Surgical resection is considered the curative treatment par excellence for patients with primary or metastatic liver tumors. However, less than 40% of them are candidates for surgery, either due to non-modifiable factors (comorbidities, age, liver dysfunction…), or to the invasion or proximity of the tumor to the main vascular requirements, the lack of a future liver remnant (FLR) adequate to maintain postoperative liver function, or criteria of tumor size and number. In these last factors, hepatic radioembolization has been shown to play a role as a presurgical tool, either by hypertrophy of the FLR or by reducing tumor size that manages to reduce tumor staging (term known as "downstaging"). To these is added a third factor, which is its ability to apply the test of time, which makes it possible to identify those patients who present progression of the disease in a short period of time (both locally and at distance), avoiding a unnecessary surgery. This paper aims to review RE as a tool to facilitate liver surgery, both through the experience of our center and the available scientific evidence.
La resección quirúrgica se considera el tratamiento curativo por excelencia para los pacientes con tumores hepáticos primarios o metastásicos. Sin embargo, menos del 40% de ellos son candidatos a cirugía, ya sea por factores no modificables (comorbilidades, edad, disfunción hepática…), como por la invasión o proximidad del tumor a los principales pedículos vasculares, la falta de un futuro remanente hepático (FRH) adecuado para mantener una función hepática postoperatoria, o criterios de tamaño y numero tumoral. En estos últimos factores, la radioembolización hepática ha mostrado tener un papel como herramienta prequirúrgica, ya sea mediante la hipertrofia del FRH o mediante la reducción del tamaño tumoral que consigue disminuir la estadificación tumoral (término conocido como “downstaging”). A estos se suma un tercer factor, que es su capacidad de aplicar el test del tiempo, que permite identificar aquellos pacientes que presenten en un plazo corto de tiempo progresión de la enfermedad (tanto a nivel local como a distancia), evitándoles una cirugía innecesaria. En este trabajo se pretende hacer una revisión de la RE como herramienta facilitadora de la cirugía hepática, tanto a través de la experiencia de nuestro centro como de la evidencia científica disponible.
Locoregional therapies play an important role in the treatment of liver tumors, especially hepatocellular carcinoma (HCC) in both early and advanced stages. Transarterial chemoembolization (TACE) continues to be the standard treatment and is more globally used for intermediate stage HCC.1 However, there is increasingly more evidence demonstrating the efficacy of other locoregional therapies, such as radioembolization (RE) with Yttrium-90 (90Y) or with Holmium-166 (166Ho), in the treatment of primary and metastatic liver tumors.
The term RE refers to the application of high doses of short range ß radiation using microspheres loaded with 90Y or 166Ho. At present, two types of microspheres loaded with 90Y are available, those of resin (Sir-Spheres®, Sirtex Medical Ltd., Sydney, Australia), which are on what the experience of our center is based, and those of glass (Therasphere®, Boston Scientific, Boston, MA, USA)]. Only poly(l-lactic acid) (PLLA) microspheres are labeled with 166Ho (QuiremSpheres®, Quirem Medical B.V., Deventer, The Netherlands).
Liver tumors can be selectively reached by intraarterial administration of the microspheres (taking advantage of their arterial vascularization), minimizing radiation of the non-tumoral liver. Both 90Y and 166Ho present a mean range of low penetration (2.5mm and 2.2mm, respectively), and thus, very high doses can be achieved in the tumor with minimum radiation in the tissue located more than 1cm from the tumor.2 This allows maximizing the dose absorbed by the tumoral tissue and minimizes the dose received by the adjacent tissue, improving the response observed while reducing the complications derived from the radiation of normal liver tissue. However, this review will show that the benefits of RE go beyond their tumoricidal capacity, converting it into a locoregional therapy with greater therapeutic potential.
It is important to note that depending on the type of microspheres used, the values of the doses absorbed that are prescribed in the planning study differ (single photon emission computerized tomography/computerized tomography [SPECT/CT] with 99mTc-albumin macroaggregates [MAA] or with 166Ho-PLLA). It seems that these differences are largely due to the difference in the number of particles injected, which conditions differences in the biological effect in both terms of efficacy and toxicity. Thus, for the same activity prescribed, a greater number of resin microspheres can potentially provide a more uniform distribution of the dose with a greater biological effect.3 Therefore, the necessary doses absorbed to produce a tumoricidal effect with this type of microspheres are generally lower than with glass and even 166Ho microspheres.
