Grupos de interés en el Estudio de las Espondiloartropatías: Español, Francés, Belga y Alemán
*J.D. Cañete y R. Sanmartí (Hospital Clínic, Barcelona),
E. Collantes y M.C. Muñoz (Hospital Reina Sofía, Córdoba), C. González (Hospital Gregorio Marañón, Madrid),
J. Gratacós (Corporació Sanitària Parc Taulí, Sabadell),
J.C. Torre-Alonso (Hospital Monte Naranco, Oviedo)
y P. Zarco (Fundación Hospital de Alcorcón, Madrid).
LA EXPERIENCIA ESPAÑOLA. GRUPO ESPAÑOL DE INTERÉS EN EL ESTUDIO DE LAS ESPONDILOARTROPATIAS*
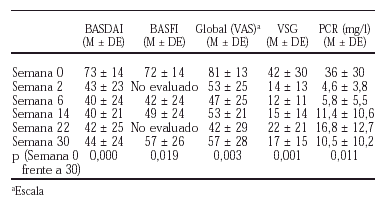
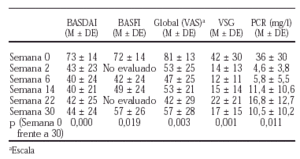
En la actualidad disponemos de los datos correspondientes a los 22 primeros pacientes con espondiloartropatía activa y refractaria a terapias habituales (AINE, FAME) según criterios definidos previamente por nuestro grupo de trabajo, que han concluido el estudio a 30 semanas. El esquema terapéutico consistió en 3 dosis de carga 0,2 y 6 semanas con 5 mg/kg para seguir con una infusión cada 8 semanas de forma similar a los pacientes con AR. La administración concomitante de metotrexato no se contemplaba en estos pacientes (los resultados previos se han comunicado en los resúmenes del Congreso Nacional de la Sociedad Española de Reumatología (SER 2001) y al Congreso Internacional de la European League Against Rheumatism (EULAR 2001).
Conclusiones
1. La dosis empleada de 5 mg/kg de peso parece adecuada para el tratamiento de estos pacientes.
2. El intervalo entre infusiones durante la fase de mantenimiento (cada 8 semanas) parece adecuado, aunque se precisan estudios longitudinales mayores para establecer su duración.
3. La mejoría se observa desde la primera dosis.
4. La respuesta clínica y analítica es francamente satisfactoria.
5. En general, el fármaco es bien tolerado.
No obstante, quedan aún algunas cuestiones por dilucidar que precisarán estudios más amplios. Entre ellas, destacamos los siguientes interrogantes:
1. ¿Durante cuánto tiempo debe seguirse administrando el fármaco para seguir manteniendo una respuesta clínica satisfactoria?
2. ¿Cuál es la pauta de administración más idónea, en la fase de mantenimiento de la respuesta?
3. ¿Pueden aparecer más efectos adversos al prolongarse la administración del fármaco?
THE FRENCH EXPERIENCE. RESULTS OF A 6 MONTHS FOLLOW-UP OPEN-LABEL STUDY
French Group. M. Breban, E. Vignon, P. Claudepierre, A. Saraux, D. Wendling, E. Delespessailles, L. Euller Ziegler, J. Sibilia, A. Perdiger, C. Alexandre, M. Dougados
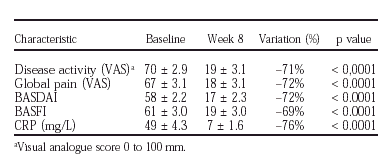
We enrolled 50 patients (38 M/12 F; mean age = 36 ± 9 yr; 87% HLA-B27+). 47 (94%) completed the treatment, as scheduled, whereas 1 and 2 received only 1 and 2 infusions, respectively. Altogether, 49 patiens (94%) were responders, as defined by ASAS criteria. Maximum improvement was observed at week 8 for most parameters, as is shown in table.
