted to hospital during the first three months following surgery.
DiscussionThe most important finding from our study is that the administration of a single intravenous dose of 15mg/kg of TXA in primary KRS has 100% rate of non autologous and non allogeneic transfusion with no thromboembolic complications in the first three months after surgery.
The group of antifibrinolytic drugs used in blood recovery in KRS include aprotinine, epsilon-aminocaproic acid and TXA. The aprotinine has proven its efficacy in published studies.16 However, it was withdrawn from the market when the benefit-risk balance came into question in patients who had undergone cardiac surgery with extracorporeal circulation. The epsilon-aminocaproic acid also reduced bleeding and transfusional requirements in KRS,17 although the outcome of a meta-analysis does not confirm this18 and the Sevilla 2013 document does not recommend its administration to reduce bleeding and/or transfusional rates in orthopaedic surgery with a 1B4 grade of recommendation. It is also 10 times less powerful than that of TXA.4,19
Controversy surrounds the use of TXA as a means of reducing perioperative bleeding in orthopaedic surgery as it is not included as an indication on the drug data sheet.20 Justification for its use must therefore relate to the patient's medical history and informed consent form,19 which we obtained in our study.
References record a huge variability regarding dose and usage guidelines for TXA.9,21,22 The majority of studies refer to the initial intraoperative dose of between 10 and 25mg/kg, followed, if necessary, by post-operative doses. Hourlier23 compares the use of a single 30mg/kg dose to one of 10mg/kg followed by continuous infusion of 2mg/kg per hour over 20h, with no differences regarding efficacy and safety, although in hip prosthesis Alshryda21 reports in his meta-analysis that the reduction in the need for allogenic transfusion is maintained regardless of whether a single or multiple dose is administered. Aguilera7 advises 1–2g fixed intravenous doses to avoid calculation error or over dosing. Furthermore, recent articles have initiated another line of research through the intra-articular administration of TXA with results no lower than those of intravenous administration.24,25 In our study we used a single intravenous dose of 15mg/kg of TXA between 15 and 20min prior to ischaemic release.
Redon blood loss, estimated blood loss and the need for allogenic transfusion is a common outcome in studies consulted of cases,14,23 and in meta-analysis.9,21,26 Poeran10 conducted a retrospective study in 510 hospitals in USA with 872,416 patients who had undergone primary knee or hip replacement surgery with a significant reduction (7.7% compared with 21%) in the need for an autologous or allogenic blood transfusion. In order to calculate blood loss several formulas have been reported.15 In our study we used the Mercuriali formula, but using haematocrit and haemoglobin levels after 48h, which are far less precise due to haemodilution than those of 5 days. This limitation should be taken into account. The volume of blood collected in the ABR system was reduced by 35.5% in patients treated with TXA, estimated blood loss reduction was 29.5% and none of the patients required an allogenic blood transfusion.
Safety in the use of TXA in primary knee replacement surgery leads to uncertainty because of risk factors of thromboembolic disease. Poeran10 does not refer to any significant major thromoboembolic complications in patients treated with TXA in his retrospective study (0.6% compared with 0.8%). Gillete27 retrospectively reviews 2046 patients who underwent primary hip or knee surgery and who were administered TXA; they were treated with aspirin, warfarin and dalteparin as thromboembolic prophylaxis and there were no significant differences between the three groups (0.35%; 0.15%; 0.52%). Whiting28 retrospectively assesses 402 ASA III and IV patients; 240 had been administered TXA during hip or knee replacement surgery and 162 had not been administered the drug, with an incidence of thromboembolic complications of 2.5% compared with 2.6%. There were no symptoms nor signs of thromboembolic complications in our study, although we did not perform a standard venous Doppler test to detect the presence of subclinical thromboembolism.
The optimum Hb level prior to KRS to minimise transfusion risk is 13g/dl.19 According to WHO criteria, suboptimum levels presented in up to 30% of OTS patients.29 Two strategies are recommended for its correction. On the one hand, if this is an iron-deficiency anaemia then iron therapy is recommended, due to its low cost and easy administration, with a 2B19 recommendation, provided it is well tolerated by the patient and there is sufficient time to obtain the appropriate correction. Otherwise, recombinant human erythropoetin is recommended, with administration protocols which present a 1A19 level or recommendation for the reduction of allogenic transfusion and provided that there is sufficient preoperative time. In our series, 63 patients (64.2%) presented with suboptimal levels of preoperative Hb, without having applied any of the recommendations for its correction due to lack of time between the pre-anaesthetic evolution and the date of surgery. Both the administration of TXA and the use of the ABR system have prevented the need for allogenic transfusion in this group of patients at risk.
The financial impact in the healthcare process is a driver for the use of TXA in KRS. The total cost of a 1g TXA capsule is 21 Euros, a non washed ABR system between 96 and 151 Euros, a unit of concentrated red blood corpuscles is 243 Euros, 1000mg of intravenous iron between 220 and 285 Euros, and 40,000 units of recombinant human erythropoetin is 235 Euros; without considering the longer hospital stay due to the need for a blood transfusión.19 In our series none of the patients had required allogenic blood transfusions either because of the use of intravenous TXA in a single dose or the use of the ABR system; and hospital stay had not increased. We have therefore changed the multimodal protocol in our outpatient department service to reduce the rate of allogenic transfusion in patients who underwent KRS surgery, so that the patients referred for TXA usage, did not use the ABR system.
At present in its Documento Sevilla 20134 the Sociedad Española de Anestesiología y Reanimación recommends ABR in knee arthoplasty with a 1B grade of recommendation and the use of TXA with a 2A grade of recommendation in orthopaedic surgery. The European Society of Anaesthesiology and Reanimation's22 recommendation is 1A and 2A grade of recommendation, respectively.
With the limitations of our study, the use of a single 15mg/kg dose of intravenous TXA in primary KRS presented a 100% rate of no autologous or allogenic transfusion, with no increase in the incidence of thrombotic events. We therefore recommend its use in this patient group, on the premise that usage should be personalised, justified by the patient's medical history and their informed consent.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments on humans or animals have been used in this research.
Data confidentialityThe authors declare that they are adhered to the protocols of their place of work regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FinancingThe authors declare they received no financing for this project.
Conflict of interestThe authors have no conflicts of interest to declare relating to this article.
Please cite this article as: Sanz-Reig J, Parra Ruiz B, Ferrández Martínez J, Martínez López JF. Dosis única intravenosa de ácido tranexámico como medida de ahorro transfusional en prótesis total primaria de rodilla. Rev Esp Cir Ortop Traumatol. 2016;60:106–112.