Since the first studies performed at the beginning of the 1960s, the development and investigation of the clinical applications of RE have led this treatment to be included within the therapeutic armamentarium of the main primary and metastatic liver tumors. Depending on the intention for which it is applied, RE with 90Y has shown to be effective not only as a purely ablative treatment (and potentially curative in initial stages) but also palliative treatment in advanced stages of the disease.4
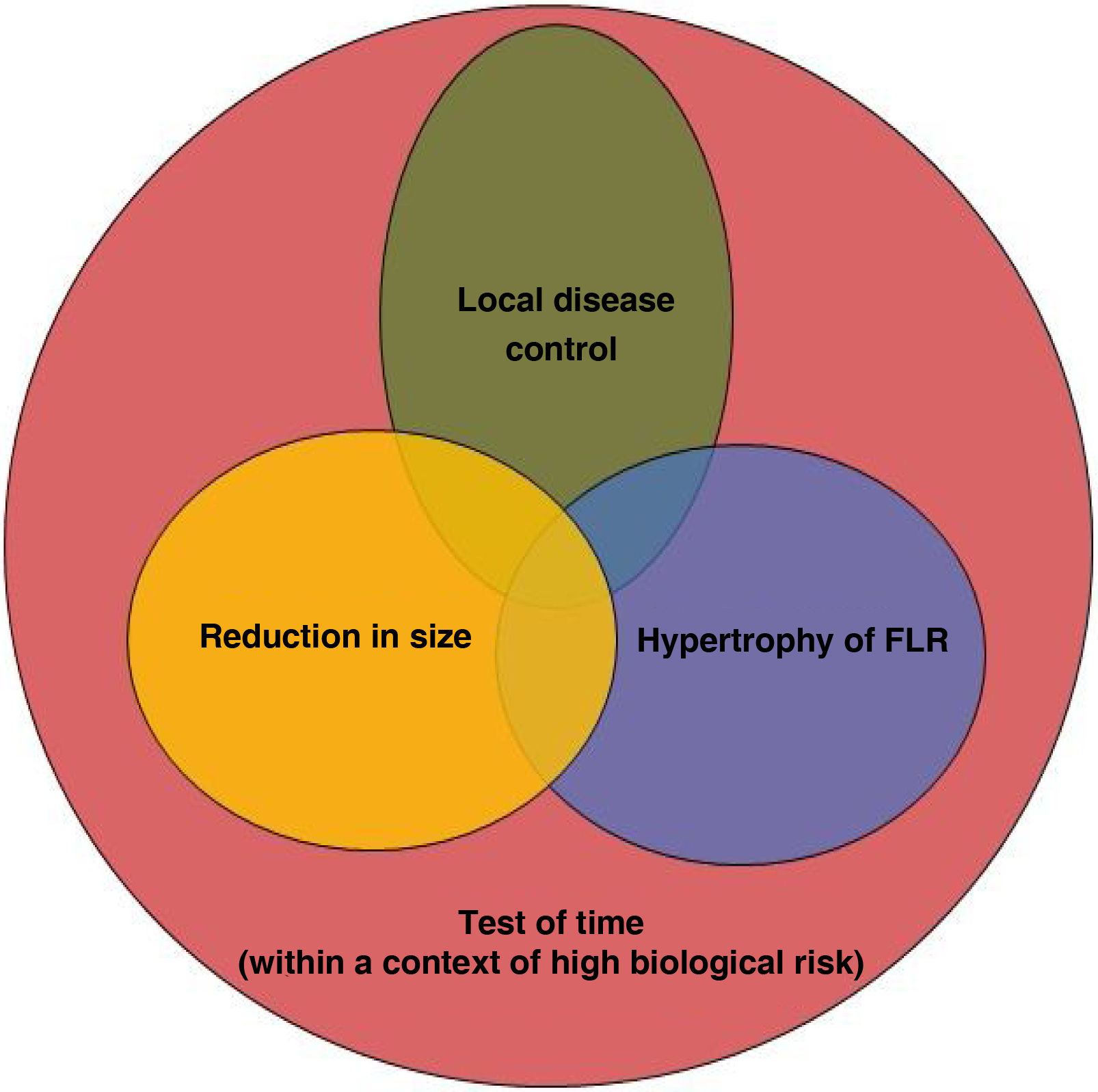
However, the role of RE as a presurgical tool should be highlighted. Compared with other intraarterial treatments, such as TACE, RE is noted for its capacity to enlarge the future liver remnant (FLR) when applied to healthy contralateral tissue. Likewise, its efficacy in decreasing tumor size allows reducing the tumoral stage (known as downstaging). Both effects are pillars that allow surgery to be performed,5,6 which is the only potentially curative treatment in these patients. Nonetheless, a third factor should be highlighted: the test of time. To achieve these effects (reduction in size (contralateral hypertrophy), it is necessary for 3–4 months to go by. The absence of distant progression during this time helps to confirm the surgical indication generally within the context of advanced or biologically aggressive tumors. The use of RE with pre-surgical intention provides the possibility of a potentially curative treatment in patients with primary and metastatic liver tumors, which would have a modest prognosis without surgery. It is important to note that to achieve adequate results, RE should be managed and indicated within the context of a multidisciplinary team. The surgical team should define what is necessary to convert the disease into one that can be resected. Oncologists and hepatologists must focus this treatment within the context of an oncological or liver disease, respectively. Lastly, the joint work of the interventional radiologist and nuclear medicine physician should allow the design of the most effective treatment to achieve these objectives.