Relapse defined by a loss of ≥ 50% of global pain improvement was observed in 96% of completers within 4 months after the last infusion, with an average delay of 7 weeks (range: 2-18 weeks).
Conclusion
Most patients suffering of inflammatory active AS promptly and dramatically respond to treatment with infliximab. A majority of responders experience relapse within 4 months of drug discontinuation.
THE BELGIAN EXPERIENCE
Belgium Group. H. Mielants, F. Van den Bosch, D. Baeten, E. Kruithof, F. De Keyser and E.M. Veys
Department of Rheumatology. Ghent University Hospital. Belgium.
Infliximab was first given in 4 patients with Crohn's disease who also presented peripheral arthritis or axial involvement; a significant decrease of articular as well as axial inflammation, together with a resolvement of gut symptoms was found in all these patients.
Based on these data, an open-label study in 21 patients suffering from long-standing therapy resistant spondyloarthropathies, (including different subgroups of SpA) was started. After a loading dose of 3 infusions of infliximab (5 mg/kg) at week 0, 2 and 6, patients were treated every 14 weeks, resulting from day 3 on in a statistical improvement of all measured parameters (global disease activity, peripheral arthritis, axial disease) and maintained during 84 days (3 months), without important side-effects.
In a double-blind placebo-controlled study of 40 patients with SpA, a highly statistical significant improvement of all clinical and biological variables in the infliximab treated group was found during the 20 weeks treatment. However, in this group in one treated patient a severe drug-related adverse event appeared: a form of disseminated tuberculosis.
The patients of the first open-label study were followed for one year to determine whether repeated infusions would effectively and safely maintain the observed effect. By giving repeated infusions, a sustained significant decrease of all disease manifestations was observed.
Before retreatment, recurrence of symptoms was observed in 16% of the patients at week 20, 68% at week 34 and 79% at week 48, indicating that an interval of 14 weeks is too long to obtain an adequate disease control; however, no loss of efficacy was observed after retreatment. During this follow-up 12 patients (57%) developed antinuclear antibodies and 4 of them developed double-stranded DNA antibodies, however without lupus-like symptoms.
Conclusions
These data indiate that blockade of TNF-* is highly effective in reducing signs and symptoms in different froms of SpA, suggesting that for the first time, there may be an effective therapeutic option for all disease manifestations of severe SpA. However, the recurrence of tuberculosis observed in 1 patient, necessitates strict inclusion criteria. Moreover, long term experience and follow-up is needed to determine the optimal maintenance regimen (dose and interval) and to detect possible long term side-effects.
THE GERMAN EXPERIENCE
J. Braun.
Rheumazentrum Ruhrgebiet. Herne. Germany.
There is now overhelming evidence that infliximab is effective in the short-term treatment of severe active AS. In a randomized controlled multicenter trial performed in Germany with 70 AS patients over 3 months, 50% improvement of BASDAI was the primary outcome parameter: infliximab was significantly more effective than placebo in more than half of the patients. These strongly positive results have been reproduced in many countries in open studies. Several patients with undifferentiated spondyloarthritis, a possible early form of AS, have been successfully treated with infliximab. There is clearly reason to think that infliximab therapy of AS is a milestone in the treatment of severe AS.
However, there are still questions to be answered. There is hope but we do not know how long the effects last and wheter we can prevent progression to ankylosis. Our own preliminary results using MRI suggest that this is indeed the case. The optimal dose is not known yet. In the vast majority of the studies 5 mg/kg have been used but 3 mg/kg may well work in patients as well. Individual dosing might finally turn out to be superior to treat individual patients. There are first studies suggesting that etarnercept is also effective to treat AS patients.
The effects of infliximab treatment of the synovium and the immune system have been examined by the Belgian colleagues from Gent. There are indications that the synovial inflammation is effectively suppressed and that the ability to secrete cytokines is reinstituted.