Liver surgery as curative treatment of primary liver tumorsSurgical resection is considered the curative treatment par excellence for patients with primary liver tumors.7,8 Liver resection (LR) must ensure complete removal of the tumor with free margins (R0 resection) and the preservation of a sufficient hepatic remnant. Under these conditions, the 5-year survival rate post-LR of correctly selected patients with HCC or intrahepatic cholangiocarcinoma (iCC) is around 70–80% and 25–40%, respectively. On the other hand, liver transplantation (LT) is considered an alternative to LR with curative potential. According to the Milan criteria, in patients with HCC (a tumor nodule less than 5cm or 2–3 nodules less than 3cm), the rates of 5-year post-LT survival are as high as 75–80%, with the advantage of low risk of recurrence and the elimination of underlying liver cirrhosis compared to resection.9,10 The role of LT in patients with iCC is more limited, although in the last years it has emerged as a promising therapeutic strategy. At present, in the beginning, LT is reserved for patients with very early iCC who are not susceptible to LR normally due to significant underlying liver dysfunction.11
However, most patients with primary liver tumors are diagnosed in an advanced state and are not candidates for surgery (LR or LT). In fact, only 10–40% of all the patients with primary liver tumors are candidates to LR at diagnosis.7 The comorbidities, age, liver dysfunction or complications derived from cirrhosis are, among others, non-modifiable factors that contraindicate LR or LT in these patients. On the other side, factors such as tumor invasion or proximity to the principle vascular pedicles, the lack of a FLR adequate to maintain sufficient post-operative liver function or criteria of tumoral size and number also impede access to LR and LT in a large proportion of patients. It is in this last subgroup of patients, who are not candidates to surgery, in whom RE can be applied with the final goal of achieving some criteria that will allow performing safe, oncologically adequate and potentially curative surgery.
Liver resection and radioembolizationRE can be applied prior to surgery with a combination of the following objectives according to each case:
- 1.
Reduction of tumoral stage (downstaging)
- 2.
Hypertrophy of the FLR
- 3.
Local disease control
- 4
Test of time
RE treatment is generally performed with the intention of achieving two or more of these objectives at the same time (Fig. 1). Since the objectives sought at the hepatic level are generally applied within a context of high biological tumoral risk, the last factor (test of time) is usually present in most of the cases. The aim of this time is to identify the patients who present disease progression within a short period of time (at both a local and distant level) and thus, avoid unnecessary surgery.
Depending on the main objective for which RE is applied and the arterial anatomy of each patient, the design of the treatment must be personalized. This includes the location(s) of the arterial tree in which the microspheres should be administered as well as the planning of the dose administered at each site and the estimation of the dose absorbed by the tumor and the healthy hepatic parenchyma.
The following is a description of the main objectives of RE:
- 1.
Tumor dowstaging
The concept of tumor downstaging refers to a reduction of the viable tumoral load with the aim of fulfilling the criteria of access to surgical treatment (LT or LR). Essentially, downstaging acts as a tool to select a subgroup of patients with a favorable tumoral biology that responds to treatments of stage reduction and whose oncological results have shown to be favorable following LT or LR. As in other centers, our center prioritizes RE as the principal tool of downstaging above other locoregional treatments such as TACE.12
In our center downstaging of the tumor with the intention of achieving LT criteria is performed in selected patients with multinodular HCC, preserved liver function, without extrahepatic disease and who do not have the Up-to-7 criteria (the sum of the number of tumoral nodules and the diameter in centimeters of the largest tumoral nodule is less than or equal to 7). With this treatment modality, RE provides downstaging rates to LT of between 50 and 60%. The posterior oncological results are excellent, observing a 5-year post-LT survival of 78% in patients in whom even Milan criteria were reduced, which is similar to that of patients who initially fulfilled these criteria and did not require downstaging.13
RE has great applications when it is used for downstaging with the intention of performing posterior LR. The tumoricidal capacity of RE in patients with primary and secondary liver tumors allows reducing the tumoral size of the lesions found close to vascular or biliary structures (Fig. 2). When these tumors bilobally affect these structures or are close to the bifurcation of the liver hilum, most are considered unresectable. In these patients, RE has shown to achieve high rates of significant tumoral response (between 60–90% of complete radiological response) and disease control, allowing posterior R0 resection.4,14
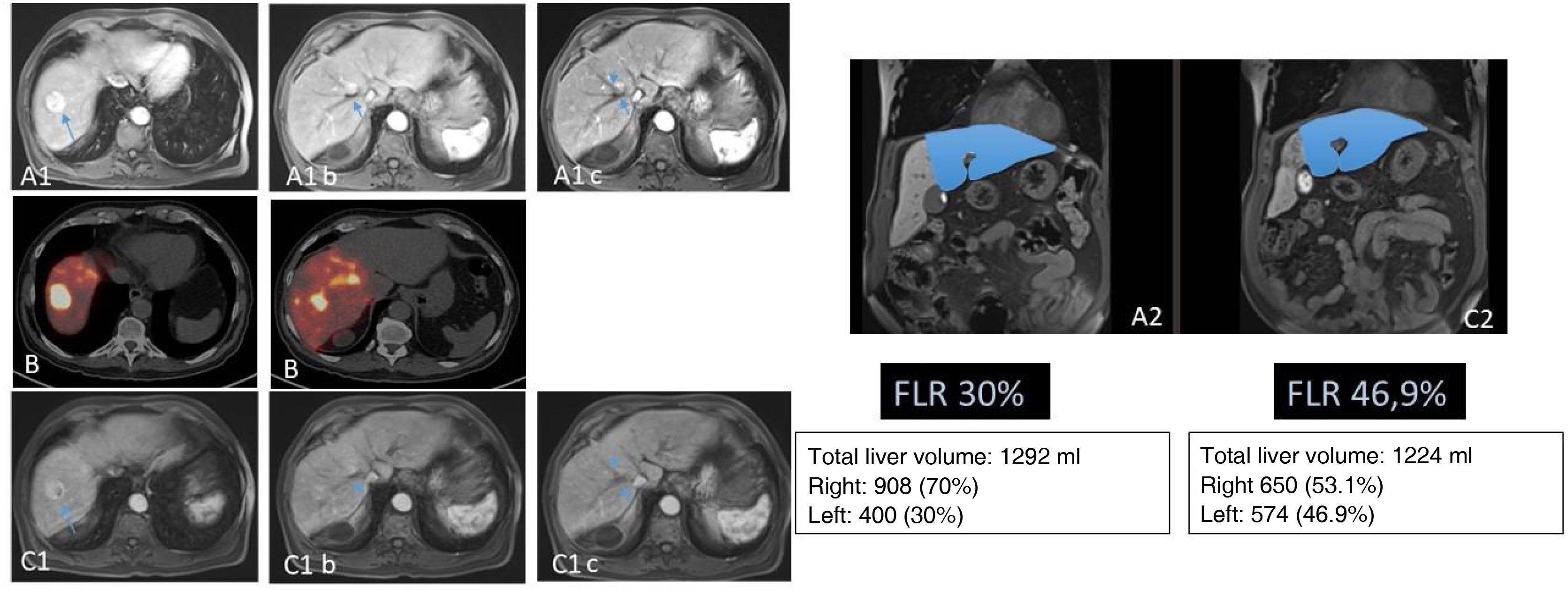
69-year-old patient with binodular hepatocarcinoma, with no distant disease and preserved liver function. Two lesions can be seen in the MR in segments VII and VIII (A). Images A1b and A1c show that the lesion of segment VIII is in contact with the right suprahepatic vein. This, together with the fact that the liver remnant after right hepatectomy would be 30% (A2), led to considering the performance of RE with the double objective of downstaging and hypertrophy of the liver remnant. In the 90Y PET study (B), after the administration of 1.5GBq of 90Y resin microspheres into the hepatic artery, adequate arrival of the treatment to the tumoral lesions (mean dose absorbed of 185Gy) and to the right non-tumoral liver tissue (mean dose absorbed of 76Gy; V30 56%) was observed. Five months after RE the separation of the VIII lesion from the adjacent vein (C1) was checked (C1b and C1c) together with adequate hypertrophy of the remnant (C2). The patient underwent laparoscopic right hepatectomy (not requiring transfusion; 3 days of hospitalization). At the last check-up (72 months after surgery) the patient remained alive and disease-free.