There are rare but significant undesired effects of anti-TNF treatment.
Tuberculosis, allergic reactions and lupus-like dis-ease have occurred in single patients. As it stands now, the impressive positive effects for the patients which are mostly felt as early as one day after the initiation of therapy, seem to clearly outweigh these shortcomings. However, caution must be taken with this effective treatment and cooperation with experienced rheumatologic centers to monitor therapy is strongly recommended.
Bibliografía relevante
Amor B, Dougados M, Khan M. Management of refractory ankylosing spondylitis and related spondyloarthropathies. Rheum Dis Clin North Am 1995; 21: 117-128.
Antoni C, Dechant C, Lorenz H, Olgivie A, Kalden-Nemeth D, Kalden J et al. Successful treatment of severe psoriatic arthritis with infliximab. Arthritis Rheum 1999; 42 (Supl): 371.
Baeten D, Kruithof E, Van den Bosch F, Demetter P, Van Damme N, Cuvelier C et al. Immunomodulatory effects of anti-tumor necrosis factor alpha therapy on synovium in spondyloarthropathy: histologic findings in eight patients from an open-label pilot study. Arthritis Rheum 2001; 44: 186-195.
Baeten D, Van Damme N, Van Den Bosch F, Kurithof E, De Vos M, Mielants H et al. Impaired Th1 cytokine production in spondyloarthropathy is restored by anti-TNFalpha. Ann Rheum Dis 2001; 60: 750-755.
Brandt J, Haibel H, Cornely D, Golder W, González J, Reddig J et al. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum 2000; 43: 1346-1352.
Brandt J, Haibel H, Reddig J, Sieper J, Braun J. Successful treatment of severe undifferentiated spondyloarthropathy with the anti-tumor necrosis factor αmonoclonal antibody infliximab. J Rheumatol 2002. En prensa.
Brandt J, Haibel H, Reddig J, Sieper J, Braun J. Treatment of patients with severe ankylosing spondylitis with infliximab a one year follow up. Arthritis Rheum. En prensa.
Braun J, Sieper J. Anti-TNFalpha: a new dimension of the pharmacotherapy of the spondyloarthropaties. Ann Rheum Dis 2000; 59: 404-407.
Braun J, Xiang J, Brandt J, Maetzel H, Haibel H, Wu et al. Treatment of spondyloarthropathies with antibodies against tumor necrosis factor alpha: first clinical and laboratory experiences. Ann Rheum Dis 2000; 59 (Supl 1):185-189.
Collantes E, Muñoz-Villanueva MC, Sanmartí R, Cañete JD, Gratacós J, Zarco P et al. Infliximab in refractory spondyloarthropaties; preliminary report in a spanish population. Ann Rheum Dis 2001; 60 (suppl): 59.
Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001; 357: 1842-1847.
Kruithof E, Van den Bosch F, Baeten D, Herssens A, De Keyser F, Mielants H et al. Repeated infusions of infliximab, a chimeric anti-TNF-α monoclonal antibody, in patients with active spondyloarthropathy: one-year follow-up. Ann Rheum Dis (submitted).
Mease PJ, Goffe BS, Metz J, Vander Stoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000; 356: 385-390.
Van den Bosch F, Kruithof E, Baeten D, De Keyser F, Mielants H, Veys EM. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor alpha (infliximab) in spondyloartrhropathy: an open pilot study. Ann Rheum Dis 2000; 59: 428-433.
Van den Bosch F, Kruithof E, Baeten D, Herssens A, De keyser F, Mielants H et al. Randomised, double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (infliximab) versus placebo in actie spondyloarthropathy. Arthritis Rheum (submitted).
Van den Bosch F, Kruithof E, De Vos M, De Keyser F, Mielants H. Crohn's Disease Associated with Spondyloarthropathy: effect of TNF-alpha Blockade with Infliximab on Articular Symptoms. Lancet 2000; 356, 9244: 1821-1822.