In patients with resectable tumors with high biological tumoral risk who require greater or extended LR, segmentary RE can be applied with the aim of reducing the size of the tumor. This modality allows evaluation of local response and the evolution of the disease while also facilitating the performance of less extensive hepatectomies, which is a factor that is especially useful in patients with metastasis of colorectal cancer (CRCm) (Fig. 3). This leads to a reduction in the risk of complications associated with the extension of the hepatectomy as well as saving the hepatic parenchyma. In these cases, the planning of RE should be as selective as possible with the aim of generating tumoral response while also preserving the maximum quantity of the non-tumoral liver parenchyma possible, and thereby allowing the potential resection to be safer.
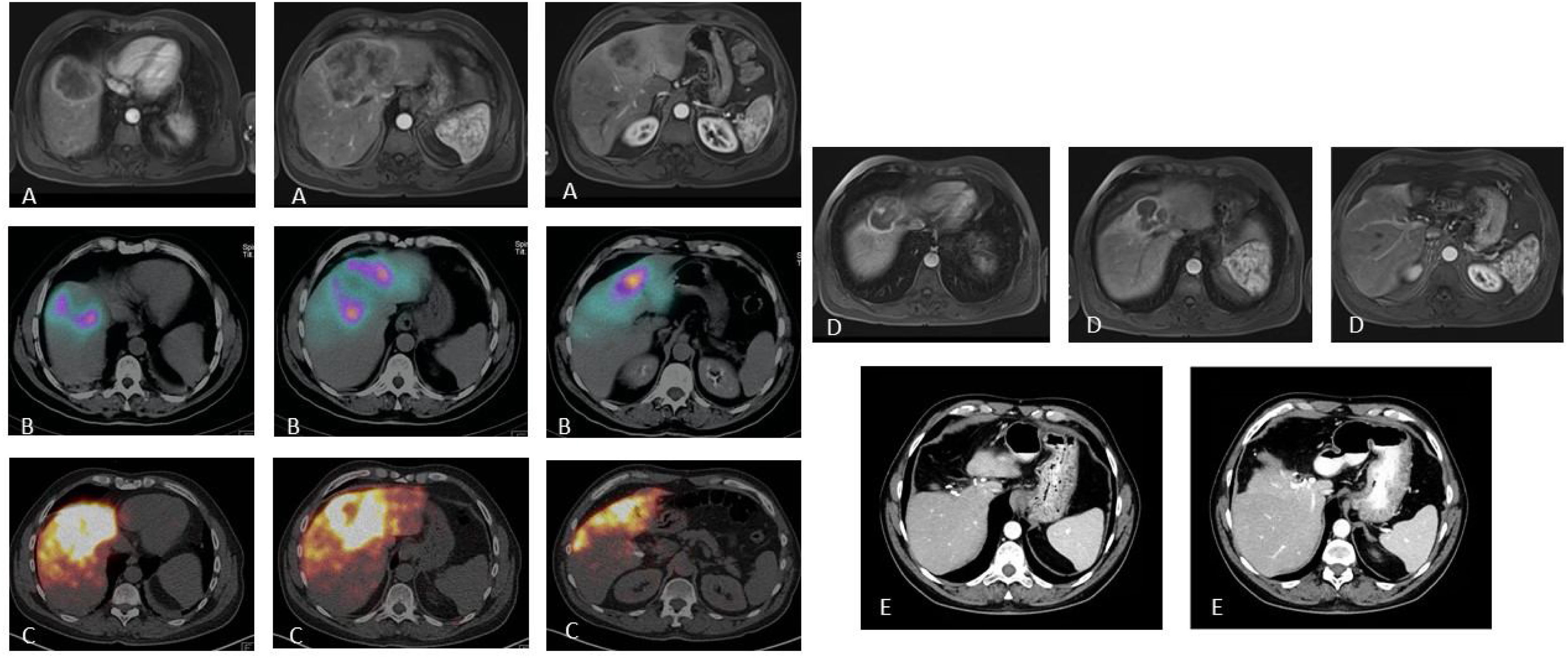
Patient with stage IV rectal carcinoma by single metastasis affecting the central IVa, IVb and VIII segments treated with neoadjuvant chemotherapy (FOLFOXIRI) with minor response (A). With the intention of reducing tumor size (requiring significant liver surgery) and as a test of time (primary non-resected and uncertain tumoral behavior), the patient was evaluated as a candidate for RE. The SPECT/CT after the injection of MAA through the segmentary arteries of the VIII and accessory of the left hepatic artery (B) shows adequate access to the tumoral tissue and reaching part of the non-tumoral tissue but with preservation of all the posterior hepatic segments. 2.3GBq of 90Y resin microspheres were administered. The dosimetric study in the 90Y-PET (C) calculated a mean dose absorbed in the tumor of 120Gy and of 74.7Gy in the non-tumoral tissue. The MR study performed 8 months after RE (C) shows a significant reduction in tumor size, which allowed laparoscopic central hepatectomy (C) with minimum blood loss and 3 days of hospitalization. The patient remains alive 72 months after RE and remained hepatic tumoral disease-free 54 months after surgery (presented metastatic lesion in the segment VC/VIII which was again resected).
When the main objective of RE is a reduction in tumoral size, the infusion of the microspheres should ideally be performed selectively in the segmentary or subsegmentary artery(ies) to which the tumor depends. In this way, the radiation administered to the tumor is maximum, achieving greater necrosis and a consequent reduction in tumoral mass. When the angiographic and SPECT/CT-MAA confirm the possibility of administering the treatment almost exclusively to the tumor or at least to two liver segments in which it is located, the aim of the treatment is to administer a tumoricidal or ablative dose to the segments (radiation segmentectomy). However, when selective administration of RE is not possible due to the localization of the tumor(s), the dose that the non-tumoral tissue will receive must be largely taken into account, which, on occasions, will condition the dose absorbed by the tumor and even the adequacy of the patient as a candidate to RE.
As mentioned previously, depending on the type of sphere used, the dose absorbed that should be used may significantly differ. In the case of glass spheres, in a subgroup of patients in whom the dose was related to pathological response of the piece resected or transplanted, the LEGACY study demonstrated that when the dose absorbed in the liver volume that included the tumor was greater than 400Gy, complete pathological necrosis was found.4 This value was supported by a phase 2 study in which the investigators found a significant difference in the dose absorbed by the responders versus the non-responders (490Gy versus 275Gy).15 These values referred to a unicompartmental calculation in which only the volume of the liver tissue to receive the dose was considered without distinguishing between the dose absorbed by the tumor and by the non-tumoral tissue. However, the use of personalized multicompartmental dosimetry is recommended (which distinguishes between the dose received by each tissue compartment) since it allows treatment optimization. For this, it must be taken into account that the optimal established dose absorbed by the tumoral tissue for this type of spheres is >200Gy.18
In the case of resin spheres, to date, the dose recommended to achieve tumoral response is >100–120Gy, while to produce ablation of the segments in which the tumor is localized, the dose is > 150Gy. Nonetheless, it is important to note that this latter value was defined through the experience of the different authors involved in the international recommendations for this type of spheres, since at that time there was not sufficient scientific evidence.16 However, in a recent study comparing the efficacy, safety and dosimetry of both 90Y spheres for producing radiation segmentectomy, it was determined that the mean threshold of dose absorbed by the tumor able to predict objective and complete tumoral response was 176Gy and 247Gy, respectively, for resin and 290Gy and 481Gy, respectively, for glass.17
In the case of 166Ho spheres, the current recommendation is to prescribe a dose absorbed by the tumor > 150Gy in the case of HCC and > 90Gy for CRCm.3 However, up to now, there are no results to allow establishing dosimetric limits for these sphere in radiation segmentectomy.
If, to the contrary, the localization of the tumoral lesions does not allow performing selective RE and the volume to treat is foreseeably around two thirds of the total liver volume, the dose absorbed that the non-tumoral liver tissue will receive must be taken into account to avoid liver toxicity secondary to treatment. This situation, which is less usual than the previous, could be found in the context of the use of RE for disease downstaging with the intention of achieving criteria for LT, or as will be seen in section 3, when the principle objective is disease control but it is not possible to selectively access the tumor. For glass spheres, the recommendation is that the mean dose absorbed received by the total non-tumoral tissue (treated and not treated) should be <75Gy (range: 50−90Gy) or <120Gy in treated healthy tissue in patients with characteristics similar to those included in the DOSISPHERE study (Child-Pugh A, large lesions and with at least 30% of preserved liver reserve).3 Other therapeutic alternatives should be considered in patients in whom it is not possible for the tumoral tissue to receive at least 200Gy (205Gy in the case of macrovascular invasion) in order not to surpass these limits in the healthy tissue.18 Given that these thresholds have mostly been established in cirrhotic patients with HCC, they can also be considered safe for patients with tumors other than HCC. However, special precaution is needed in patients with iCC with underlying cirrhosis and after chemotherapy.3 With respect to resin spheres, the accepted recommendation is that the non-tumoral tissue should receive <40Gy, reducing to < 30Gy in patients who have previously undergone chemotherapy or present compromised liver function. Similar to the glass spheres, if these limitations do not allow the administration of sufficient radioactivity for the tumoral tissue to receive > 100Gy, alternative therapies other than RE should be considered.16 In the case of 166Ho glass spheres, the accepted value in healthy unilobar tissue is <60Gy, considering the patient as a candidate to RE only if it is possible for the tumoral tissue to receive > 150Gy (for HCC and iCC) or >90Gy (for CRCm).3
The contraindication of surgery is sometimes due to the infiltration of these structures in addition to the absence of an adequate FLR. In these cases, planning plays a crucial role in which selective application of RE to the tumor must be combined with lobar application. In this way, the same patient achieves an effective tumoral response while also favoring atrophy of the lobe treated and contralateral hypertrophy. This mixed administration technique, known as modified radiation lobectomy, combines selective administration targeting the tumor and another that is lobar directed to the healthy tissue. This makes it possible to achieve the tumoricidal dose established for each type of microsphere without surpassing the limited recommended as safe for healthy tissue.19
Lastly, RE also seems to be useful for downstaging treatment for laparoscopic surgery. This novel concept, which has not previously been described in the literature, is based on the benefits that laparoscopic liver surgery has shown compared to open surgery. The laparoscopic approach has shown to be superior to open surgery in intraoperative results and morbidity and mortality as well as being associated with better long-term oncological results.20–22 Thus, in patients in whom major extended open hepatectomy is considered due to the need for vascular or biliary resection and posterior vascular anastomosis or hepaticojejunostomy, RE sometimes allows a reduction in tumor size and the release of these structures, thereby enabling less extensive laparoscopic surgery and ensuring adequate local disease control and facilitating a time for confirming the absence of disease progression. In our center we have used this indication of RE in four patients to date. The results in the four have been satisfactory and the surgeries were performed laparoscopically with excellent results.
- 2.
Hypertrofy of the future liver remnant
To avoid liver failure following LR it must be assured that the FLR is of at least 40% of the total liver volume in patients with hepatic cirrhosis or previous chemotherapy treatment and of between 20–30% in patients with a healthy liver. In our center, in patients with liver tumors who are candidates for LR and in whom the FLR would be insufficient according to these criteria, we mainly choose lobar RE over other treatments (such as portal embolization or associating liver partition and portal vein ligation for staged hepatectomy) when there is high biological risk or in the context of a cirrhotic liver. When the intention is to produce compensatory hypertrophy of the FLR, RE through one of the principle hepatic arterial branches has demonstrated the capacity to achieve significant atrophy of the treated hepatic lobe as well as rates of increase in contralateral volume of between 20 and 100% compared to the initial volume.5 This response is achieved not only in healthy but also cirrhotic livers and is also not only volumetric, since lobar RE also achieves changes in metabolic liver function of the FLR, which have not been demonstrated with other hypertrophy techniques in liver surgery.23,24 A differential characteristic of the volumetric changes produced by RE is that they are produced more slowly than with the use of other techniques of hypertrophy induction. In contrast to the techniques based on embolization or portal vein ligation, maximum compensatory hypertrophy following RE takes between 6–9 months to be achieved.25 In our experience, this is an advantage since it allows evaluation of tumoral response and progression of the hypertrophy and, overall, provides a valuable period for observing the evolution of the disease (test of time). Thus, the aggressiveness of the tumor can be assessed by identifying the local progression in the untreated liver or extrahepatic progression. In this way, LR that would not provide benefits in survival would be avoided in some patients and the disease can be reevaluated and other more adequate treatments can be considered to improve their prognosis.
In the case of the generation of contralateral compensatory hypertrophy by RE, also called radiation lobectomy, the dosimetric values are especially focused on the non-tumoral liver tissue. For the glass spheres, the recommended dose absorbed in the treated non-tumoral tissue is ≥88 Gy (<75Gy in the treated and non-treated non-tumoral liver tissue). In the case of resin spheres, it is recommended that the prescribed dose absorbed in the treated non-tumoral tissue be > 70 Gy.16 Nonetheless, the retrospective study of Grisanti et al. found that for the resin spheres, not only the mean dose absorbed was important but also the uniformity of the distribution of the radiation in the healthy parenchyma. Therefore, when half of the healthy volume treated received at least 30Gy (V30), the FLR increased to ≥ 40 %, with greater accuracy in the patients with FLR<30% in the beginning.26 A prospective study, which will be recruiting patients until 2024, is currently ongoing with the aim of establishing the maximum tolerated dose of 166Ho microspheres absorbed by the healthy liver in patients with HCC who undergo radiation lobectomy as a bridge to surgery.27
- 3.
Disease control
There are certain circumstances in which the objective of local treatment is not so much to reduce tumor size but rather to exclusively achieve local control without other requisites. RE has demonstrated a rate of cessation of tumoral progression of more than 90% and stabilization of the disease of between 75–100% at the hepatic level.4 This capacity of disease control and arrest of progression is especially relevant in two situations prior to liver surgery. In candidates to LR who present giant primary liver tumors with high biomarker values or primary tumors that develop early recurrence following previous treatments, RE stabilizes the disease while evaluating response or local progression. These characteristics are also applicable to secondary tumors with high risk of extrahepatic or peritoneal progression, especially in patients with CRCm refractory to standard chemotherapy or in whom the debut of the disease was perforation or locoregional lymph node involvement of the primary tumor was extensive. In these patients, RE provides local disease control and safe valuable time for evaluating the evolution of the extrahepatic disease before performing hepatic surgery.
On the other hand, in candidates to LT with a foreseen prolonged time on the waiting list or with an elevated risk of progression, this capacity of disease control is used as a treatment bridge until undergoing LT.13 In patients who are candidates to LT, the progression of HCC is one of the main causes of drop out from the waiting list. RE is especially useful in these patients, with clinical trials reporting success rates of approximately 90% as a treatment bridge to transplantation.28 RE has demonstrated that it can prolong the time to progression by a median of >26 months and can reduce drop outs from the waiting list compared with TACE.28
In this scenario of disease control, dosimetric planning is defined (as in the section on downstaging) from the possibility of administering a selective treatment (and thus, with tumoricidal or ablative aims) or not (in which the volume of the treated non-tumoral tissue determines the limit of activity to administer).
Surgical experience in our centerOur center began to implement RE in 2003, being one of the first centers worldwide. At that time, RE was mainly considered a palliative treatment. However, over time, the effectiveness of RE in rescuing the first patients was observed, giving them the possibility of undergoing potentially curative surgery. After 2005, we entered the unexplored area of LR after RE. Although these resections were more difficult due to post-RE adherences, large tumor size and fibrosis of the liver tissue, especially in cases with fibrosis close to the pedicles, the postoperative results were satisfactory.29,30
Later, in March 2011, we performed the first laparoscopic LR - segmentectomy of segment VI in a patient with cirrhosis. The result was successful and the patient was discharged without complications three days after surgery. As our team gained experience, we were able to perform more complex laparoscopic surgeries. Consequently, between 2011 and 2014, our center performed eight LR following RE, six of which were open surgeries and 2 were laparoscopic, with only one being a major resection. After carrying out this first major resection (which was the first after RE published in the medical literature31), laparoscopy became our approach of choice. From 2015–2020, 13 LR were performed following RE, 10 of which were laparoscopic (77%) and only three were open interventions (23%). The open hepatectomies were exclusively carried out in cases requiring complex vascular reconstructions, making this the only exclusion criteria for laparoscopic surgery.
We have recently described our experience in post-RE surgery in the publication of our results, focusing on the major laparoscopic resections. The results obtained demonstrate that when these procedures are performed by an expert team, they are safe and achieve optimal postoperative results that are not inferior to those obtained following LR without previous RE. In addition, they are within the current standards of laparoscopic liver surgery.32
In another recent article, we highlighted how both LR and LT after RE have become treatment strategies with curative potential for patients with HCC and iCC. The use of RE prior to resection in patients with unresectable HCC or iCC has shown overall survival rates at 10 years of 57% for HCC and 60% for iCC. These results are promising and demonstrate the positive impact of RE on the long-term survival of these patients. In regard to the results in patients who were rescued by LT or in those in whom RE was applied as a therapeutic bridge, the 10-year overall survival results were optimal with rates of 51.3%. In this group only two patients died due to disease recurrence, highlighting the good oncological control and adequacy of the combined strategy of RE and LT.33
These findings support the efficacy of both LR and LT in patients with previous RE and demonstrate that this strategy provides promising results in terms of long-term survival. To achieve optimal results it is important to carry out RE within a multidisciplinary setting in which the patient is correctly selected and the intention of administering RE is carefully planned. This implies defining the best strategy to achieve the resectability of the tumor or proceed to LT, and based on this, planning the application site of the microspheres and the radiation dose to be administered to the tumor and the healthy liver tissue. Under these conditions, RE allows safely performing rescue surgery in patients with primary and metastatic liver tumors, which were previously not considered as candidates for surgery, and offers a potentially curative strategy with excellent results in terms of safety, complications and long-term survival.